Abstract
Background
Daily oral antiretroviral pre-exposure prophylaxis (PrEP) is a promising strategy for prevention of HIV-1 acquisition. Three clinical trials demonstrated PrEP efficacy; however, two PrEP trials among women did not find protection against HIV-1. One hypothesis proposed for these divergent results is that PrEP efficacy may be reduced in populations with higher HIV-1 incidence.
Methods
Using data from the Partners PrEP Study, a randomized, placebo-controlled trial of daily oral tenofovir (TDF) and emtricitabine/tenofovir (FTC/TDF) PrEP among heterosexual HIV-1 serodiscordant couples from Kenya and Uganda, we assessed PrEP efficacy among subgroups at higher risk for HIV-1 acquisition, including subgroups of women with high HIV-1 incidence.
Results
The overall placebo arm HIV-1 incidence was 2.0 per 100 person-years. Among higher-risk subgroups, placebo arm HIV-1 incidence ranged from 3.9 to 6.6 per 100 person-years. In all subgroups, PrEP was protective against HIV-1 acquisition, with efficacy point estimates ranging from 64% to 84%. Among subgroups of women with placebo-arm HIV-1 incidence >5.0, efficacy estimates ranged from 64% to 84%. Monthly visit attendance for PrEP refills and tenofovir detection in plasma were high.
Conclusions
Among higher-risk subgroups in the Partners PrEP Study, including groups solely of higher-risk women, both TDF alone and combined FTC/TDF PrEP had consistently high efficacy for HIV-1 protection. PrEP, when used with high adherence, is a highly-effective prevention strategy for higher-risk heterosexuals. Prioritizing PrEP for persons at high risk of HIV-1 will maximize its prevention impact.
Keywords: antiretroviral agents, HIV-1 acquisition, primary prevention, women, Africa
Introduction
Randomized clinical trials of a daily oral regimen of antiretrovirals used as pre-exposure prophylaxis (PrEP) for the prevention of HIV-1 acquisition have demonstrated efficacy in women and men from diverse settings [1–3]. However, two trials of daily oral PrEP, both among African women, did not find efficacy for protection against HIV-1 [4, 5]. Determining the reasons for these divergent results is a priority. Low adherence to PrEP has been proposed as the primary explanation for the null findings in two trials [4, 5], but further investigation is needed to clarify whether other factors contributed. One hypothesis is that biological or behavioral risk factors for HIV-1 acquisition may have been more prevalent in the trials that did not find efficacy, potentially suggesting PrEP’s protection is reduced when challenged with greater or more frequent HIV-1 exposure. Notably, HIV-1 incidence in the placebo arm, an indicator of study population background risk, was 2–4 events per 100 person-years in the trials that demonstrated PrEP efficacy [1–3] compared to 4–5 per 100 person years in those that did not [4, 5]. Additionally, the two trials with null findings enrolled exclusively women.
To evaluate the hypothesis that PrEP efficacy might be reduced in populations with increased risk of HIV-1 acquisition, we identified higher-risk subgroups within a trial of PrEP that overall demonstrated efficacy for HIV-1 prevention [2], including subgroups of women alone, and estimated PrEP efficacy among these higher-risk subgroups.
Methods
Study population
Data were from the Partners PrEP Study, a randomized, placebo-controlled trial which enrolled and followed 4747 HIV-1 serodiscordant couples at nine sites in Kenya and Uganda between July 2008 and November 2010. The study design and results have been detailed previously [2]. Briefly, HIV-1 seronegative partners were randomized to tenofovir disoproxyl fumarate (TDF), co-formulated emtricitabine (FTC)/TDF, or placebo, and were followed and tested for HIV-1 seroconversion monthly (with two rapid tests; positives were confirmed by HIV-1 Western blot and RNA PCR) for up to three years; their HIV-1 infected partners, who were not yet eligible for antiretroviral treatment (ART) at enrollment, were followed quarterly.
Higher-risk subgroups
We considered known HIV-1 risk factors to define five subgroups of uninfected participants at higher HIV-1 risk. Baseline data were used to categorize 1) those with an HIV-1 infected partner with plasma HIV-1 RNA >50,000 copies/mL, which has been strongly associated with higher transmission risk [6] and 2) those identified as higher-risk using a recently-reported composite risk score for HIV-1 serodiscordant couples [7]. Time-dependent characteristics were used to classify 3) participants who reported unprotected sex within three months prior to each visit and 4) participants who themselves or their partners had signs, symptoms, or a diagnosis of a sexually transmitted infection (STI), also within the three months prior to each visit. Among women alone, we defined the same four subgroups and also classified 5) women under 30 years of age, chosen as comparable to the populations in the two PrEP trials conducted solely among women [4, 5].
