Abstract
Background: In this study, we evaluated the incidence of apoptosis at the ultrastructural levels and expression of some apoptosis-related genes in vitrified human ovarian tissue just after warming. Methods: Human ovarian tissue biopsies from 23 women after caesarean section were transported to the laboratory within 2 hours, and then they were cut into small pieces. Some pieces were vitrified and warmed and the other samples were considered as control. Apoptosis was assessed by a transmission electron microscope and also by molecular analysis of pro-apoptotic (Fas, FasL, Bax, p53, caspase8, and caspase3) and antiapoptotic (Bcl-2 and BIRC5) genem RNA levels using real-time RT-PCR before and after vitrification. Results: No sign of apoptosis was shown ultrastructurally in vitrified samples. The level of FasL, Bcl-2, Bax, p53, and caspase3 mRNA and Bax:Bcl-2 ratio were similar in non-vitrified and vitrified groups; however, the expression of Fas and caspase8 genes was higher and BIRC5 was lower in vitrified samples compared to non-vitrified group (P<0.05). Conclusion: The fine structure of human vitrified ovarian tissue was well preserved; moreover, vitrification was shown to affect the expression of some apoptosis-related genes. However, additional study is needed to confirm this observation.
Key Words: Vitrification, Apoptosis, Gene expression, Ovary, Humans
Introduction
There are two methods for cryopreservation of human ovarian tissue: conventional slow freezing and cryopreservation by high concentration of cryoprotectant and direct plunging into liquid nitrogen (vitrification). The first attempt for cryopreservation of human ovarian tissue carried out by Zhang et al. [1] using ultrarapid freezing with DMSO and sucrose. To evaluate and compare the outcome of different cryopreservation protocols (safety of technique), some investigations were focused on the assessments of the normality of follicles and the incidence of cell death after thawing or warming [-]. Apoptosis may occur not only in normal ovarian tissue [6] but also during cryopreservation [7, 8].
The viability of ovarian follicles and the incidence of apoptosis were assessed after warming of vitrified human ovarian tissue, and there were some controversy in this regards. Different studies have shown the vitrification of human ovarian tissue provides similar results compared to the conventional slow-freezing technique [-]. Some studies have reported some cryodamage in ovarian tissue [4, 5]. Gandolfi et al. [4] have shown that vitrification causes an extensive damage to preantral follicles of ovarian tissue. The efficiency of vitrification as a cryopreservation method for human ovarian tissue has been supported by several studies [7, -].
In different studies, Xiao et al. [13] and Chang et al. [7] have recently shown the fewer TUNEL-positive cells in vitrified human ovarian tissue as compared with fresh or slow-cooled tissue. However, Zhou et al. [18] demonstrated that the incidence of apoptotic cell in vitrified ovarian tissue was significantly higher than fresh tissue.
Recently, we compared the incidence of apoptosis in human ovarian tissue by two cryopreservation methods not only after warming but also after 24 hours in vitro culture. In addition, we observed no sign of apoptosis in both cryopreservation groups regarding to different methods for apoptosis assessment, such as DNA laddering, TUNEL assay, and transmission electron microscopy [8]. However, it has not been reported so far whether vitrification of human ovarian tissue induces the incidence of apoptosis at the molecular level.
According to our knowledge, there is poor report about the evaluation of apoptosis at the molecular levels (mRNA) after vitrification of human ovarian tissue, even there is very limited attention to another mammalian models in this regard [19, 20]. Mazoochi et al. [19] demonstrated some changes in expression of genes related to apoptosis in mouse vitrified ovarian follicles after in vitro culture [19]. There are at least two broad pathways that lead to ovarian tissue apoptosis: intrinsic pathway that involved several apoptotic genes, such as Bcl-2, Bax, p53, and BIRC5 [-] and extrinsic pathway such as Fas/FasL system [25, 26]. In both pathways, signaling results in the activation of a family of cysteine proteases such as caspase 8, and caspase 3 that act in a proteolytic cascade to remove the dying cell [27, 28]. Among these genes, some are antiapoptotic (Bcl-2 and BIRC5) and some are pro-apoptotic (Fas, FasL, Bax, p53, caspase8, and caspase3).
