Abstract
Background : Oocyte cryopreservation is one of the most important topics in the field of assisted reproductive technology to preserve women fertility, but relationship between cryopreservation and apoptosis is still a matter of debate. The present study was aimed to investigate the effects of vitrification on apoptosis in mouse oocytes by Cryotop method. Method: A total of 200 germinal vesicle (GV) and 200 metaphase II (MII) oocytes were obtained from ovaries and fallopian tubes of NMRI mice, respectively and divided into control and experimental groups. Oocytes in experimental group were vitrified by Cryotop using vitrification medium and were kept in liquid nitrogen for one month. The survival rate of oocytes was evaluated after 2 hour incubation time. Then, the oocyte apoptosis was evaluated by TUNEL technique and compared with those in control group. The data was compared statistically using SPSS software and chi-square test. Results: The survival rates of vitrified GV (93%) and MII oocytes (88%) showed a significant decrease compared with the control group (P<0.05), but there was no significant difference in survival rate of both vitrified oocyte groups. The incidence of apoptosis in vitrified and control GV oocytes showed no significant difference (13% vs. 7%), but the rate of apoptosis in vitrified MII oocytes increased significantly not only in comparison with MII control group (25% vs. 5%) but also with vitrified GV oocytes (P<0.05). Conclusion: The results indicate that vitrification increases apoptosis in mouse MII oocytes and apoptosis may play a role in MII oocyte injury after vitrification.
Key Words: Vitrification, Apoptosis, Oocytes
INTRODUCTION
Oocyte cryopreservation is one of the most important topics in the field of assisted reproductive technology, which is performed prior to radiotherapy, chemotherapy , and ovary damaging surgeries to preserve women fertility [1]. However, oocyte cryopreservation has been faced with many obstacles. Low survival and fertility rates combined with the rupture of meiosis spindle and the possibility of fetal aneuploidy limit the researches on human oocyte cryopreservation [2]. Cryopreservation methods can be divided into three categories: slow freezing, rapid freezing and vitrification [2]. Formation of ice crystals in the slow and rapid freezing methods makes the results unfavorable. The freezing process damages the cellular structure and in particular, the cell membranes through transformation of intracellular water into ice crystals [3]. Hence, another study has been carried out to reduce the time, remove the ice crystals, adjust the concentration of solutes and exclude the expensive equipments required for slow freezing [4].
One of the techniques to avoid damage through ice crystallization is the conversion of a liquid into a glass; a method known as vitrification, which was initially proposed by Luyet in 1937 for cryopreservation of oocytes [4].
Vitrification is known as a physical process by which a highly concentrated solution of cryoprotectant rapidly (5-8 s) transforms into a glassy vitrified state, from the liquid phase by a tremendous increase in the viscosity while cooling at a low temperature (-196ºC). This process is similar to the situation of molecules and ions in the liquid state with their natural disperse unchanged [5, 6]. Two issues are of prime importance in vitrification phenomenon: the type and the concentration of cryoprotectant. These two factors should be chosen in a manner to prevent the formation of intracellular and extracellular ice crystal and also avoid a concentration of cryoprotectant that is fatal for the cell. Usage of high concentrations of cryoprotectant in large cells such as oocytes, embryos, tissues or organs is necessary to avoid the lethal effects of ice crystallization. Nevertheless, an increase in the concentration of cryoprotectant itself can also be lethal to cells [7]. Studies have shown that despite the undeniable role of freezing procedures on the treatment of infertile people, the application of different concentrations of cryoprotectant and thermal-cooling changes can produce adverse effect on cell ultrastructure [8] and even the phenomenon of apoptosis [9].
In apoptosis, the DNA cleaves to oligonucleosomal pieces which is a characteristic currently used for staining of apoptotic cells. In this technique, called TUNEL method, the DNA fragments produced by terminal deoxytransferase are replaced by a new modified nucleotide (fluorescein-dUTP), allowing the broken ends of double-stranded DNA fragments to be labeled [10, 11]. Using this method, the cells are labeled even in early stages of apoptosis. It has been shown that the application of this technique, even in cases with low number of cells and small amount of DNA, is of great benefit to quantitative measurement of cell death in mouse and human embryos.
