Abstract
Airway protection is the prevention and/or removal of material by behaviors, such as cough and swallow. We tested the hypothesis that cough and swallow, in response to aspiration, are a “meta-behavior” and thus are coordinated and have alterations in excitability to respond to aspiration risk and maintain homeostasis. Anesthetized animals were challenged with a protocol that simulated ongoing aspiration and induced both coughing and swallowing. Electromyograms of the mylohyoid, geniohyoid, thyrohyoid, thyroarytenoid, thyropharyngeus, cricopharyngeus, parasternal, rectus abdominis muscles together with esophageal pressure were recorded to identify and evaluate cough and swallow. During simulated aspiration, both cough and swallow intensity increased and swallow duration decreased consistent with a more rapid pharyngeal clearance. A phase restriction between cough and swallow was also observed; swallow was restricted to the E2 phase of cough during chest wall and abdominal motor quiescence. These results support the conclusion that the cough and swallow pattern generators are an airway protective meta-behavior. The resulting alterations in swallow drive during the simulated aspiration protocol also supports the conclusion that the trachea provides feedback on swallow quality, informing the brainstem about aspiration incidences. The overall coordination of cough and swallow led to the additional conclusion that mechanically the larynx and upper esophageal sphincter act as two separate valves controlling the direction of positive and negative pressures from the upper airway into the thorax.
Keywords: dysphagia, dystussia, airway protection, geniohyoid, mylohyoid, thyrohyoid, thyroarytenoid, thyropharyngeus, inferior pharyngeal constrictor, cricopharyngeus, upper esophageal sphincter, parasternal, inspiratory, inspiration, rectus abdominis, expiratory, expiration, compression, pharyngeal clearance, electromyography, EMG, pressure, esophageal, pharynx, esophagus, oral cavity
1.0 Introduction
Airway protection is the coordination of several behaviors to prevent and/or correct the aspiration of material into the lungs. Two important behaviors in airway protection are swallow and cough. Swallowing is a coordinated behavior that is dependent upon afferent feedback for initiation and modulation. Touch, pressure, and/or liquid on the tongue, faucial pillars, soft palate, uvula, epiglottis, pharyngeal wall, and/or junction of the pharynx/esophagus can induce swallowing (Miller & Scheeington, 1916; Pommerenke, 1928; Storey, 1968; Miller, 1982). Cough is a reflex which responds to material entering the airway by producing high velocity airflows creating shearing forces in larger airways and squeezing actions in smaller airways to remove mucus and foreign matters (Ross et al., 1955; Fontana & Lavorini, 2006; Widdicombe & Chung, 2007).
Disordered airway protection, is clinically defined as intrusion of material below the level of the vocal folds during swallowing (dysphagia), (DePippo et al., 1992; Aviv et al., 1996; Rosenbek et al., 1996a; Robbins et al., 1999; McCullough et al., 2001a; Kalia, 2003; Robbins et al., 2008; Cichero & Altman, 2012), and/or an impaired/lack of cough response to aspiration (dystussia) (Muz et al., 1989; Martin et al., 1994; Smith Hammond et al., 2001; Kelly et al., 2007). Cough is the most noticeable response to aspiration; however there are a host of responses including swallowing, expiration reflex, increased mucous secretions, and/or alterations contractions of the smooth muscle lining the airway (Bolser et al., 1995; Belvisi & Bolser, 2002; Bolser & Davenport, 2007; Vovk et al., 2007). The patient may also exhibit other clinical indicators such as postural changes and changes in voice quality (McCullough et al., 2001b; McCullough et al., 2005; Logemann et al., 2008).
Cough and swallow can both be elicited in experimental models. Cough can be initiated by mechanical stimulation of the trachea or larynx, (Bolser & DeGennaro, 1994; Bolser et al., 2006; Wang et al., 2009; Poliacek et al., 2011) or inhalation of an irritant aerosol (Bolser et al., 1995); and swallow by injection of water into the oropharynx, mechanical stimulation of the pharynx, and/or electrical stimulation of the superior laryngeal nerve (Miller & Sherrington, 1915). Swallow is proposed to be generated by a dorsal and ventral medullary network that may share upper airway motor outputs with that of the respiratory pattern generator (Jean, 2001). The central initiation and rhythmogenesis of swallow is thought to be restricted to the dorsal swallow group and not controlled by the ventral respiratory pattern generator (Jean, 2001). On the other hand, the available evidence supports reconfiguration of existing elements of the respiratory pattern generator in the production of coughing (Shannon et al., 1996; Shannon et al., 1998; Shannon et al., 2004), although some control functions for cough are mediated by brainstem systems that are not required for breathing (Bolser & Davenport, 2002). As such, preclinical data support some sharing of neural elements between the pattern generators for swallow, cough, and breathing but the core network for swallow appears to be anatomically separate within the brainstem from that for cough and breathing (Dick et al., 1993; Oku et al., 1994; Shannon et al., 1996; Baekey et al., 2001).
There are clear clinical associations between dysphagia (disordered swallow) and dystussia (disordered cough) in those with Parkinson’s disease and stroke (Smith Hammond et al., 2001; Pitts et al., 2008; Pitts et al., 2009; Smith Hammond et al., 2009). Training paradigms to influence or prevent episodes of aspiration have been intensely studied (Rosenbek et al., 1991; Schmidt et al., 1994; Ali et al., 1996b; Rosenbek et al., 1996a; Rosenbek et al., 1996b; Aviv et al., 1997; Rosenbek et al., 1998; Aviv et al., 2002; Miller et al., 2006; Clave et al., 2008; Miller, 2008; Troche et al., 2010; Voytas & Al Rifai, 2012), however, few treatments have demonstrated therapeutic effectiveness in modifying or preventing aspiration across patient populations. This may be because the primary treatment is for dysphagia with little intervention for dystussia (Bath et al., 1999; Foley et al., 2008; Wheeler-Hegland et al., 2009).
The clinical association between dysphagia and dystussia could be explained simply by the fact that the minimal neural elements for both cough and swallow are located in the brainstem. In this scenario, disease processes such as stroke, would be expected to affect each behavior similarly because of this anatomical association. However, dystussia and dysphagia can occur in patients with neurologic diseases that do not directly affect the brainstem. Alternatively, a more complex, but not mutually exclusive, hypothesis could account for co-depression of cough and swallow in neurological diseases. A central control system could exist that that coordinates the expression of these behaviors to optimize airway protection. . Additionally, the coordinated expression of several behaviors, each with unique regulation, to achieve a common goal – such as cough and swallow - is consistent with the hypothesis that response to aspiration is a “meta-behavior.” This is analogous to the behavior of autonomous agents used to schedule responses when two or more components are combined to react to incoming stimuli (Guessoum & Briot, 1999). Features of the behavior include “precedence” in which the actions that have little to no central processing take precedence over actions which require additional processing, and “blocking” in which any of the components (behaviors) can block any other action until it is completed. An additional assumption is that the gain or excitability of the components (behaviors) can also be altered without sacrificing homeostasis (Fibla et al., 2010). If these hypotheses are true, this system may be affected and/or impaired by multiple neurologic disease states, which may account for the known clinical associations between disordered cough and swallow. However, the evidence for a coordinating mechanism between reflexive swallow and cough is based solely on inferences from clinical observations.
The aims of this study were to determine if the cough and swallow motor patterns are coordinated and, if so, identify operational principles which govern their interactions following an aspiration event. We hypothesized that during a simulated aspiration, there will be minimal overlap of the cough and swallow behaviors.. Furthermore, we speculated that the behaviors interact spatially to optimize mechanical effectiveness during aspiration.