Therefore, the present study was the first attempt to evaluate the expression of the apoptosis-related genes (Fas, FasL, Bcl-2, Bax, p53, BIRC5, caspase 8, and caspase 3) in vitrified-warmed human ovarian tissue in comparison with non-vitrified using real-time RT-PCR.
MATERIALS AND METHODS
Reagents and materials were obtained from Sigma-Alderich (Germany) except mentioned otherwise.
Ovarian tissue collection. An informed consent was given by 23 women aged between 24-35 years old (median 28) under a protocol approved by the Ethics Committee of the Faculty of Medical Science of Tarbiat Modares University (Ref. No. 5274856), Tehran, Iran. Ovarian cortical tissue fragments of approximately 5 × 5 × 1 mm were obtained from women undergoing elective caesarean sections. Then, they were transported to the laboratory within 1-2 h with pre-warmed and equilibrated Leibovitz'sL-15 medium supplemented with 10 mg/ml human serum albumin, 100 IU/ml penicillin and 100 µg/ml strepto-mycin. The ovarian cortexes were cut into small pieces approximately 2.5 × 1 × 1 mm under a sterile laminar hood with transfer medium. Then, these fragment tissues were randomly divided into non-vitrified and vitrified groups.
Vitrification and warming procedure. The tissues were vitrified according to the protocol described by Salehnia et al. [29] with some modifications. The vitrification solution was made of Ethylene glycol, Ficoll and Sucrose that named EFS40% containing 40% ethylene glycol (v/v), 30% Ficoll 70 (w/v), and 1 M sucrose supplemented with 0.21% human serum albumin instead of BSA. The human ovarian tissues were equilibrated in three changes of vitrification solutions for 5 minutes. Following the last incubation, individual tissue samples were placed into aseptic cryovials containing 100 µl vitrification solutions. The tubes were then put under the nitrogen vapor for 30 s finally immersed and stored in liquid nitrogen at least for two months. The tissues were thawed by immersing the vials in 37°C bath water with gentle agitation until melting the samples. Then, they were washed serially in 1, 0.5, 0.25 M sucrose and phosphate buffer I (PBI) containing 10 mg/mL human serum albumin at room temperature for 5 min. The samples were equilibrated in McCoy’s culture media for 30 min.
Transmission electron microscopy. For assessment of nuclear fragmentation regarding to apoptosis or cell death, the tissue samples including non-vitrified (n = 3) and vitrified (n = 3) human ovarian tissues in 2.5% glutaraldehyde in PBS (pH 7.4) for 2 hours and post-fixed with 1% osmium tetroxide in the same buffer for 2 hours. After dehydration in an ascending series of ethanol, specimens were placed in acetone and embedded in epoxy resin. Semithin sections were stained with toluidine blue and studied under light microscopy. The thin sections were stained with uranyl acetate and lead citrate using a transmission electron microscope (Zeiss, 911, Germany).
RNA extraction and cDNA synthesis for molecular assessment. Total RNA was extracted from non-vitrified (n = 3), vitrified (n = 3), and apoptosis-induced human ovarian tissue groups using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The RNA concentration was determined by spectrophotometry and adjusted to a concentration of 250 ng/ml. Using oligo dT, RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase. Using specified primers (Table 1), the Fas, FasL, Bcl-2, Bax, p53, BIRC1, caspase8, and caspase3 genes were amplified. GAPDH gene was used as an internal control.
Table 1.