The objectives of the present study were to evaluate the effects of vitrification of mouse oocytes in germinal vesicle (GV) and metaphase II (MII) stages on their DNA damage in order to clarify the mechanism of injury to the oocytes after cryopreservation.
MATERIALS AND METHODS
Preparation of immature and mature oocytes. Adult female NMRI mice (6-8 weeks old) obtained from Razi Institute for Serums and Vaccines (Karaj, Iran) were used in the present study. Animals were housed under a 12 h light/dark cycle in a room with controlled temperature (22 ± 2°C) and humidity of 40-50%. Food and water were available ad libitum. Induction of ovulation was initially performed by intraperitoneal injection of 10 units pergnant mare serum gonadotropin (Sigma, UK), followed by 10 IU hCG after 48 hour. Mice were sacrificed by cervical dislocation approximately 13 hours after hCG injection. GV oocytes were collected from ovarian follicles by dissection method and MII oocytes were obtained from fallopian tube by flushing method. To eliminate sampling biases, oocytes of morphologically similar sizes with clear cytoplasm and uniform zona pellucida were allocated to each experimental group. The GV oocytes without cumulus cells were obtained by removal of the surrounding cumulus cells using a Pasteur pipette through frequent pipetting. The MII stage oocytes were also separated from the cumulus-oocyte complexes by the enzymatic action of intra cytoplasmic sperm injection (ICSI) Cumulase (Origio, Denmark), which is for the removal of the cumulus complex and corona radiate surrounding the oocyte in preparation for ICSI. Oocytes with partial remnants or no cumulus cells were transferred to fresh culture medium following several washings. A total of 400 oocytes (200 GV stage oocytes and 200 MII stage oocytes) were divided into two control and experimental groups and used for survival and apoptosis assessments.
Vitrification and thawing of oocytes. The GV and MII oocytes were vitrified using a two-step exposure to equilibrium and vitrification solutions. In the first step (equilibrium/dehydration), the GV and MII oocytes of both groups were exposed to the equilibrium solution (Origio, Denmark) for 10 minutes [12]. Then, the oocytes were exposed to vitrification solution (Origio, Denmark), followed by loading the 1-3 oocytes onto the propylene strip of Cryotop (Kitazato, Japan) within less than 60 seconds [13]. During thawing, the protective coating of Cryotops was initially removed, while the propylene strips were still in liquid nitrogen, followed by immediate and rapid transfer of Cryotops from LN2 into the thawing medium (Origio, Denmark). Later, the oocytes were transferred to culture medium (Origio, Denmark) and after 2 hours of incubation, the survival rate of oocytes was evaluated using an inverted microscope. Oocytes with spherical shape, intact membranes, clear zone without rupture, and uniform cytoplasm were considered healthy and alive. After vitrification and thawing, the survived MII and GV oocytes randomly were selected for subsequent investigations.
TUNEL staining. TUNEL and propidium iodide staining, performed by the method of Brison and Schultz [14], were presented in 1997 to investigate the phenomenon of DNA fragmentation in oocyte. Following several washings in PBS (Gibco, Grand Island, NY, USA), the oocytes were fixed in 3.7% paraformaldehyde solution (Wako, Japan), treated with 0.1% Triton X-100 solution (Sigma, Germany) for 40 min and exposed to blocking solution at 4°C overnight. Then, the oocytes were primarily incubated in TUNEL solution (Roche, Germany) at 37°C for an hour according to the manufacturer's instructions. Negative control oocytes were incubated only in fluorescent solution without enzyme to ensure the absence of labeling. For the positive control, a number of oocytes prior to incubation with TUNEL staining solution was incubated with 50 µg/ml DNase I solution (Sigma, Germany) for one hour and then treated with TUNEL solution. The oocytes were stained with 50 µg/ml propidium iodide (Sigma, Germany) solution for 20 min to label nuclei and examined using a fluorescent microscope (Labomed, USA).