2.0 Methods
Experiments were performed on 17 spontaneously breathing adult male cats. Ethical approval of the protocol was confirmed by the University of Florida Intuitional Animal Care and Use Committee (IACUC). The animals were initially anesthetized with sodium pentobarbital (35-40 mg/kg i.v.); supplementary doses were administered as needed (1-3 mg/kg i.v.). A dose of atropine sulfate (0.1-0.2 mg/kg, i.v.) was given at the beginning of the experiment to reduce secretions from repeated tracheal stimulation. Cannulas were placed in the femoral artery, femoral vein, and trachea. An esophageal balloon was placed via an oral approach to measure pressure in the midthoracic esophagus. Arterial blood pressure and end-tidal CO2 were continuously monitored. Body temperature was monitored and maintained at 37.5 ± 0.5 °C using a heating lamp and pad. Arterial blood samples were periodically removed for blood gas analysis. PO2 was maintained using air mixtures with enriched oxygen (25-60%) to maintain values above 100 mm Hg.
Electromyograms (EMG) were recorded using bipolar insulated fine wire electrodes. Seven muscles were used to evaluate cough and/or swallow function: mylohyoid, geniohyoid, thyrohyoid, thyropharyngeus, thyroarytenoid, cricopharyngeus, parasternal, and rectus abdominis. The digastric muscles were dissected away from the surface of the mylohyoid and electrodes were placed on the left mylohyoid. A small horizontal incision was made at the rostral end of the right mylohyoid followed by an incision following the midline for approximately 1cm to reveal the geniohyoid underneath. Electrodes were placed 1cm from the caudal insertion of the geniohyoid muscle. The thyroarytenoid electrodes were inserted through the cricothyroid window into the anterior portion of the vocal folds, which were visually inspected post-mortem. Rotation of the larynx and pharynx counterclockwise revealed the superior laryngeal nerve, which facilitated placement of the thyropharyngeus muscle electrodes. The thyropharyngeus is a fan shaped muscle with the smallest portion attached to the thyroid cartilage; electrodes were placed in the ventral, caudal portion of the muscle overlaying thyroid cartilage within 5 mm of the rostral insertion of the muscle. To place the electrodes within the cricopharyngeus muscle, the larynx and pharynx were rotated counterclockwise to reveal the posterior aspect of the larynx. The tissue was palpated for the edge of the cricoid cartilage and electrodes were placed just cranial to the edge of this structure. Thyrohyoid electrodes were inserted approximately one cm rostral to the attachment to the thyroid cartilage; those for the parasternal muscle were placed in the third intercostal space, just adjacent to the sternum. The rectus abdominis electrodes were located approximately two cm caudal to the xiphoid process just medial to the margin of the rectus abdominis. The positions of all electrodes were confirmed by visual inspection and EMG activity patterns during breathing, cough and swallow.
Cough was induced by mechanical stimulation of the extra and intra-thoracic trachea using a thin polyethylene catheter (diameter 1.27mm). The catheter was manually rotated along the length of the intrathoracic trachea. Cough was defined as a burst of activity in the parasternal EMG, followed by (and partially overlapping) a burst in the thyroarytenoid and rectus abdominis, along with a negative to positive change in esophageal pressure. To initiate swallowing, a one-inch long, thin polyethylene catheter (diameter 2.37 mm), attached to a 6cc syringe was placed into the oropharynx. Water was injected into the pharynx via a syringe (3 cc’s). Swallowing was defined as a quiescence of the cricopharyngeus with overlapping activity in the mylohyoid, geniohyoid, thyropharyngeus, thyrohyoid, thyroarytenoid and the parasternal (representing the schluckatmung or swallow breath) (Wilson et al., 1981; Gestreau et al., 2000; Saito et al., 2002; Bonis et al., 2011).
The protocol included non-overlapping stimulus intervals for sequential induction of cough and swallow behaviors followed by temporally overlapped stimulation trials. Mechanical stimulation of the trachea mimics aspiration of material into the trachea and readily provokes vigorous coughing in this model (Bolser et al., 2001; Belvisi & Bolser, 2002; Poliacek et al., 2007; Wang et al., 2009; Poliacek et al., 2011). Injection of water into the oropharynx is a reliable stimulus for swallow. The purpose for this temporal overlap was to simulate aspiration in the presence of a water filled pharyngeal airway. As such, this protocol closely approximated not just a single aspiration event, but the risk of further ingestion of material into the tracheal airway from the pharynx. This process is similar to that experienced by humans with pathologies that predispose them to significant risk of aspiration during a meal (Horner & Massey, 1988; Bushmann et al., 1989; Coates & Bakheit, 1997; Potulska et al., 2003; Prosiegel et al., 2004; Daniels et al., 2006; Miller, 2008; Cabre et al., 2010). The following sequential protocol was used: two trials with mechanical stimulation of the trachea for 20s each, two trials with pharyngeal injection of water, two combined stimulation trials with mechanical stimulation of the trachea for 20s and water injection into the pharynx 5s after the onset of the cough stimulus.
All EMG signals were amplified, filtered (200-5000 Hz), rectified, and integrated (time constant 50 ms). EMG amplitude measures were normalized to the largest cough or swallow respectively. Cough phase durations were measured using the definitions from Wang et al (2009) . The inspiratory phase (CTI), the expiratory phase with active muscle activity (CTE1), passive expiratory phase (CTE2), and total cough (CTtot) durations were measured. CTI was defined as the onset of parasternal activity to the maximum burst of the parasternal EMG, CTE1 was defined as the maximum burst of the parasternal EMG to the end of the abdominal EMG activity, and CTE2 was defined as the end of the abdominal motor burst to the onset of the parasternal EMG activity for the next cough in the epoch. Swallow duration measures were defined as laryngeal elevation: the onset of the mylohyoid to the end of the EMG burst in the geniohyoid; upper esophageal opening: from the sharp decrease in cricopharyngeus activity to its resumption; and total swallow duration: the onset of the mylohyoid activity to the resumption of the cricopharyngeus activity.
Results are expressed as means ± standard error. For statistical analysis Student’s paired t-tests were used to identify differences. Results were corrected for multiple comparison by controlling false discovery rate to 0.05 (Benjamini & Hochberg, 1995). Relationships between normalized burst amplitudes of the cricopharyngeus, parasternal, abdominal, and esophageal pressure during cough was evaluated by linear regression analysis. Relationships between swallow and cough phase were analyzed for randomness using the runs test. A difference was considered significant if the p-value was less than 0.05.