Oligonucleotide primers
| Accession numbers | Gene | Primer sequence | PCR product size (bp) |
|---|---|---|---|
| NC_000012.11 | GAPDH | Forward:5`CTGGGCTACACTGAGCACC 3` Reverse:5`AAGTGGTCGTTGAGGGCAATG3` |
101 |
| NC_000010.10 | Fas | Forward: 5`TGAAGGACATGGCTTAGAAGTG 3` Reverse:5`GGTGCAAGGGTCACAGTGTT3` |
118 |
| NC_000001.10 | FasL | Forward: 5´GCAGCCCTTCAATTACCCAT 3` Reverse:5`CAGAGGTTGGACAGGGAAGAA3` |
101 |
| NC_000018.9 | Bcl-2 | Forward:5`TTGCTTTACGTGGCCTGTTTC3` Reverse:5`GAAGACCCTGAAGGACAGCCAT3` |
94 |
| NC_000019.9 | Bax | Forward: 5`CCCGAGAGGTCTTTTTCCGAG3` Reverse:5`CCAGCCCATGATGGTTCTGAT3` |
155 |
| NC_000017.10 | p53 | Forward: 5´GAGGTTGGCTCTGACTGTACC3` Reverse:5`TCCGTCCCAGTAGATTACCAC3` |
133 |
| NC_000017.10 | BIRC5 | Forward:5`AGGACCACCGCATCTCTACAT3` Reverse:5`AAGTCTGGCTCGTTCTCAGTG 3` |
118 |
| NC_000002.11 | Caspase 8 | Forward: 5`ATTTGCCTGTATGCCCGAGC 3` Reverse:5`CCTGAGTGAGTCTGATCCACAC3` |
105 |
| NC_000004.11 | Caspase 3 | Forward: 5´AGAGGGGATCGTTGTAGAAGTC 3` Reverse:5`ACAGTCCAGTTCTGTACCACG3` |
81 |
Real-time RT-PCR. After extraction of total RNA and cDNA synthesis, one-step RT-PCR was performed on Applied Biosystems (UK) real-time thermal cycler according to QuantiTect SYBR Green RT-PCR kit (Applied Biosystems, UK, Lot no:1201416). Prior to the quantitative analysis, optimization procedures were performed by running real-time RT-PCR with or without template to verify the reaction conditions, including the annealing temperatures of the primers and specific products. For target sequence amplifications, 100 ng (5 µl) of RNA was used per 20 µl reaction volume. After completing the PCR run, melt curve analysis was used to confirm the amplified product. For each sample, the reference gene (GAPDH) and the target genes were amplified in the same run. Standard curves were obtained using the logarithmic dilution series of total RNA. Real-time thermal condition included holding step at 95ºC for 5 min, cycling step at 95ºC for 15 s, 58ºC for 30 s, and 72ºC for 30 s, and it was continued by a melt curve step at 95ºC for 15 s, 60ºC for 1 min, and 95ºC for 15 s. Then, relative quantification of target genes was determined using the Pfaffl method [30]. The real-time RT-PCR experiments were repeated three times.
Statistical analysis. Statistical analysis was carried out with SPSS 19.0 software. Quantitative variables were expressed as mean ± SD. The results of real-time RT-PCR were compared by one-way ANOVA test and post hoc Turkey's test (P≤0.05).
RESULTS
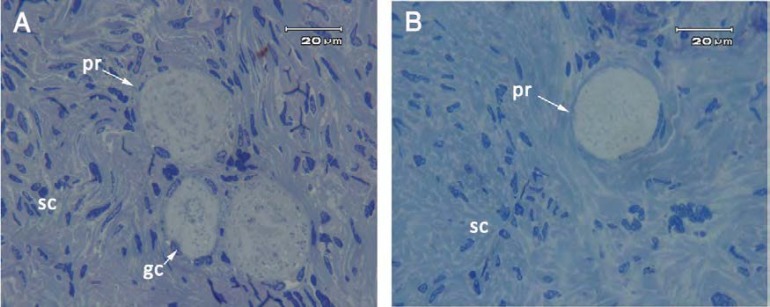
The follicular morphology of vitrified and non-vitrified ovarian tissue in semithin sections. The morphology of human ovarian tissue after vitrification and warming were well preserved. No morphological sign of apoptosis, including pyknotic and fragmented nuclei and cytoplasmic vacoules in ovarian follicles and stromal cells were observed in the follicles after semithin section preparation (Fig. 1). There were some small primordial and primary follicles within these tissues.
Fig. 1.