Statistical Analysis. The viability and apoptosis rates between the oocytes in both control and experimental groups were analyzed using chi-square test.
Differences at P value of 0.05 or less were considered to be significant.
RESULTS
Qualitative observations. Figures 1 and 2 indicate that all positive control oocytes were labeled by TUNEL staining and appeared as green. None of the negative control oocytes were labeled by TUNEL staining and only counterstained by propidium iodide and appeared as red. As seen in Figure 1, the oocytes in experimental group after vitrification and thawing were stained for apoptosis, i.e., the apoptotic cells were well labeled with TUNEL staining and quite distinct from the non-apoptotic cells.
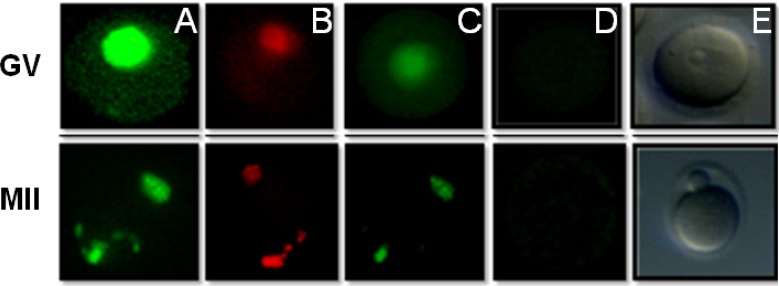
Fig. 1.
Microscopic view of mouse GV and MII stage oocytes. (A) Cell nuclei were marked green after incubation with DNase I (positive control). (B) Microscopic view of mouse oocytes under a fluorescent microscope; no cell was marked in the absence of terminal deoxytransferase but the cell nucleus stained with the propidium iodide (negative control). (C) Microscopic view of an apoptotic cell subjected to vitrification. (D) Microscopic view of a non-apoptotic cell subjected to vitrification. (E) Image of GV-stage oocytes under a stereo microscope
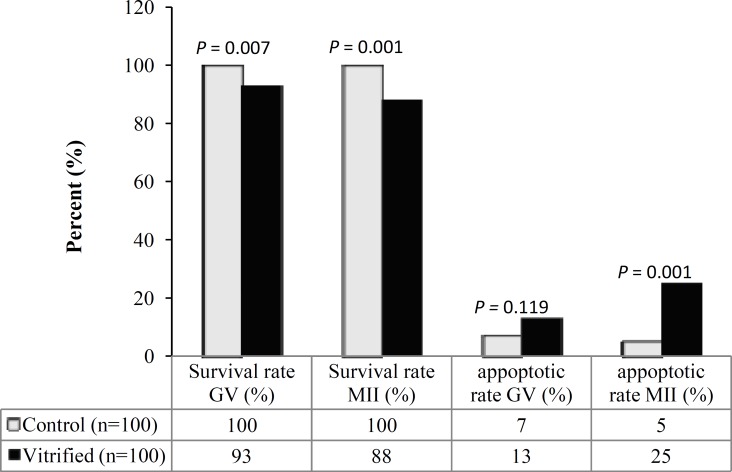
Fig. 2.
Survival and apoptosis rates in GV and MII oocytes of control and vitrified groups. There was a significant difference in the survival rate of GV and MII oocytes in vitrified and control groups. Apoptosis rate was significantly different in vitrified and control groups of MII oocytes. However, there was insignificant difference in apoptosis of vitrified GV oocytes and control groups
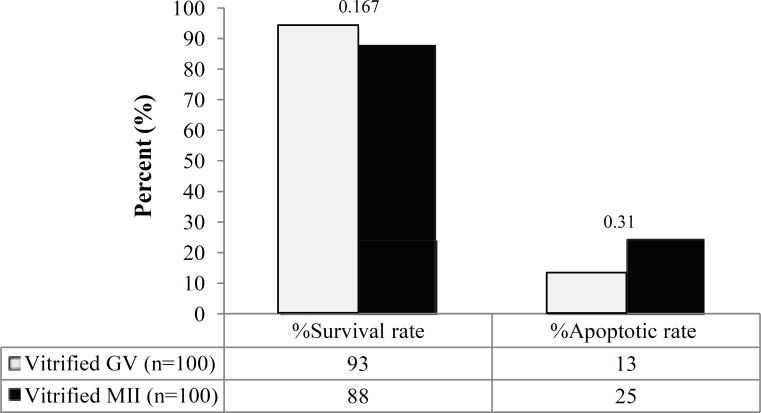
The survival rate in GV and MII oocytes after vitrification. As seen in Figure 2, the survival rate of GV oocytes after vitrification showed a significant decrease compared with control group (93% vs. 100%, P<0.05). Similarly, the survival rate of MII oocytes following vitrification also demonstrated a significant decrease compared with control group (88% vs. 100%, P<0/05). There was no significant difference in survival rates of both vitrified oocyte groups (P = 0.167).