3.0 Results
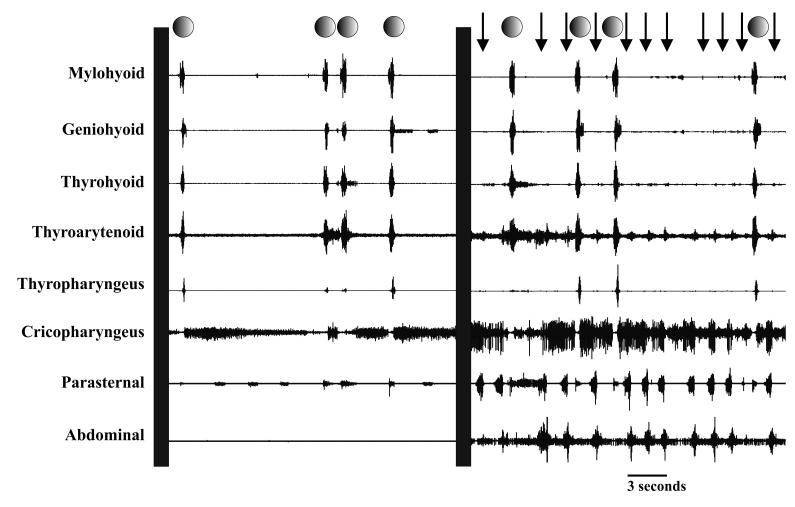
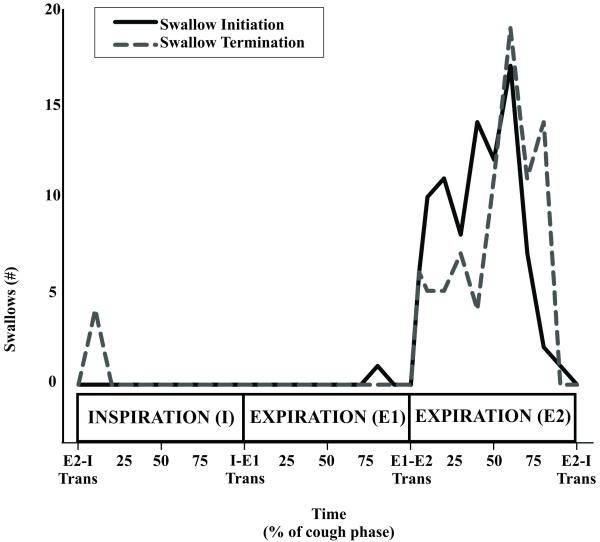
Injection of a water bolus into the oropharynx elicited an average of 2.1± 0.2 swallows with water alone and significantly more swallows 2.9± 0.4 when combined with mechanical stimulation of the trachea (p< 0.01). Mechanical stimulation of the trachea alone elicited an average of 7.5± 0.9 coughs per trial. Raw EMG traces for cough and swallow are represented in Figure 1. Water alone elicited swallow during breathing, and 3% of swallows (2 of 73) were in the inspiratory phase of breathing, 76% of swallows (56 of 73) were during the expiratory phase of breathing, 11% of swallows (8 of 73) were in the transition from inspiration to expiration, and 10% of swallows (7 of 73) were in the transition of expiration to inspiration. The combined stimulation modality produced swallows during sequential cough efforts and 95% of the swallows (87 of 92) were completed during the E2 cough phase. There were four instances of a swallow occurring from the transition of E2 to a cough I phase and one instance of swallow occurring from the transition of E2 to a eupneic inspiration. Figure 2 is a histogram of swallow initiation and termination within the cough phases during the aspiration protocol. Each cough phase (I, E1, and E2) duration measures were normalized to 100, and each phase was segmented into 10 bins (30 bins total over the three phases). The runs test for swallow onset (p < 0.001) and swallow termination (p < 0.001) during the cough phases was non-linear.
Figure 1.
Raw EMG traces of the coordination of cough and swallow. Swallow is denoted by circles and cough by arrows. The first panel is injection of water into the pharynx resulting in four swallows, and the second panel is coordinated coughs and swallows resulting from the aspiration protocol.
Figure 2.
Histogram of swallow initiation and termination within the cough phases during the aspiration protocol. The occurrence and termination of swallows were plotted across cough phases that were segmented into quartiles. Swallows were executed primarily in the E2 cough phase. The solid line is swallow initiation as demarked by elevation of the hyoid and relaxation of the upper esophageal sphincter, and swallow termination was identified by increased tone to the upper esophageal sphincter following relaxation. There is one occurrence of a swallow being initiated during the E1 phase and four occurrences swallows being completed during the I phase of the subsequent cough (n=73 swallows from 17 animals).
The swallows which occurred during the combined stimulus modality had significantly greater EMG amplitudes for the parasternal (p < 0.04), geniohyoid (p < 0.01), thyrohyoid (p< 0.01), thyropharyngeus (p < 0.001), and cricopharyngeus (p ≤ 0.01) (see Table 1). There was also a significant decrease in the parasternal burst duration (p< 0.04). The burst duration of the laryngeal, pharyngeal, and submental muscles did not change significantly in the combined stimulus modality, however the duration of the laryngeal elevation (p ≤ 0.01), the opening of the upper esophageal sphincter (p < 0.01), and the total swallow duration (P < 0.01) were significantly decreased.
Table 1.
Effect of water and water plus tracheal stimulation (TS + Water) on normalized EMG amplitudes and durations (ms) of selected swallow-related muscles.
| Amplitude (% of maximum)^ | Water | TS + Water |
|---|---|---|
| Parasternal | 51 ± 6 | 67 ± 4* |
| Mylohyoid | 68 ± 5 | 78 ± 5 |
| Geniohyoid | 69 ± 4 | 82 ± 3** |
| Thyroarytenoid | 75 ± 3 | 80 ± 3 |
| Thyrohyoid | 78 ± 3 | 88 ± 1** |
| Thyropharyngeus | 61 ± 4 | 74 ± 2** |
| Cricopharyngeus (post-relaxation burst) | 52 ± 6 | 70 ± 4** |
|
| ||
| Duration (ms) | Water | TS ± Water |
|
| ||
| Laryngeal Elevation□ | 507 ± 38 | 430 ± 30** |
| Total Swallow | 572 ± 43 | 508 ± 36** |
| Upper Esophageal Sphincter Open | 455 ± 25 | 386 ± 14** |
| Parasternal | 419 ± 46 | 331 ± 43* |
| Mylohyoid | 468 ± 39 | 420 ± 31 |
| Geniohyoid | 366 ± 33 | 360 ± 32 |
| Thyroarytenoid | 440 ± 25 | 418 ± 34 |
| Thyrohyoid | 398 ± 39 | 392 ± 24 |
| Thyropharyngeus | 182 ± 24 | 185 ± 25 |
p ≤ 0.05
P ≤ 0.01
Onset of mylohyoid activity to offset of geniohyoid activity.
EMG’s were normalized to the maximum EMG amplitude.
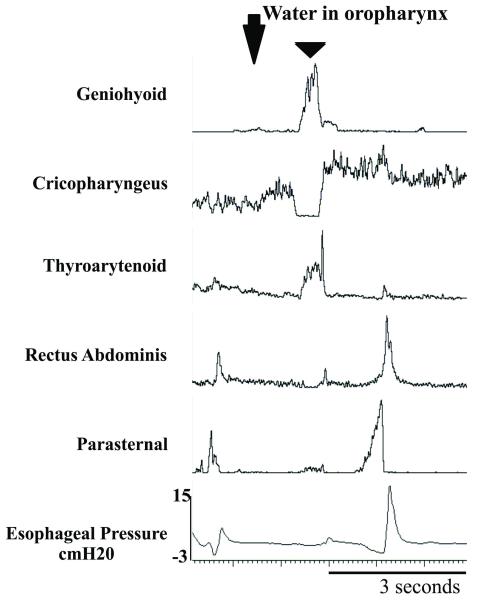
Repetitive coughs were compared before and after the introduction of the swallow stimulus into the oropharynx. The coughs which occurred after the introduction of the swallow stimulus had significantly greater EMG amplitudes for the parasternal (p < 0.05), rectus abdominis (p < 0.001), thyropharyngeus (p < 0.01), and the positive component of esophageal pressure (p < 0.02) significantly increased (Table 2 and Figure 3). There were no significant changes in CTI and CTE1 phases of cough. Additionally, CTE2 durations with and without a swallow present were compared. Following the injection of water into the oropharynx, CTE2 with a swallow was significantly longer (p < 0.05) than those without a swallow.
Table 2.