Semithin section of human ovarian tissue, Non-vitrified (A) and vitrified (B) groups. There were some primordial follicles (pr) with normal morphology. gc, granulosa cell; sc: stromal cell
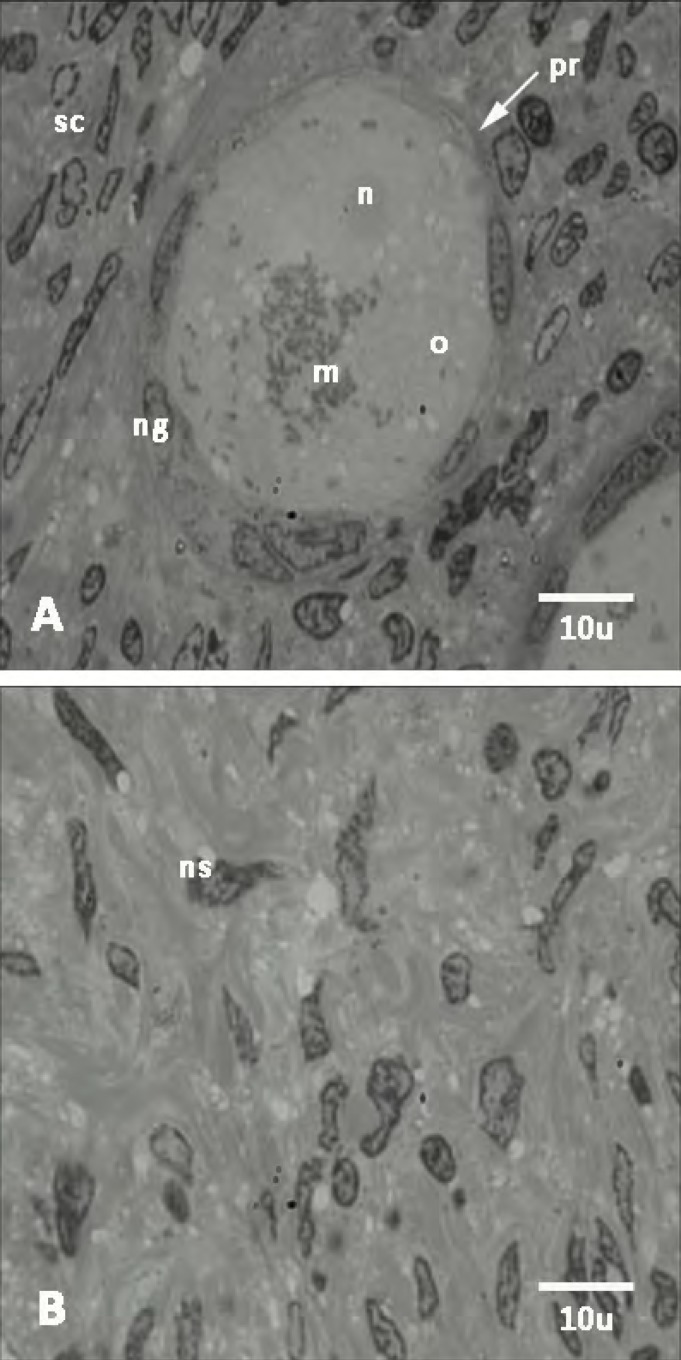
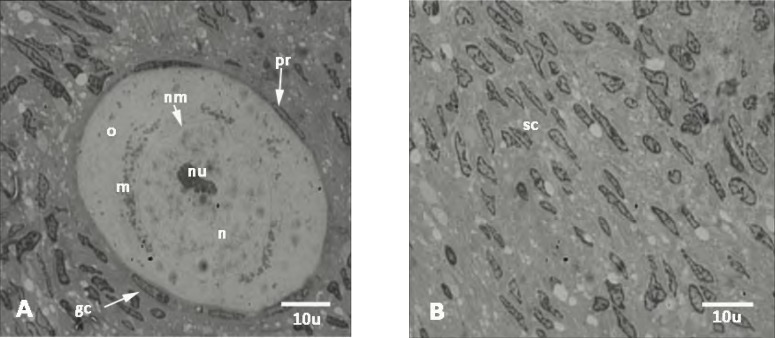
Ultrastructural observation. The ultrastructure of nucleus of oocyte and follicular cells of primary follicles in vitrified group were well preserved and seemed to be similar to the non-vitrified group (Fig. 2). The oocyte had a euchromatic nucleus at the germinal vesicle stage. The follicular cells in both groups had normal ultrastructure. No nucleus fragmentation, shrinkage of cell membrane, and vacuoles were shown within cytoplasm of oocyte and follicular cells. The euchromatic nuclei showed peripheral aggregates of heterochromatin. However, the ultrastructure of stromal cells within both vitrified and non-vitrified tissue has been shown in Figure 3. There were not any sign of fragmentation of nucleus within the stromal cells.
Fig. 2.