The apoptosis rate in GV and MII oocytes after vitrification. As shown in Figure 3, the rate of apoptosis in GV oocytes after vitrification and warming showed no significant difference compared with control group (13% vs. 7%, P = 0.119). In contrast, the rate of apoptosis in vitrified MII oocytes demonstrated a significant increase compared to control MII oocytes (25% vs. 5%, P<0.05). A higher incidence of apoptosis was observed in vitrified MII oocytes compared to GV oocytes (P<0.05).
Fig 3.
Comparison of survival and apoptosis rates in vitrified GV and MII oocytes. No significant difference in the survival rate of both vitrified GV and MII oocytes groups was observed. In contrast, apoptosis rate in vitrified GV oocytes increased when compared to MII oocytes
DISCUSSION
According to the results of the present study, the survival rates of GV and MII oocytes showed a significant decrease following vitrification and thawing using Cryotop strips compared to the control group. Yazdanpanah et al. [15] demonstrated that the viability capacities of immature human oocytes following vitrification (56.0 %) were significantly reduced when compared with the control group (86.8 %). Similarly, Cao and his colleagues [16] in their study on human oocytes showed a significant reduction in the survival rates of GV and MII stage oocytes following thawing; however, the difference between the survival rate of GV and MII stage oocytes after vitrification and thawing was insignificant. In a study by Galeati and his colleagues [17], the pig oocytes at MII stage were found to be very sensitive to vitrification and thawing when using Cryotop. They also concluded that vitrification leads to reduction in viability of oocytes, changes in the organization of microtubules and damage to fertilization process [17]. These findings are consistent with the results of the present study.
In 2011, Soo et al. [18] found that there is no significant difference in survival rates of both GV and MII oocytes after vitrification and thawing compared with control group, a finding different from that obtained in our study. The difference observed between the results of these two studies could be attributed to application of different carriers, vitrification protocols, and diverse animal species. In the study by Soo and his colleagues [18] mentioned above, electron microscope grid and straw were used at freezing stage. Also, there is a report by Succu and his colleagues [19] in which they showed that the carriers used for freezing not only influence the spindle structure and the ability to survive but also affect the development potential due to the molecular change produced during vitrification process. Considering the conventional vitrification protocols, there are several major concerns regarding the relatively high concentration of cryoprotectant, toxicity of cryoprotectant, and osmotic changes due to vitrification solution [20]. Obviously, the exposure time, the equilibrium, and the vitrification solutions used in the studies described above are different from those employed in the present study. The species of the mouse in which the oocytes were obtained was different so that the species in the present study was NMRI while in the recently mentioned research was ICR. Research has revealed that the survival rate of oocytes is related to not only with the vitrification method but to the animal species and even the quality of oocytes and the evolutionary stage of embryos [21]. In addition, the present study demonstrated that apoptosis in vitrified MII oocyte group significantly increased compared to the control group. However, apoptosis in vitrified GV oocytes group showed no significant difference when compared with the control group. Likewise, Rao and his colleagues [22] in their study on goat immature oocytes showed that vitrification by Cryotop is one of the best techniques available, and the difference in rate of apoptosis in immature oocytes of both vitrified and control groups was statistically insignificant. Moreover, Mazoochi et al. [23] demonstrated that the expression of apoptotic genes in the follicles obtained from vitrified-warmed ovarian tissue strips has no significant difference when compared with the control group. In addition, Ebrahimi et al. [24] showed that apoptotic gene expression in GV oocytes of sheep after vitrification by cryotop was similar to the control group. Also in another study by Tharasanit and his colleagues [25] comparable findings were reported.