We confirmed that our subgroups were at higher HIV-1 risk by assessing HIV-1 incidence in those randomized to placebo, comparing the incidence rate to that in the full study placebo arm (2.0 per 100 person-years). In addition, we included the subgroup in the analysis if there was reasonable statistical power to assess PrEP efficacy. For a subgroup with a placebo arm incidence of 3.0 per 100 person-years, a sample size of 313 per arm would provide 70% power to detect 70% efficacy. Similarly, groups with placebo arm incidence rates of 4.0, 5.0, and 6.0 would require 236, 190 and 176 in each arm, or an equivalent amount of person-time for time-dependent characteristics. We included groups sized to approximate 70% power.
Adherence
In addition to evaluating PrEP efficacy, for subgroups defined by baseline characteristics, we assessed both monthly study visit attendance (a marker of consistent return for study medication) and study medication use. Periods when participants were on protocol-defined study drug holds (e.g., during pregnancy) were excluded. Medication use was estimated using a randomly selected cohort (n=198) from the trial’s active PrEP arms who had tenofovir concentrations measured in plasma at months 1, 3, 6 and bi-annually [2].
Statistical methods
We used Cox proportional hazards regression with HIV-1 seroconversion as the outcome. Participants who were later confirmed to have seronegative acute HIV-1 infection at the time of randomization were excluded. For groups defined by baseline characteristics, models were run within each subgroup including all follow-up time and treatment assignment was the only predictor. For groups defined by time-varying exposures, we modeled the full study sample with treatment arm, the time-varying exposure (unprotected sex or STIs), and the interaction between arm and exposure as predictors, excluding follow-up time after partner ART initiation. We also calculated the number needed to treat [NNT] to avert one infection. The analysis includes study visits through July 10, 2011, when the trial’s placebo arm was stopped [2]. All analyses were conducted in SAS 9.2 (SAS Institute).
Results
For participants who were not found to be HIV-1 infected at enrollment (n=4733, of whom 1780 were female), the median age was 33 years (interquartile range [IQR] 28–40), median partnership duration was 7.6 years (IQR 3.3–14.3), and median time couples were aware of their serodiscordancy was 0.4 years (IQR 0.1–2.0). Participants reported an average of 4 sex acts in the prior month (IQR 2–8).
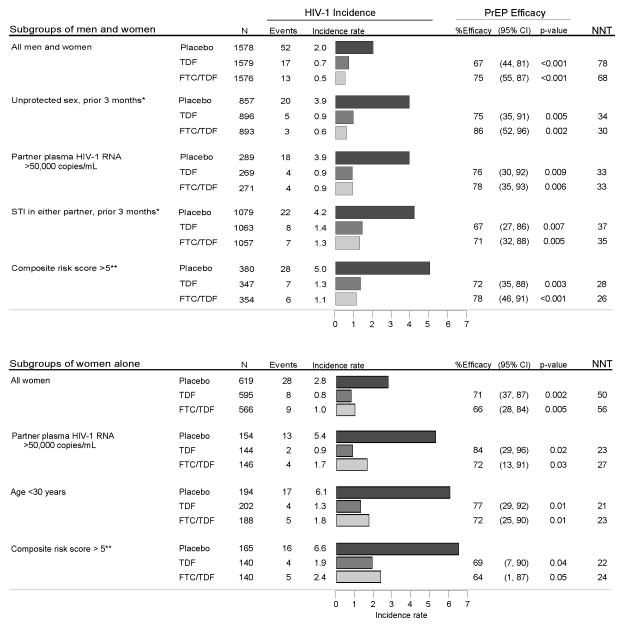
Overall HIV-1 placebo arm incidence in the Partners PrEP Study was 2.0 per 100 person-years, and PrEP efficacy was 67% (95% confidence interval [CI] 44–81, p<0.001) for TDF and 75% (95% CI 55–87, p<0.001) for FTC/TDF [2]. In higher-risk subgroups containing both men and women, placebo arm incidence ranged from 3.9 to 5.0 per 100 person-years (Figure 1). In all subgroups, PrEP was protective against HIV-1 acquisition, with efficacy point estimates ranging from 67% to 86% (all p<0.05). In the highest-incidence subgroup, those with a high composite risk score (22.8% of the study population), TDF efficacy was 72% (95% CI 35–88, p=0.003) and FTC/TDF efficacy was 78% (95% CI 46–91, p<0.001).