Transmission electron micrographs of non-vitrified ovarian tissue. Ultrastructure of primordial follicles (pr) (A) and stroma (B). n, nucleus; o, ooplasm; m, mitochondria; ng, granulosa cell nucleus; sc, stromal cell; ns, stromal cell nucleus
Fig. 3.
The fine structure of human ovarian tissue. Primordial follicles (A) and stroma (B) of vitrified-warmed group. Pr, primordial follicle; nu, nucleolus; nm, nuclear membrane; n, nucleus; o, ooplasm; m, mitochondria; gc, granulosa cell; sc, stromal cell. The ultrastructure of nucleus of oocyte and granulosa cells of primary follicle was well preserved and was similar to the non-vitrified group. The oocyte had a euchromatic nucleus at the germinal vesicle stage. No nucleus fragmentation, shrinkage of cell membrane and vacuoles within cytoplasm of oocyte and granulosa were seen
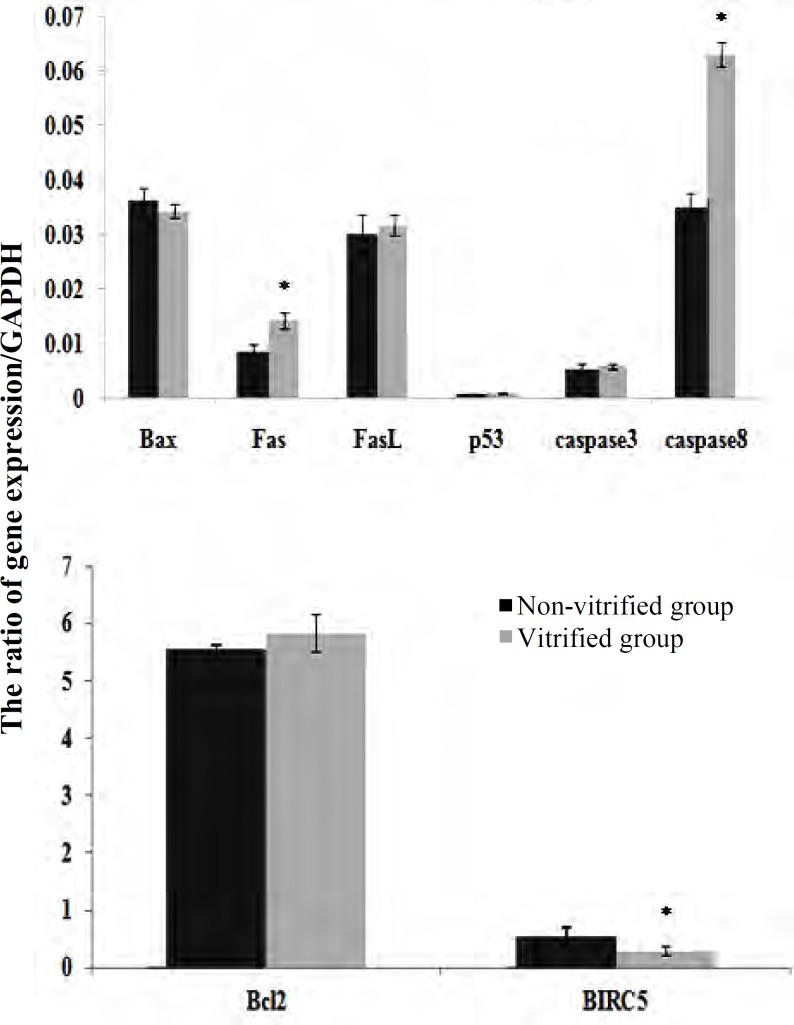
Expression of apoptosis-related genes in non-vitrified and vitrified human ovarian tissue. The mRNA levels of several apoptosis-related genes, including Fas, FasL, Bax, p53, caspase8, and caspase3, Bcl-2, and BIRC5 genes were evaluated in non-vitrified and vitrified human ovarian tissue by real-time RT-PCR. The expression ratio of the target gene to housekeeping (GAPDH) gene in vitrified and non-vitrified samples has been shown in Figure 4. As the results demonstrated, among the studied genes, the expression of FasL, Bcl-2, Bax, p53, and caspase3 was not changed significantly in both groups. The level of Fas and caspase8 mRNA was significantly higher in the vitrified group compared with the non-vitrified sample, but the BIRC5 mRNA level was significantly lower in the vitrified group in comparison with its control (P<0.05).