The results of this study showed that the number of oocytes with normal shape and arrangement of meiosis spindle in GV group were significantly higher than that of MII group. Studies have shown that the GV stage oocytes due to lack of meiotic spindle are less sensitive to environmental factors and also are more resistant compared to other cells [26]. Furthermore, the high permeability of the membrane and the absence of meiosis spindle in the immature oocytes can reduce the damage caused by vitrification [27].
The results of the present study are consistent with the findings of a recent research [28] regarding the absence of undesirable effect of vitrification on apoptosis of GV oocytes. Future studies are needed to comprehensively investigate all aspects of cryopreservation effects on immature oocytes at both cellular and molecular.
The results of the present study indicate that vitrification increases apoptosis in mouse MII oocytes and apoptosis may play a role in MII oocyte injury after vitrification.
ACKNOWLEDGEMENTS
We would like to thank Deputy of Research Department of Qazvin University of Medical Sciences for funding the present study.
References
- 1.Wang Z, Sun Z, Chen Y, He F. A modified cryoloop vitrification protocol in the cryopreservation of mature mouse oocytes. Zygote (Cambridge, England) 2009;17(3):217–24. doi: 10.1017/S0967199409005309. [DOI] [PubMed] [Google Scholar]
- 2.Gualtieri R, Laccarino M, Mollo V, Prisco M, Laccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril. 2009 Apr;91(4):1023–34. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 3.Chen SU, Yang YS. Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwan J Obstet Gynecol. 2009 Mar;48(1):15–22. doi: 10.1016/S1028-4559(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 4.Luyet BJ. The vitrification of organic colloids and of protoplasme. Biodynamica. 1937;1(29):1–14. [Google Scholar]
- 5.Magli MC , Lappi M, Ferraretti AP, Capoti A, Ruberti A, Gianaroli L. Impact of oocyte cryopreservation on embryo development. Fertil Steril. 2010 Jan;93(2):510–6. doi: 10.1016/j.fertnstert.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 6.Kasai M , Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod Biomed Online. 2004 Aug;9(2):164–70. doi: 10.1016/s1472-6483(10)62125-6. [DOI] [PubMed] [Google Scholar]
- 7.Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007 Jan;67(1):81–9. doi: 10.1016/j.theriogenology.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006 Nov;73(11):1454–1462. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]
- 9.Rajaei F, Karja NW, Agung B, Wongsrikeao P, Taniguchi M, Murakami M, Sambuu R et al. Analysis of DNA fragmentation of porcine embryos exposed to cryoprotectants. Reprod Domest Anim. 2005 Oct;40(5):429–32. doi: 10.1111/j.1439-0531.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamadori I, Yoshino T, Kondo E, Cao L, Akagi T, Matsuo Y, et al. Comparison of two methods of staining apoptotic cells of leukemia cell lines. Terminal deoxynucleotidyl transferase and DNA polymerase I reactions. J Histochem Cytochem. 1998 Jan;46(1):85–90. doi: 10.1177/002215549804600111. [DOI] [PubMed] [Google Scholar]
- 11.Neubar E, Luetjens CM, Chan AW, Schatten GP. Analysis of DNA fragmentation of in vitro cultured bovine blastocysts using TUNEL. Theriogenology. 2002 Jun;57(9):2193–202. doi: 10.1016/s0093-691x(02)00901-9. [DOI] [PubMed] [Google Scholar]
- 12.Elnahas A, Alcolak E, Abu Marar E, Elnahas T, Elnahas K, Palapelas V et al. Vitrification of human oocytes and different development stages of embryos: An overview. J Middle East Fertil Soc J. 2010 Jan;15:2–9. [Google Scholar]