Figure 1. HIV-1 incidence and PrEP efficacy overall and among higher-risk subgroups.
Incidence rates are per 100 person years. STI = sexually transmitted infection. NNT = number needed to treat to avert 1 infection, calculated as 1 divided by the difference in the placebo arm incidence rate and active arm incidence rate. Plasma HIV-1 RNA concentrations were measured by PCR in samples collected at enrollment from HIV-1 infected study partners.
* For time-dependent variables, N in these subgroups represents participants who were categorized as high-risk during at least one month of follow up. For unprotected sex, data were from the three months prior to each visit. The STI risk group included participants who themselves or their partners had signs (genital discharge or ulcers), symptoms (genital burning, discharge, sores, or lower abdominal pain in women), or a diagnosed STI (Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis) within the three months prior to each visit. Symptoms of sexually transmitted infections (STIs) were evaluated quarterly and also recorded if reported in the interim. A clinical evaluation of STI signs and laboratory testing for STIs [13] were conducted annually and as indicated. The total person-time for time-varying exposures (i.e., unprotected sex and STI within the prior three months) was too small to estimate efficacy among women alone.
** Composite risk score includes age of the uninfected partner, number of children, circumcision status of male HIV-1 uninfected partner, married/cohabiting, unprotected sex, and HIV-1 infected partner viral load [7]
Among women enrolled in the Partners PrEP Study, overall HIV-1 incidence in the placebo arm was 2.8 per 100 person-years, and TDF efficacy was 71% (95% CI 37–87, p=0.002) and FTC/TDF efficacy was 66% (95% CI 28–84, p=0.005) [2]. In subgroups of higher-risk women, placebo arm incidence ranged from 5.4 to 6.6 per 100 person years. TDF efficacy estimates ranged from 69% to 84% and FTC/TDF efficacy ranged from 64% to 72% (all p<0.05). For two subgroups, placebo arm incidence was >6 events per 100 person-years: women under 30 years of age (placebo incidence 6.1, 32.8% of women in the cohort), among whom TDF efficacy was 77% (95% CI 29–92, p=0.01) and FTC/TDF efficacy was 72% (95% CI 25–90, p=0.01), and women with a high composite risk score (placebo incidence 6.6, 25.0% of women), among whom TDF and FTC/TDF efficacy were 69% (95% CI 7–90, p=0.04) and 64% (95% CI 1–87, p=0.05). In this highest-risk subgroup, the NNT to avert one infection was 22 for TDF and 24 for FTC/TDF.
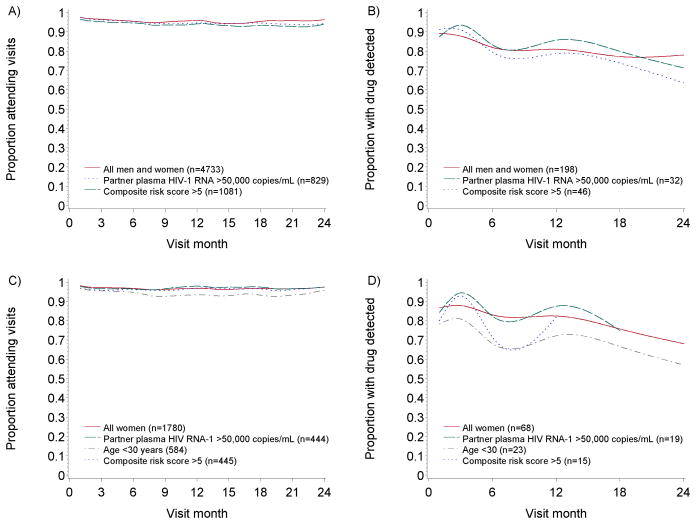
Visit attendance was high, with month-to-month attendance >94% throughout the study period in all subgroups (Figure 2a and c). Tenofovir detection was >70% of samples for all subgroups (Figure 2b and d).
Figure 2. Visit attendance and medication adherence by visit month.
A) visit attendance among higher-risk subgroups including both men and women; B) proportion with tenofovir detected, among a subgroup of randomly-selected participants from the trial’s active PrEP arms, as detailed previously [2]; C) visit attendance among higher-risk subgroups of women; D) proportion with tenofovir detected among higher-risk of women from within the randomly-selected participants in figure B. For figures B and D, tenofovir was measured in plasma at months 1, 3, 6, 12, 18, and 24 in 198 randomly selected active arm participants. Ns represent the number at enrollment for visit attendance figures and the number with any tenofovir measured for medication adherence figures. Subgroups with ≤5 plasma samples in a month are excluded for that month. For all figures, follow-up is presented through 24 months due to limited numbers thereafter.