Effect of tracheal stimulation (TS) and water plus tracheal stimulation (TS + Water) on normalized EMG amplitudes and durations (ms) of selected cough-related muscles and pressure. Change in CTE2 duration when no swallow was present versus swallow present.
| Amplitude (% of maximum)^ | TS | TS + Water |
|---|---|---|
| Parasternal | 58 ± 4 | 71 ± 3* |
| Rectus Abdominis | 48 ± 5 | 69 ± 3*** |
| Thyroarytenoid | 52 ± 5 | 62 ± 5 |
| Cricopharyngeus | 71 ± 3 | 73 ± 3 |
| Thyrohyoid | 54 ± 6 | 65 ± 5** |
| Esophageal Pressure (cm H20) | 19 ± 3 | 31 ± 5** |
|
| ||
| Duration (ms) | TS | TS ± Water |
|
| ||
| Inspiratory | 823 ± 93 | 835 ± 115 |
| Compression | 139 ± 19 | 147 ± 16 |
| Expiratory 1 (E1) | 437 ± 38 | 408 ± 28 |
| Total Cough Cycle | 5286 ± 1169 | 4338 ± 1006 |
|
| ||
| Duration (ms) | No swallow | With swallow |
|
| ||
| Expiratory 2 (E2)□ | 1497 ± 890 | 3097 ± 2783* |
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
E2 durations after water injected into the pharynx
EMG’s were normalized to the maximum EMG amplitude.
Figure 3.
Change in cough motor drive with injection of water into the oropharynx. Triangle denotes swallow. Note the increased rectus abdominis and parasternal electromyographic activity and expiratory esophageal pressure in the second cough.
3.1 Additional observations on pharyngeal muscle activities during eupnea and cough
The cricopharyngeus (upper esophageal sphincter) was active during repetitive cough (Figure 1, 3, and 4). It had an augmenting pattern over the inspiratory phase, peaked at the transition from the inspiratory to the expiratory phase, and declined in magnitude during the expiratory phase. The cricopharyngeus EMG amplitude was not correlated with the peak in the positive esophageal pressure (r2 = 0.005), rectus abdominis amplitude (r2 = 0.08), or parasternal amplitude (r2 = 0.04) during coughing. The thyropharyngeus muscle had expiratory phasic activity during eupnea which decreased during the cough stimulus and repetitive coughing (Figure 4).
Figure 4.
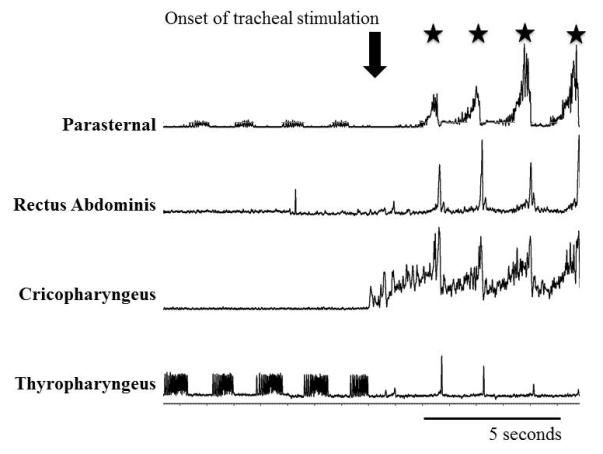
Cannula was inserted into the trachea at the downward arrow. Cough cycles are noted with stars. Expiratory phasic thyropharyngeus EMG activity was suppressed, and cricopharyngeus EMG activity was increased at the onset of the tracheal stimulation. The cricopharyngeus EMG has dynamic activity during cough with the peak during the transition from the inspiratory to the expiratory phase.
4.0 Discussion
This is the first study to examine the coordination of cough and swallow during the aspiration response. The aspiration protocol elicited significantly more swallows than the water bolus without concurrent tracheal stimulation. Most (95%) of the aspiration swallows occurred during the E2 phase of cough. The total swallow duration was decreased without decreasing the burst duration of any pharyngeal/laryngeal/submental muscles, and there was an increase in the EMG magnitude of the pharyngeal muscles, inspiratory muscle activity for schluckatmung production, and hyoid elevators (geniohyoid and thyrohyoid) for swallows that occurred during repetitive coughing episodes. The duration of cough E2 phases which contained a swallow were significantly longer than in control trials. Moreover, chest wall and abdominal (inspiratory and expiratory) and thyrohyoid muscle electromyographic activity increased during coughs following the injection of the water into the pharynx as did expiratory esophageal pressures. Cricopharyngeus activity was also elevated during coughing.
4.1 Phase restriction of swallow
Swallowing that is normally executed during eupnea has been intensely studied, and there is a phase preference for swallows to occur during the expiratory phase of breathing; specifically that 80% of swallows occur during the expiratory phase of breathing in humans (Martin-Harris et al., 2003; Wheeler Hegland et al., 2009; Wheeler Hegland et al., 2011), cats (Dick et al., 1993), goats (Feroah et al., 2002a; Feroah et al., 2002b; Bonis et al., 2011), and rats (Saito et al., 2003). Our observations are indicative of a more rigid regulatory control of swallowing during aspiration promoting events, i.e. swallow during repetitive coughing. We propose the concept of phase restriction to explain the fixed occurrence of swallows during the quiescent period following the cough expulsion (cough E2) (Figure 1 and 2). This idea is reinforced by cough E2 prolongation, ensuring adequate time for swallow initiation and completion before the onset of the next inspiratory phase (Figure 1 and 2). This rigid control system is necessary because both cough and swallow share the pharyngeal airway, and the presence of food or liquid in this airway segment represents a significant aspiration risk.
Forssberg, Grillner and Rossignol (1975), Sillar (1991), Watson (1992), and Pearson (1993) all proposed a filtering of afferent information to ensure an appropriate motor response within the context of the ongoing motor activity. This concept includes roles for afferent feedback in the establishment of the temporal order of motor behaviors and in controlling transitions from one behavior to another. Furthermore the decision to produce a behavior, based on afferent information, is dependent on the state of the ongoing motor behaviors, like breathing and/or coughing (Forssberg et al., 1975; Sillar, 1991; Watson, 1992; Pearson, 1993). This effect has been established in other systems including: chick hatching and stepping (Bekoff et al., 1987), hand movements in primates (Sanes et al., 1985), and flight in the locust (Wolf & Pearson, 1987) by using models of deafferentation. Our results support a theory of filtering of pharyngeal and laryngeal afferent information by brainstem networks to inhibit the swallow pattern generator during the inspiratory and active expiration phases of cough.
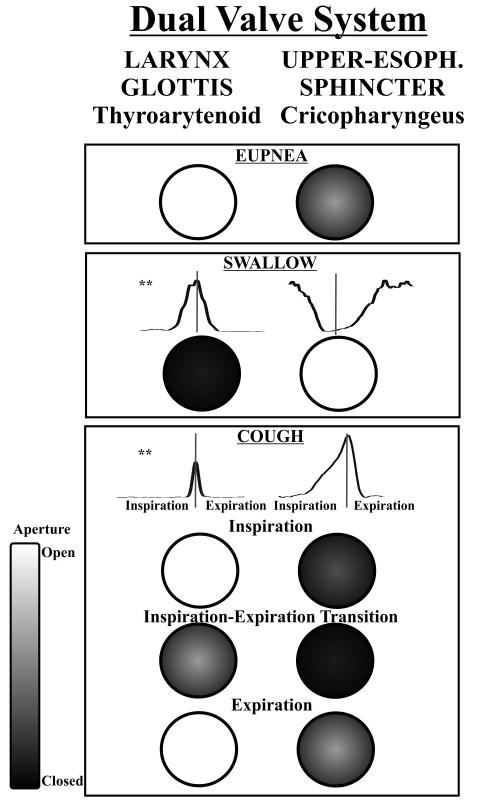
4.2 Dual valve system
Our results also suggest a highly coordinated control of both the laryngeal airway and upper esophageal sphincter such that they may represent a dual valve system regulating pressure between upper airway and the thoracic cavity (Figure 5). To our knowledge, prior work on cough or swallowing did not observe signatures of a unified control system for the larynx and upper esophageal sphincter. The laryngeal adductor/abductor and upper esophageal sphincter control air/bolus flow into or out-of the lungs or esophagus. For example, during swallowing there is maximal activity of the laryngeal adductor muscles and maximum relaxation of the upper esophageal sphincter, and thus pressures move the bolus into the esophagus and not into the larynx/trachea. Our data support the presence of a reciprocal relationship for cough as well. During our experiments the EMG activity of the cricopharyngeus muscle was very sensitive to mechanical stimulation in the trachea (Figure 4). EMG activity of this muscle steadily increased over the inspiratory period, peaking during the transition from inspiration to E1 and decreased during the E1 and E2 phases. This mechanism mechanically “seals” the upper esophageal sphincter during cough to prevent loss of intra-thoracic pressure and thus maximizes cough effectiveness. This increased cricopharyngeus muscle activity also reduces the risk of esophageal reflux into the pharynx.