- 13.Chian RC, Son WY, Huang JY, Cui SJ, Buckett WM, Tan SL, et al. High survival rate and pregnancies of human oocytes following vitrification: preliminary report. Fertil Steril. 2005 Sep;84(1):108–111. [Google Scholar]
- 14.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997 May;56:1088–96. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- 15.Yazdanpanah F, Khalili MA, Eftekhar M, Karimi H. The effect of vitrification on maturation and viability capacities of immature human oocytes. Arch Gynecol Obstet. 2013 Mar; doi: 10.1007/s00404-013-2777-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Xing Q, Zhang ZG, Wei ZL, Zhou P, Cong L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod Biomed Online. 2009 Sep;19(3):369–73. doi: 10.1016/s1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 17.Galeati G, Spinaci M, Vallorani C, Bucci D, Porcu E, Tamanini C. Pig oocyte vitrification by Cryotop method: Effects on viability, spindle and chromosome configuration and in vitro fertilization. Anim Reprod Sci. 2011 Aug;127(1-2):43–9. doi: 10.1016/j.anireprosci.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Cha SK, Kim BY, Kim MK, Kim YS, Lee WS, Yoon TK, et al. Effects of various combinations of cryoprotectants and cooling speed on the survival and further development of mouse oocytes after vitrification. Clin Exp Reprod Med. 2011 Mar;38(1):24–30. doi: 10.5653/cerm.2011.38.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Succu S, Leoni GG, Berlinguer F, Madeddu M, Bebbere D, Mossa F, et al. Effect of vitrification solutions and cooling upon in vitro matured prepubertal ovine oocytes. Theriogenology. 2007 Jul;68(1):107–14. doi: 10.1016/j.theriogenology.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Fahy GM. Theoretical considerations for oocyte cryopreservation by freezing. Reprod Biomed Online. 2007 Jun;14(6):709–14. doi: 10.1016/s1472-6483(10)60672-4. [DOI] [PubMed] [Google Scholar]
- 21.Niemann H, Lucas-Hahn A, Stoffregen C. Cryopreservation of bovine oocytes and embryos following microsurgical operations. Mol Reprod Dev. 1993 Oct;36(2):232–5. doi: 10.1002/mrd.1080360217. [DOI] [PubMed] [Google Scholar]
- 22.Rao BS, Mahesh YU, Charan KV, Suman K, Sekhar N, Shivaji S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology. 2012 Jun;64(3):176–84. doi: 10.1016/j.cryobiol.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mazoochi T, Salehnia M, Pourbeiranvand S, Forouzandeh M, Mowla SJ, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009 Mar;15(3):155–64. doi: 10.1093/molehr/gap002. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010 May;27(5):239–46. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tharasanit T, Colleoni S, Lazzari G, Colenbrander B, Galli C, Stout TA. Effect of cumulus morphology and maturation stage on the cryopreservability of equine oocytes. Reproduction. 2006 Nov;132(2):759–69. doi: 10.1530/rep.1.01156. [DOI] [PubMed] [Google Scholar]
- 26.Cocchia N, Ciani F, Russo M, El Rass R, Rosapane I, Avallone L, et al. Immature cat oocyte vitrification in open pulled straws (OPSs) using a cryoprotectant mixture. Cryobiology. 2010 Apr;60(2):229–34. doi: 10.1016/j.cryobiol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Sathananthan AH, Selvaraj K, Girijashankar ML, Ganesh V, Selvaraj P, Trounson AO. Trounson. From oogonia to mature oocytes: inactivation of the maternal centrosome in humans. Microsc Res Tech. 2006 Jun;69(6):396–407. doi: 10.1002/jemt.20299. [DOI] [PubMed] [Google Scholar]
- 28.Rao BS, Mahesh YU, Charan KV, Suman K, Sekhar N, Shivaji S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology. 2012 Jun;64(3):176–84. doi: 10.1016/j.cryobiol.2012.01.005. [DOI] [PubMed] [Google Scholar]