Discussion
Among higher-risk subgroups within the Partners PrEP Study, including groups solely of women, both daily oral TDF and combined FTC/TDF PrEP had consistently high efficacy for HIV-1 protection. Thus, our results do not support the hypothesis that PrEP efficacy is reduced in women or other higher-risk populations. Although the Partners PrEP Study population was composed of HIV-1 serodiscordant couples who had not yet transmitted HIV-1, which could suggest lower biological or behavioral risk, we identified several subgroups with placebo arm HIV-1 incidence rates over 5 per 100 person-years and across these groups PrEP efficacy ranged from 64% to 84%. The range of results from daily oral PrEP trials [1–5] has raised questions about PrEP efficacy and consequently concern regarding PrEP implementation. A number of hypotheses have been proposed that might explain some of these differences [8] including adherence to the daily regimen, biological differences between men and women, and increased risk due to increased exposure, including exposure to persons with acute HIV-1 infection. Our results do not indicate reduced PrEP efficacy in persons with high HIV-1 exposure. Across PrEP trials, a key factor related to efficacy has been adherence to the daily medication. Plasma tenofovir levels in the two trials that failed to demonstrate PrEP efficacy indicated that ≤30% participants were consistently taking the study medication [4, 5]. In contrast, about 80% of participants in trials with the highest PrEP efficacy estimates, including the Partners PrEP Study, had tenofovir detected in plasma [2, 3], and detection of tenofovir was associated with an approximately 90% reduction in HIV-1 risk [1, 2]. In the present study, among all subgroups, including younger women, visit-to-visit attendance (indicating obtaining medication refills) and medication use (as measured by tenofovir detection) were high throughout follow up, and, most importantly, PrEP efficacy was high. Qualitative research suggests that the commitment within an HIV-1 serodiscordant partnership to avoid transmission may have contributed to high PrEP adherence in our study population [9], but further investigation of factors that promote PrEP adherence among persons at risk will benefit not only PrEP implementation but HIV-1 prevention measures more broadly.
Pharmacokinetic studies have suggested that TDF metabolite concentrations in cervicovaginal tissues after oral dosing are lower than in rectal tissues [10, 11], which potentially could indicate reduced PrEP efficacy in women. Nonetheless, we observed comparable PrEP efficacy in women compared to the entire study population, suggesting that daily oral TDF and FTC/TDF, when taken with high adherence, appear to provide sufficient drug concentrations for high HIV-1 protection for women. Further work is needed to identify tenofovir levels required for protection in relevant sites of exposure and what factors might affect these levels.
In our study population, exposure to acutely HIV-1 infected sexual partners is likely less frequent than other trial populations, although HIV-1 risk from outside partnerships is significant in serodiscordant couples [12] and some HIV-1 infected partners in our study may have been recently infected. In addition, accurately quantifying participants’ HIV-1 exposure is challenging; therefore, we used high placebo arm incidence as a proxy for high exposure.
In conclusion, daily oral PrEP was highly efficacious for the prevention of HIV-1 acquisition in the Partners PrEP Study, both overall and among higher-risk subgroups. In populations with HIV-1 incidence ≥5, the NNT to avert one infection was below 30. Prioritizing PrEP for persons at higher risk of HIV-1, who will adhere to the intervention, will maximize the number of potential new infections averted.
Acknowledgments
Funding: The United States National Institutes of Health (grant R01MH095507) and the Bill and Melinda Gates Foundation (grant OPP47674)
We thank the couples who participated in this study for their motivation and dedication. We are grateful to the Clinical Pharmacology Analytical Laboratory at Johns Hopkins University for performing the tenofovir plasma measurements.
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories
Seattle, USA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath.
Study sites and site principal investigators
Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Author contributions
P.M.M. and J.M.B. conceived the study and wrote the first draft of the manuscript. P.M.M. performed the statistical analysis. C.C., N.M., J.D.C., D.D., E.B., A.M., J.T, E.M.K., and K.T.T. contributed critical revisions to the analysis and interpretation. All authors contributed to the writing of the final draft. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003), #26LB. Presented at: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 3–6, 2013. [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahle EM, Hughes JP, Lingappa JR, John-Stewart G, Celum C, Nakku-Joloba E, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1 serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr. 2012;62:339–347. doi: 10.1097/QAI.0b013e31827e622d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 9.Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix CW. The clinical pharmacology of antiretrovirals for HIV prevention. Curr Opin HIV AIDS. 2012;7:498–504. doi: 10.1097/COH.0b013e32835847ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6:e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]