Figure 5.
Dual valve system hypothesis. The side by side circles represent the two valves, the larynx and the upper esophageal sphincter. Closure of the two valves is controlled by the thyroarytenoid and the cricopharyngeus muscles, respectively. The valve aperture is represented on a scale from white (maximum opening) to black (maximum closure). Note: during the expiratory phase of eupnea there is some thyroarytenoid activity. ** Thyroarytenoid and cricopharyngeal electromyogram waveform averages across multiple behavior occurrences during cough and swallow. The vertical gray line in the second and third panels is the midline marker of the behavior execution, and during cough this represents the transition from inspiration to active expiration.
Given this dual valve system (Figure 5), swallowing can occur during the inspiratory phase of breathing because trans-laryngeal flows and intra-thoracic pressure are relatively low. We propose that swallow-related laryngeal adductor activity (glottic and supra-glottic) is sufficient to close the airway during a eupneic breathing cycle; however the much higher inspiratory and expiratory flows and pressure during coughing would make it mechanically difficult for a successful bolus transfer across the esophageal sphincter. Even during the compression phase (a time of little or no trans-laryngeal flow) the pressure in the thoracic cavity is high and hindering bolus movement.
Shannon, et al (1996; 1998; 2004), and Bolser and Davenport (2002) proposed that the temporal regulation of the expiratory phase of cough is altered by excitability of the expiratory-augmenting late neurons (i.e neurons which fire during the expiratory phase of breathing with significantly more action potentials in the second half of the expiratory period as compared to the first half) within the Bötzinger Complex. We propose that the swallow pattern generator interacts with these neurons, increasing their excitability to prolong the cough E2 phase. These control mechanisms may represent a neural substrate that is essential for airway protection, and may help to explain how a wide range of neurologic diseases results in related dysphagia and dystussia (Smith Hammond et al., 2001; Pitts et al., 2008; Pitts et al., 2009; Smith Hammond et al., 2009; Pitts et al., 2010).
An absent cough response to aspiration is a hallmark of dysphagia, and may be a manifestation of an impaired sensory feedback control system. During a clinical evaluation of swallow, material can penetrate to the level of the vocal folds without a cough or expiration response in young adults during swallowing (Daniels et al., 2004; Daggett et al., 2006). We hypothesize that the trachea, with or without the laryngeal afferent activation, provides feedback on swallow quality, on a cycle by cycle basis. Additionally, dysphagia caused by neurotrauma (Aviv et al., 1996) or damage to the vagus (Halum et al., 2003) can result in a condition known as cricopharyngeal bar and laryngeal dysfunction. Cricopharyngeal bar is defined as hyperactivity of the cricopharyngeus during swallow, resulting in obstruction of the esophageal opening during swallowing leading to residue in the pharynx (Aviv et al., 1996; Halum et al., 2003). These clinical findings are consistent with the dual valve system, representing a control system that when dysfunctional, results in pathological behavior of both valves.
4.3 Pharyngeal clearance
Jean (1984, 2001) discussed oral and pharyngeal afferent feedback as a primary modulator of the swallow motor pattern generator. More specifically the size, texture, taste, temperature of the bolus or pharyngeal distention can alter the swallow pattern (Kahrilas & Logemann, 1993; Logemann et al., 1995; Ali et al., 1996a; Rademaker et al., 1998; Ertekin et al., 2000; Hiss et al., 2001; Jean, 2001; Kendall & Leonard, 2001; Kendall et al., 2001; Butler et al., 2004; Chee et al., 2005; Leow et al., 2007; Troche et al., 2008; Humbert et al., 2009; Thexton et al., 2009; Yamamura et al., 2010). This effect has not been previously demonstrated by stimulation of tracheal afferents. Mechanical stimulation of the trachea activates afferent receptors (c-fibers and rapidly adapting receptors) with axons in the recurrent laryngeal nerve (Kalia & Mesulam, 1980). Our results thus support the hypothesis that tracheal receptors, in addition to the pharyngeal and esophageal receptors proposed by Jean, (1984) modulate the central pattern generator for swallow
Increased swallow EMG activity of the geniohyoid, thyrohyoid, thyropharyngeus, parasternal and post-swallow cricopharyngeus during mechanical stimulation of the trachea is evidence of increased pharyngeal clearance. This is manifested by increased swallow intensity and increased swallow occurrence during the aspiration protocol. These results indicate that mechanical stimulation of the trachea alters activity patterns of submental and pharyngeal muscles. We hypothesized this increased drive would result in increased pharyngeal clearance, because it was also accompanied by decreases in total swallow and laryngeal elevation time. Note these changes were not perpetuated by a decrease in individual muscle activation time, but a faster activation of the oral-pharyngeal-upper esophageal sphincter wave (Table 1).
This is the first report of modulation of the posterior pharyngeal constrictor, the thyropharyngeus muscle during coughing. The implications of this finding extend beyond the activity pattern of a single muscle upper airway muscle during cough or breathing. The activity of this muscle is an additional manifestation of novel coordinating mechanisms between cough and swallow. We believe that effective pharyngeal clearance is also accomplished through a complex interplay of material ejected by cough and subsequently swallowed. Figure 4 shows an example of phasic expiratory activity of the thyropharyngeus muscle. The thyropharyngeus muscle controls the diameter of the pyriform sinus, adjacent to the laryngeal vestibule, which acts as a reservoir for material within the pharynx during swallowing. Accumulated material in the pyriform sinus is similar to bolus accumulation before/during the pharyngeal phase of swallow, mucus ejected from the lower airways by coughing could pool in the pyriform sinus. We hypothesize that the pyriform sinus remains open to accommodate material ejected by coughing by a reduction in phasic activity of the thyropharyngeus muscle. During subsequent swallows contraction of the thyropharyngeus then collapses the sinus, emptying the contents into the esophagus.
We note that due to the open trachea in this preparation, the alterations in thyropharyngeus muscle EMGs were likely the result of modifications of central mechanisms driving this motoneuron pool rather than sensory feedback from material deposited in the pharynx or pharyngeal airflow. The extent, to which these motor responses of the thyropharyngeus muscle were due to inhibition from the cough pattern generator or due to activation of tracheal afferents, or both, is unknown. Cough motor drive was also increased in the cycles immediately following a swallow occurrence (Figure 2). This observation is explained by one of at least two alternate hypotheses: a) prolonged excitatory relationships exists between the swallow and cough pattern generators in addition to short-term phase restriction, and/or b) prolongation of the preceding cough E2 phase enhances synaptic drive to spinal and upper airway motoneurons. This dynamic interplay between these behaviors and may be a central motor program in anticipation of increased cough-related airflow shear forces in the pharyngeal airway following the swallow.