Fig. 4.
Comparison of expression of apoptosis-related genes to GAPDH in non-vitrified and vitrified human ovarian tissue. The ratio of genes expression of Bax, Fas, FasL, p53, caspase 3, and caspase 8 to GAPDH(A) and the ratio of Bcl-2, BIRC5 to GAPDH (B) using real-time RT-PCR in non-vitrified and vitrified groups. *Significant differences between groups (P<0.05).
DISCUSSION
The vitrification method is a simple alternative method for cryopreservation of human ovarian tissue. Results of the present study showed no ultrastructural changes, including nuclear fragmentation, mito-chondrial disruption, shrinkage of cell membrane, and sytoplasmic vacuolein the oocyte as well as follicular and stromal cells after vitrification and warming of ovarian tissue. On the other hand, there were not any apoptotic changes in ovarian cells at the ultrastructural level in vitrified samples compared to the control group. Moreover, our observation confirmed the safety of vitrification procedure using EFS40 for human ovarian preservation based on morphological and ultrastructural study [8].
Also, the results of our previous study showed no significant changes in chromatin condensation in nucleus of different types of cells within human ovarian tissue which subjected to vitrification or ultrarapid cryopreservation [8]. However, similar conclusion was published by some other researchers in this regard. Keros et al. [10] compared vitrification and slow programmed freezing of human ovarian tissue. They revealed by electron microscopy that the ovarian stroma was significantly better preserved after vitrification compared to slow freezing.
Sheikhi et al. [14] have shown that vitrification is an excellent method to cryopreserve ovarian tissue. They did not observe any differences in the ultrastructure of oocytes between non-vitrified and vitrified tissues. Moreover, they have recently demonstrated that the fine structure of oocytes, granulosa, and ovarian stromal cells in vitrified human ovarian tissue are well preserved by electron microscopic analysis. They used ethylene glycol as a permeating cryoprotectant and compared it with different solutions [16].
Wang et al. [11] showed that the primordial follicles in vitrified human ovarian tissues by needle immersed vitrification were well preserved and the ultrastructure of the stromal cells was better preserved in vitrified group than the slow-freezing or the dropping vitrification. In contrast, Zhou et al. [18] compared different vitrification procedures using several cryoprotectant solutions for human ovarian tissue. Their ultrastructural observation showed irregularly shaped or swollen mitochondria in the cytoplasm of both oocytes and follicular cells. However according to our knowledge, there was not any report regarding to apoptotic changes of human ovarian tissue after vitrification.
In the other part of this study, we evaluated, for the first time, the expression of some apoptosis-related genes in vitrified human ovarian tissue. Our observation showed no significant difference between the mRNA levels of some pro-apoptotic genes, including FasL, Bax, p53, and caspase3 and antiapoptotic gene Bcl-2 in non-vitrified and vitrified samples. We concluded that the vitrification of human ovarian tissue using EFS40 did not induce apoptosis cell death at the gene expression levels. According to this panel of genes, we suggested that after vitrification none of intrinsic and extrinsic pathways of apoptosis were induced.
There are two mechanisms for ovarian tissue apoptosis: one mechanism is triggered by the binding of death molecules to cell surface receptors such as Fas/FasL system (cell death signaling or extrinsic pathway). The other one (intrinsic or mitochondrial pathway of apoptosis) is generated by the signals occurring within the cell, mitochondria and Bcl-2 family members (Bcl-2 and Bax) [-]. In both pathways, signaling resulted in the activation of a family of cysteine proteases named caspases, that act in a proteolytic cascade to remove the degenerating cell [34]. The p53 protein is an antiproliferative transcription factor that controls genomic integrity by inducing cell cycle arrest or apoptosis. This protein is expressed in the apoptotic granulosa cells of atretic follicles [35]. Parallel with our observation, Hussein et al. [36] demonstrated p53 expression was not altered after slow freezing of human ovarian tissue.