4.4 Meta-behavior
The results support the idea that the production of cough and swallow in response to aspiration is a “meta behavior” We observed alterations in gain of cough and swallow when the behaviors were induced in the aspiration protocol. In this context, alterations in behavioral gain are consistent with allostasis, or the maintenance of stability through change (Fibla et al., 2010). Allostasis describes a process by which organisms adjust to predictable and unpredictable events. Meta-behavioral responses, such as the coordination of cough and swallow reported in this study, provide tools by which the central nervous system achieves allostasis. Our data also support precedence and blocking as important control mechanisms in airway protection. The specific brainstem mechanisms that underlie these control features are unknown. However, they likely represent a substrate for pathological processes that result in dysphagia and dystussia.
One implication of this designation is that no single behavior is sufficient to protect the airway from aspiration. In patients that are unable to swallow, feeding occurs via a stomach tube to bypass the pharynx (Norton et al., 1996; Britton et al., 1997; Meng et al., 2000; Heffernan et al., 2004). If untreated, these patients will aspirate and are likely to acquire pneumonia (Wada et al., 2000; Kaplan et al., 2002; Cabre et al., 2010). While some of these patients might be able to cough, the presence of coughing does not alter the clinical strategy for their management. Clinical decision-making de facto discounts the sufficiency of coughing alone to prevent pneumonia. It is much more common to encounter patients who have impairment of both cough and swallow [stroke (Smith Hammond et al., 2001; Smith Hammond et al., 2009), PD (Pitts et al., 2008; Pitts et al., 2009; Pitts et al., 2010; Troche et al., 2010; Pitts et al., 2012), etc.], consistent with a linked control system for these behaviors in humans.
4.5 Limitations of the experimental design
A limitation of the experimental design was the use of sodium pentobarbital anesthesia, and its effects on respiratory motor drive. Warner and colleagues (1992), in sodium pentobarbital anesthetized dogs, demonstrated acute suppression of expiratory motor drive during breathing, but the effects were ameliorated over time in spite of constant plasma levels of this anesthetic. Warner et al (1992) concluded that the expiratory depressant effects of this anesthetic were transient. Our laboratory has shown that vigorous cough expiratory motor responses occur in cats anesthetized with sodium pentobarbital (Bolser et al., 2000; Bolser and Davenport, 2000).
An additional limitation was the use of a single stimulus modality to induce each behavior (mechanical for cough and water for swallow). It is not yet known if cough induced by chemical stimuli and/or swallow induced by mechanical/chemical stimuli (e.g., various bolus types of different size, texture, taste, etc.) would be coordinated. However, we hypothesize that these coordinating mechanisms are primarily central in nature, and not dependent on afferent modality. As such, we predict that this meta-behavioral response will be observed regardless of stimulus modality.
5.0 Conclusion
Cough and swallow are highly coordinated through defined excitatory and inhibitory central interactions. This inter-behavior control system minimizes the risk of aspiration and is consistent with the existence of a meta-behavioral control system. These operating principles provide a framework for integrating models of dysphagia and dystussia. Furthermore, increased cough and swallow excitability during simulated aspiration suggests a novel role of tracheal afferent feedback for informing this meta-behavioral control system for airway protection regarding aspiration.
Highlights.
This manuscript describes novel mechanisms which regulate the coordination of cough and swallow specifically in response to aspiration.
Our work demonstrates the existence of common system that is sophisticated and exerts control over these behaviors at several different levels.
This knowledge will stimulate research aimed to understand the control of these behaviors as integrated and coupled.
Acknowledgments
NIH Institute of Heart Lung and Blood; HL89104, HL103415, HL109025, and HL107745
Footnotes
Author Contributions: TEP: Experimental design, performing experiments, analyzing data, interpreting data, and manuscript preparation
MJR: Experimental design, performing experiments, analyzing data
ANM: Experimental design, performing experiments, analyzing data
IP: Experimental design, performing experiments, interpreting data, and manuscript preparation
CMS: Experimental design, interpreting data, and manuscript preparation
BGL: Experimental design, interpreting data, and manuscript preparation
KFM: Experimental design, interpreting data, and manuscript preparation
PWD: Experimental design, performing experiments, interpreting data, and manuscript preparation
DCB: Experimental design, performing experiments, analyzing data, interpreting data, and manuscript preparation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ali GN, Laundl TM, Wallace KL, deCarle DJ, Cook IJS. Influence of cold stimulation on the normal pharyngeal swallow response. Dysphagia. 1996a;11:2–8. doi: 10.1007/BF00385791. [DOI] [PubMed] [Google Scholar]
- Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996b;110:383–392. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Martin JH, Sacco RL, Zagar D, Diamond B, Keen MS, Blitzer A. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Annals of Otology, Rhinology and Laryngology. 1996;105:92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Sacco RL, Mohr JP, Thompson JL, Levin B, Sunshine S, Thomson J, Close LG. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107:1254–1260. doi: 10.1097/00005537-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. Journal of Physiology. 2001;534:565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath P, Bath F, Smithard D. Interventions for dysphagia in acute stroke. Cochrane Database System Review. 1999;2 doi: 10.1002/14651858.CD000323. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Nusbaum MP, Sabichi AL, Clifford M. Neural control of limb coordination. I. Comparison of hatching and walking motor output patterns in normal and deafferented chicks. The Journal of Neuroscience. 1987;7:2320–2330. [PMC free article] [PubMed] [Google Scholar]
- Belvisi MG, Bolser DC. Summary: animal models for cough. Pulmonology Pharmacology and Therapeutics. 2002;15:249–250. doi: 10.1006/pupt.2002.0349. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulmonology Pharmacology and Therapeutics. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Current Opinion in Allergy and Clinical Immunology. 2007;7:32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC. Effect of codeine on the inspiratory and expiratory burst pattern during fictive cough in cats. Brain Research. 1994;662:25–30. doi: 10.1016/0006-8993(94)90792-7. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O’Reilly S, Hey JA, Chapman RW. Pharmacological studies of allergic cough in the guinea pig. European Journal of Pharmacology. 1995;277:159–164. doi: 10.1016/0014-2999(95)00076-w. [DOI] [PubMed] [Google Scholar]
- Bolser DC, McLeod RL, Tulshian DB, Hey JA. Antitussive action of nociceptin in the cat. European Journal of Pharmacology. 2001;430:107–111. doi: 10.1016/s0014-2999(01)01244-4. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respiratory Physiology and Neurobiology. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis J, Neumueller S, Marshall B, Krause K, Qian B, Pan L, Hodges M, Forster H. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respiratory Physiology and Neurobiology. 2011;175:272–282. doi: 10.1016/j.resp.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J, Lipscomb G, Mohr P, Rees W, Young A. The use of percutaneous endoscopic gastrostomy (PEG) feeding tubes in patients with neurological disease. Neurology. 1997;244:431–434. doi: 10.1007/s004150050117. [DOI] [PubMed] [Google Scholar]
- Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–1314. doi: 10.1212/wnl.39.10.1309. [DOI] [PubMed] [Google Scholar]
- Butler SG, Postma GN, Fischer E. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngology Head and Neck Surgery. 2004;131:860. doi: 10.1016/j.otohns.2004.06.706. [DOI] [PubMed] [Google Scholar]
- Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age and Ageing. 2010;39:39. doi: 10.1093/ageing/afp100. [DOI] [PubMed] [Google Scholar]
- Chee C, Arshad S, Singh S, Mistry S, Hamdy S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chemical Senses. 2005;30:393–400. doi: 10.1093/chemse/bji034. [DOI] [PubMed] [Google Scholar]
- Cichero JA, Altman KW. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources. Nestle Nutritional Institute Workshop Series. 2012;72:1–11. doi: 10.1159/000339974. [DOI] [PubMed] [Google Scholar]
- Clave P, Arreola V, Romea M, Medina L, Palomera E, Serra-Prat M. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clinical Nutrition. 2008;27:806–815. doi: 10.1016/j.clnu.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. European Neurology. 1997;38:49–52. doi: 10.1159/000112902. [DOI] [PubMed] [Google Scholar]
- Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21:270–274. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Corey DM, Hadskey LD, Legendre C, Priestly DH, Rosenbek JC, Foundas AL. Mechanism of sequential swallowing during straw drinking in healthy young and older adults. Journal of Speech and Hearing Research. 2004;47:33–45. doi: 10.1044/1092-4388(2004/004). [DOI] [PubMed] [Google Scholar]
- Daniels SK, Schroeder MF, McClain M, Corey DM, Rosenbek JC, Foundas AL. Dysphagia in stroke: Development of a standard method to examine swallowing recovery. Journal of Rehabilitative Research and Development. 2006;43:347–356. doi: 10.1682/jrrd.2005.01.0024. [DOI] [PubMed] [Google Scholar]
- DePippo KL, Holas MA, Reding MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Archives of Neurology. 1992;49:1259–1261. doi: 10.1001/archneur.1992.00530360057018. [DOI] [PubMed] [Google Scholar]
- Dick T, Oku Y, Romaniuk J, Cherniack N. Interaction between central pattern generators for breathing and swallowing in the cat. Journal of Physiology. 1993;465:715. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertekin C, Kiylioglu N, Tarlaci S, Keskin A, Aydogdu I. Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterology Motility. 2000;12:567–572. doi: 10.1046/j.1365-2982.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- Feroah TR, Forster H, Fuentes CG, Lang IM, Beste D, Martino P, Pan L, Rice T. Effects of spontaneous swallows on breathing in awake goats. Journal of Applied Physiology. 2002a;92:1923–1935. doi: 10.1152/japplphysiol.01079.2000. [DOI] [PubMed] [Google Scholar]
- Feroah TR, Forster H, Fuentes CG, Wenninger J, Martino P, Hodges M, Pan L, Rice T. Contributions from rostral medullary nuclei to coordination of swallowing and breathing in awake goats. Journal of Applied Physiology. 2002b;93:581–591. doi: 10.1152/japplphysiol.01268.2001. [DOI] [PubMed] [Google Scholar]
- Fibla MS, Bernardet U, Verschure PF. Allostatic control for robot behaviour regulation: An extension to path planning. Intelligent Robots and Systems (IROS), 2010 IEEE/RSJ International Conference on; IEEE; 2010. pp. 1935–1942. [Google Scholar]
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age and Ageing. 2008;37:258–264. doi: 10.1093/ageing/afn064. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Lavorini F. Cough motor mechanisms. Respiratory Physiology and Neurobiology. 2006;152:266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Research. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Experimental Brain Research. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- Guessoum Z, Briot J-P. From active objects to autonomous agents. Concurrency, IEEE. 1999;7:68–76. [Google Scholar]
- Heffernan C, Jenkinson C, Holmes T, Feder G, Kupfer R, Leigh PN, McGowan S, Rio A, Sidhu P. Nutritional management in MND/ALS patients: an evidence based review. Amyotrophic Lateral Sclerosis. 2004;5:72–83. doi: 10.1080/14660820410020349. [DOI] [PubMed] [Google Scholar]
- Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16:128–135. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- Horner J, Massey EW. Silent aspiration following stroke. Neurology. 1988;38:317–319. doi: 10.1212/wnl.38.2.317. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins JA. Neurophysiology of swallowing: Effects of age and bolus type. Neuroimage. 2009;44:982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. Journal of the Autonomic Nervous System. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological Review. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Logemann JA. Volume accommodation during swallowing. Dysphagia. 1993;8:259–265. doi: 10.1007/BF01354548. [DOI] [PubMed] [Google Scholar]
- Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism. 2003;52:36–38. doi: 10.1016/s0026-0495(03)00300-7. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam M. Brain stem projections of sensory and motor components of the vagus complex in the cat. Journal of Comparative Neurology. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kaplan V, Angus DC, Griffin MF, Clermont G, Watson RS, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly age-and sex-related patterns of care and outcome in the united states. American Journal of Respiratory and Critical Care Medicine. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117:1723–1727. doi: 10.1097/MLG.0b013e318123ee6a. [DOI] [PubMed] [Google Scholar]
- Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope. 2001;111:2017–2021. doi: 10.1097/00005537-200111000-00028. [DOI] [PubMed] [Google Scholar]
- Kendall KA, Leonard RJ, McKenzie SW. Accommodation to changes in bolus viscosity in normal deglutition: a videofluoroscopic study. Annals Otology Rhinology and Laryngology. 2001;110:1059–1065. doi: 10.1177/000348940111001113. [DOI] [PubMed] [Google Scholar]
- Leow L, Huckabee M, Sharma S, Tooley T. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chemical Senses. 2007;32:119. doi: 10.1093/chemse/bjl037. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Gensler G, Robbins JA, Lindblad AS, Brandt D, Hind JA, Kosek S, Dikeman K, Kazandjian M, Gramigna GD. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. Journal of Speech, Language and Hearing Research. 2008;51:173. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. Journal of Speech, Language and Hearing Research. 1995;38:556. doi: 10.1044/jshr.3803.556. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. Journal of Applied Physiology. 2003;94:1735. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- Martin BJW, Corlew MM, Wood H, Olson D, Golopol LA, Wingo M, Kirmani N. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9:1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- McCullough G, Wertz R, Rosenbek J. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. Journal of Communication Disorders. 2001a;34:55–72. doi: 10.1016/s0021-9924(00)00041-1. [DOI] [PubMed] [Google Scholar]
- McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. Journal of Speech Language and Hearing Research. 2005;48:1280–1293. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- McCullough GH, Wertz RT, Rosenbek JC, Mills RH, Webb WG, Ross KB. Inter- and intrajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia. 2001b;16:110–118. doi: 10.1007/PL00021291. [DOI] [PubMed] [Google Scholar]
- Meng N-H, Wang T-G, Lien I-N. Dysphagia in patients with brainstem stroke: incidence and outcome. American Journal of Physical Medicine and Rehabilitation. 2000;79:170–175. doi: 10.1097/00002060-200003000-00010. [DOI] [PubMed] [Google Scholar]
- Miller A. The neurobiology of swallowing and dysphagia. Developmental Disabilities. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Deglutition. Physiological Review. 1982;62:129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Miller F, Sherrington C. Some observations on the bucco-pharyngeal stage of reflex deglutition in the cat. Experimental Physiology. 1915;9:147–186. [Google Scholar]
- Miller FR, Scheeington CS. Some observations on the buccopharungeal stage of reflex deglutition in the cat. Quarterly Journal of Experimental Physiology. 1916;9:147–186. [Google Scholar]
- Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age and Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- Muz J, Mathog RH, Nelson R, Jones LA., Jr Aspiration in patients with head and neck cancer and tracheostomy. Americal Journal of Otolaryngology. 1989;10:282–286. doi: 10.1016/0196-0709(89)90009-4. [DOI] [PubMed] [Google Scholar]