Bcl-2 is an antiapoptotic and Bax is a proapoptotic factor. Bax can suppress the ability of Bcl-2 to block apoptosis. Ratio of Bax:Bcl-2 can influence the ability of a cell to respond to an apoptotic signal. The expression level of Bax:Bcl-2 in vitrified group was the same as control group. We concluded that vitrification did not change the susceptibility of the ovarian tissue to apoptotic signals. When Bcl-2 is in excess the cells are protected, and when Bax is in excess, the cells are susceptible to apoptosis [37].
A similar report was published by Depalo et al. [38] that compared the Bax/Bcl-2 ratio in fresh and slow frozen human ovarian tissue samples.
In other part of this study, the molecular analysis showed the higher expression of proapoptotic genes (Fas and caspase8) in parallel with lower expression of antiapoptotic gene (BIRC5) in vitrified samples compared to the control group. Moreover, caspase8 expression as an initiator caspase was increased in vitrified ovarian tissue samples, but it did not result in high expression of caspase3. On the other hand, the expression level of caspase8 is not sufficient to activate other effector caspases in apoptosis.
In other point of view we suggested that these changes may be reversible and do not influence the viability of ovarian tissue cells, or these different mRNA levels have not any effect on the in vitro or in vivo subsequent survival of tissue and their follicular development. Confirmation of these suggestions needs more study to analyze the incidence of apoptosis after short- and long-term culture by some complementary techniques.
According to our knowledge, there is not any report regarding the evaluation of cell death at the molecular level in human and animal models after vitrify-cation/warming. However, the similar pattern of apoptosis gene expression has been reported by Mazoochi et al. [19] during in vitro culture of isolated follicles derived from vitrified mice ovarian tissue. They showed that the expression of some apoptotic related genes (Fas and BIRC5) was changed, and that of other genes (p53, Bcl-2, Bax, and FasL) did not affect after in vitro culture of isolated follicles derived from vitrified mouse ovarian tissue.
In conclusion, no signs of apoptosis were observed at the ultrastructural levels in the vitrified and warmed human ovarian tissue, and the follicles and stromal cell integrity were well preserved. In spite of some changes in FasL, BIRC5 and caspase8 gene expression, the vitrification did not have any effect on the expression of FasL, Bcl-2, Bax, p53 and caspase3 genes. Therefore, additional studies are needed to confirm accuracy of these observations after a long-term culture or transplantation of human ovarian tissue.
References
- 1.Zhang J, Liu J, Xu K, Liu B, Dimattina M. Extra-corporeal development and ultrarapid freezing of human fetal ova. J Assist Reprod Genet. 1995 Jul;12(6):361–8. doi: 10.1007/BF02215727. [DOI] [PubMed] [Google Scholar]
- 2.Isachenko V, Isachenko E, Rahimi G, Krivokharchenko A, Alabart J, Nawroth F. Cryo-preservation of human ovarian tissue by direct plunging into liquid nitrogen: negative effect of disaccharides in vitrification solution. Cryo Letters. 2002 Sep-Oct;23(5):333–44. [PubMed] [Google Scholar]
- 3.Rahimi G, Isachenko E, Sauer H, Isachenko V, Wartenberg M, Hescheler J, et al. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15(6):343–9. doi: 10.1071/RD02063. [DOI] [PubMed] [Google Scholar]
- 4.Gandolfi F, Paffoni A, PapassoBrambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006 Apr;85:1150–6. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 5.Isachenko V, Lapidus I, Isachenko E, Krivo-kharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrine-logical, and molecular biological evaluation. Reproduction. 2009 Aug;138(2):319–27. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 6.Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005 Mar-Apr;11(2):162–77. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- 7.Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011 Aug;37(8):1092–101. doi: 10.1111/j.1447-0756.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 8.Salehnia M, Sheikhi M, Pourbeiranvand S, Lundqvist M. Apoptosis of human ovarian tissue is not increased by either vitrification or rapid cooling. Reprod Biomed Online. 2012 Nov;25(5):492–9. doi: 10.1016/j.rbmo.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008 Aug;139(2):193–8. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009 Jul;24(7):1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008 Oct;23(10):2256–65. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 12.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012 Nov;98(5):1291–8. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010 Nov;94(6):2323–8. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod. 2011 Mar;26(3):594–603. doi: 10.1093/humrep/deq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y, Xu X, Qian Y, Zhou C, Xu J. Morphology and cell proliferation evaluation of follicles from cryopreserved human ovarian tissue by vitrification. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013 Jan;42(1):75–80. doi: 10.3785/j.issn.1008-9292.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Preservation of human ovarian follicles within tissue frozen by vitrification in a xeno-free closed system using only ethylene glycol as a permeating cryoprotectant. Fertil Steril. 2013 Jul;100(1):170–7. doi: 10.1016/j.fertnstert.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed online. 2011 Aug;23(2):160–86. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhou XH, Wu YJ, Shi J, Xia YX, Zheng SS. Cryopreservation of human ovarian tissue: Comparison of novel direct cover vitrification and conventional vitrification. Cryobiology. 2010 Apr;60(2):101–5. doi: 10.1016/j.cryobiol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Mazoochi T, Salehnia M, Pourbeiranvand S, Forouzandeh M, Mowla S J, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009 Mar;15(3):155–64. doi: 10.1093/molehr/gap002. [DOI] [PubMed] [Google Scholar]
- 20.Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromo-somal abnormalities following cryotopvitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010 May;27(5):239–46. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao XJ, Martimbeau S, et al. Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ. 1998 Jan;5(1):67–76. doi: 10.1038/sj.cdd.4400316. [DOI] [PubMed] [Google Scholar]
- 22.Matsda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S. Apoptosis by death factor review. Cell. 1997 Feb;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 24.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003 Oct;197(1):8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 25.Inoue N, Maeda A, Matsuda-Minehata F, Fukuta K, Manabe N. Expression and localization of Fas ligand and Fas during atresia in porcine ovarian follicles. J Reprod Dev. 2006 Dec;52(6):723–30. doi: 10.1262/jrd.18043. [DOI] [PubMed] [Google Scholar]
- 26.Quirk SM, Cowan RG, Joshi SG, Henrikson KP. Fas antigen-mediated apoptosis in human granu-losa/luteal cells. Biolreprod. 1995 Feb;52(2):279–87. doi: 10.1095/biolreprod52.2.279. [DOI] [PubMed] [Google Scholar]
- 27.Cohen GM. Caspases: the executioners of apop-tosis. Biochem J. 1997 Aug;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AL, Bridgham JT. Caspase-mediated apoptosis in the vertebrate ovary. Reproduction. 2002 Jul;124(1):19–27. doi: 10.1530/rep.0.1240019. [DOI] [PubMed] [Google Scholar]
- 29.Salehnia M, Abbasian Moghadam E, Rezazadeh Velojerdi M. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil Steril. 2002 Sep;78(3):644–5. doi: 10.1016/s0015-0282(02)03287-9. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May;129(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003 Mar;199(3):275–88. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- 32.Hussein MR, Haemel AK, Wood GS. p53-related pathways and the molecular pathogenesis of melanoma. Eur J Cancer Prev. 2003 Apr;12(2):93–100. doi: 10.1097/00008469-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Inoue N, Manabe N, Matsui T, Maeda A, Nakagawa S, Wada S, et al. Roles of tumor necrosis factor-related apoptosis-inducing ligand signaling pathway in granulosa cell apoptosis during atresia in pig ovaries. J Reprod Dev. 2003 Aug;49(4):313–21. doi: 10.1262/jrd.49.313. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998 Aug;281(5381):1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 35.Keren-Tal I, Suh BS, Dantes A, Lindner S, Oren M, Amsterdam A. Involvement of p53 expression in cAMP-mediated apoptosis in immortalized granulosa cells. Exp Cell Res. 1995 May;218(1):283–95. doi: 10.1006/excr.1995.1157. [DOI] [PubMed] [Google Scholar]
- 36.Hussein MR, Bedaiwy MA, Falcone T. Analysis of apoptotic cell death, Bcl-2, and p53 protein expression in freshly fixed and cryopreserved ovarian tissue after exposure to warm ischemia. Fertil Steril. 2006 Apr;85(Suppl):1082–92. doi: 10.1016/j.fertnstert.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4(12):1099–109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 38.Depalo R, Lorusso F, Bettocchi S, Selvaggi L, Cavallini A, Valentini AM, et al. Assessment of estrogen receptors and apoptotic factors in cryo-preserved human ovarian cortex. Syst Biol Reprod Med. 2009 Dec;55(5-6):236–43. doi: 10.3109/19396360903046761. [DOI] [PubMed] [Google Scholar]