- Norton B, Homer-Ward M, Donnelly M, Long R, Holmes G. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13–16. doi: 10.1136/bmj.312.7022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. Journal of Physiology. 1994;480(Pt 2):309–324. doi: 10.1113/jphysiol.1994.sp020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. Common principles of motor control in vertebrates and invertebrates. Annual Review of Neuroscience. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Morris K, Lindsey B, Davenport P, Poliacek I, Bolser D. Co-ordination of cough and swallow in vivo and in silico. Experimental Physiology. 2012;97:469–473. doi: 10.1113/expphysiol.2011.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Troche MS, Carnaby-Mann G, Rosenbek JC, Okun MS, Sapienza CM. Utilizing voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in Parkinson’s disease. Chest. 2010;138:1426–1431. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- Poliacek I, Corrie LWC, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. Journal of Applied Physiology. 2007;102:1014–1021. doi: 10.1152/japplphysiol.00616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Morris KF, Lindsey BG, Segers LS, Rose MJ, Corrie LWC, Wang C, Pitts TE, Davenport PW, Bolser DC. Blood pressure changes alter tracheobronchial cough: computational model of the respiratory-cough network and in vivo experiments in anesthetized cats. Journal of Applied Physiology. 2011;111:861–873. doi: 10.1152/japplphysiol.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommerenke WT. A study of the sensory areas eliciting the swallow reflex. American Journal of Physiology. 1928;84:36–41. [Google Scholar]
- Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism and Related Disorders. 2003;9:349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- Prosiegel M, Schelling A, Wagner-Sonntag E. Dysphagia and multiple sclerosis. Internaltional Multiple Sclerosis Journal. 2004;11:22–31. [PubMed] [Google Scholar]
- Rademaker AW, Pauloski BR, Colangelo LA, Logemann JA. Age and volume effects on liquid swallowing function in normal women. Journal of Speech, Language, and Hearing Research. 1998;41:275. doi: 10.1044/jslhr.4102.275. [DOI] [PubMed] [Google Scholar]
- Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D, Baum H, Lilienfeld D, Kosek S, Lundy D. Comparison of 2 interventions for liquid aspiration on pneumonia incidence. Annals of Internal Medicine. 2008;148:509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins J, Fishback B, Levine RL. Effects of thermal application on dysphagia after stroke. Journal of Speech and Hearing Research. 1991;34:1257–1268. doi: 10.1044/jshr.3406.1257. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins J, Willford WO, Kirk G, Schiltz A, Sowell TW, Deutsch SE, Milanti FJ, Ashford J, Gramigna GD, Fogarty A, Dong K, Rau MT, Prescott TE, Lloyd AM, Sterkel MT, Hansen JE. Comparing treatment intensities of tactile-thermal application. Dysphagia. 1998;13:1–9. doi: 10.1007/PL00009542. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996a;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996b;11:225–233. doi: 10.1007/BF00265206. [DOI] [PubMed] [Google Scholar]
- Ross BB, Gramiak R, Rahn H. Physical dynamics of the cough mechanism. Journal of Applied Physiology. 1955;8:264–268. doi: 10.1152/jappl.1955.8.3.264. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I. Swallowing-related activities of respiratory and non-respiratory neurons in the nucleus of solitary tract in the rat. Journal of Physiology. 2002;540:1047–1060. doi: 10.1113/jphysiol.2001.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I, Osawa M. Activity of neurons in ventrolateral respiratory groups during swallowing in decerebrate rats. Brain and Development. 2003;25:338–345. doi: 10.1016/s0387-7604(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Sanes J, Mauritz K, Dalakas M, Evarts E. Motor control in humans with large-fiber sensory neuropathy. Human Neurobiology. 1985;4:101. [PubMed] [Google Scholar]
- Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9:7–11. doi: 10.1007/BF00262752. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulmonary Pharmacology and Therapeutics. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey D, Morris K, Nuding S, Segers L, Lindsey B. Production of reflex cough by brainstem respiratory networks. Pulmonary Pharmacology and Therapeutics. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. Journal of Applied Physiology. 1998;84:2020. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Sillar KT. Spinal pattern generation and sensory gating mechanisms. Current Opinion in Neurobiology. 1991;1:583–589. doi: 10.1016/s0959-4388(05)80032-7. [DOI] [PubMed] [Google Scholar]
- Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135:769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- Storey AT. Laryngeal initiation of swallowing. Experimental Neurology. 1968;20:359–365. doi: 10.1016/0014-4886(68)90079-4. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. Journal of Neurophysiology. 2009;101:1386–1393. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche M, Okun M, Rosenbek J, Musson N, Fernandez H, Rodriguez R, Romrell J, Pitts T, Wheeler-Hegland K, Sapienza C. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST. Neurology. 2010;75:1912–1919. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson’s disease. Dysphagia. 2008;23:26–32. doi: 10.1007/s00455-007-9090-7. [DOI] [PubMed] [Google Scholar]
- Vovk A, Bolser DC, Hey JA, Danzig M, Vickroy T, Berry R, Martin AD, Davenport PW. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulmonology Pharmacology and Therapeutics. 2007;20:423–432. doi: 10.1016/j.pupt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas J, Al Rifai A. The Use of Electrical Stimulation in Treatment of Dysphagia: A Skilled Nursing Facility Experience. Journal of the American Medical Directors Association. 2012;13:B23–B23. [Google Scholar]
- Wada H, Nakajoh K, Satoh-Nakagawa T, Suzuki T, Ohrui T, Arai H, Sasaki H. Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology. 2000;47:271–276. doi: 10.1159/000052811. [DOI] [PubMed] [Google Scholar]
- Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC. Spatiotemporal regulation of the cough motor pattern. Cough. 2009;5:12. doi: 10.1186/1745-9974-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AHD. Presynaptic modulation of sensory afferents in the invertebrate and vertebrate nervous system. Comparative Biochemistry and Physiology Part A: Physiology. 1992;103:227–239. doi: 10.1016/0300-9629(92)90573-9. [DOI] [PubMed] [Google Scholar]
- Wheeler-Hegland K, Ashford J, Frymark T, McCabe D, Mullen R, Musson N, Hammond CS, Schooling T. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part II—impact of dysphagia treatment on normal swallow function. Journal of Rehabilitative Research and Development. 2009;46:185–194. [PubMed] [Google Scholar]
- Wheeler Hegland K, Huber JE, Pitts T, Davenport PW, Sapienza CM. Lung Volume Measured During Sequential Swallowing in Healthy Young Adults. Journal of Speech, Language, and Hearing Research. 2011;54:777. doi: 10.1044/1092-4388(2010/09-0237). [DOI] [PubMed] [Google Scholar]
- Wheeler Hegland KM, Huber JE, Pitts T, Sapienza CM. Lung volume during swallowing: single bolus swallows in healthy young adults. Journal of Speech, Language, and Hearing Research. 2009;52:178. doi: 10.1044/1092-4388(2008/07-0165). [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Chung KF. Cough. Pulmonology Pharmacology and Therapeutics. 2007;20:305–306. doi: 10.1016/j.pupt.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Wilson S, Thach B, Brouillette R, Abu-Osba Y. Coordination of breathing and swallowing in human infants. Journal of Applied Physiology. 1981;50:851. doi: 10.1152/jappl.1981.50.4.851. [DOI] [PubMed] [Google Scholar]
- Wolf H, Pearson KG. Comparison of motor patterns in the intact and deafferented flight system of the locust. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1987;160:269–279. [Google Scholar]
- Yamamura K, Kitagawa J, Kurose M, Sugino S, Takatsuji H, Mostafeezur RM, Zakir HM, Yamada Y. Neural Mechanisms of Swallowing and Effects of Taste and Other Stimuli on Swallow Initiation. Biological and Pharmaceutical Bulletin. 2010;33:1786–1790. doi: 10.1248/bpb.33.1786. [DOI] [PubMed] [Google Scholar]