Abstract
The acylation step of the α-chymotrypsincatalyzed hydrolysis of N-acetyl-L (or DL)-tryptophan p-nitrophenyl, p-nitrothiophenyl, ethyl, and thiolethyl esters has been studied by the stopped-flow technique at 25°. The acylation rate constant, k2, and the enzyme substrate dissociation constant, Ks, were directly determined at pH 4, 5, and 8. Steady-state kinetics were studied at pH 7. The k2 values are nearly identical for oxygen esters and their sulfur counterparts, whereas the Ks value of the ethyl ester is larger by an order of magnitude than those of the other three. The results strongly suggest that oxygen and thiol esters of these specific substrates are hydrolyzed via the same pathway, and furthermore that acylation consists of more than one step, the formation and breakdown of a tetrahedral intermediate, the former being rate-determining. Effects of leaving-group hydrophobicity on k2 and Ks are also discussed.
Keywords: enzyme, presteady state, rate constant, binding constant, leaving-group effect
Full text
PDF
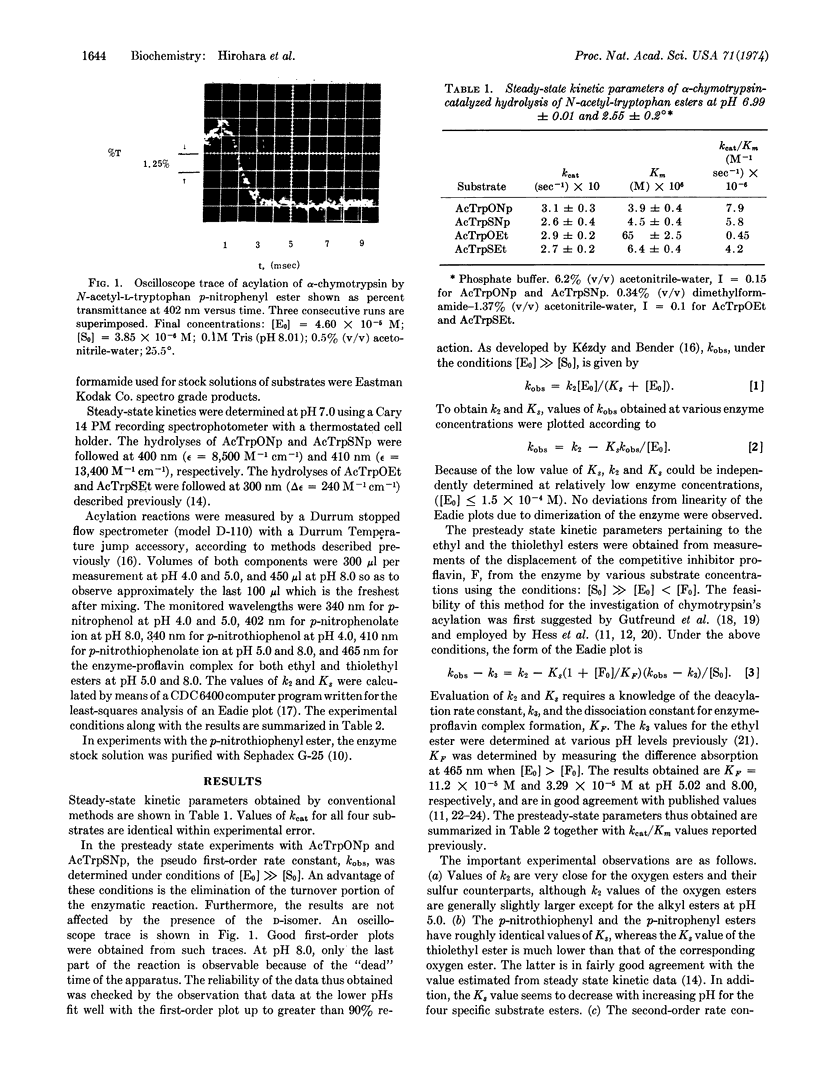

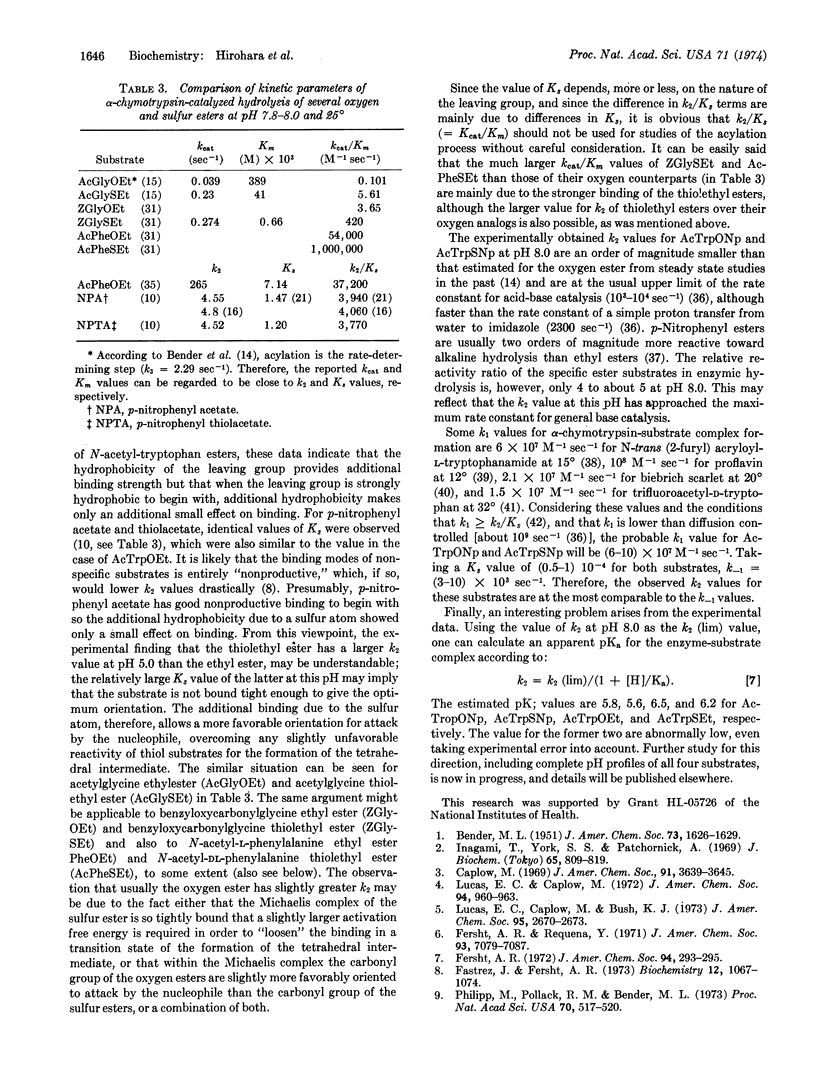

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Barman T. E., Gutfreund H. Optical and chemical identification of kinetic steps in trypsin- and chymotrypsin-catalysed reactions. Biochem J. 1966 Nov;101(2):411–416. doi: 10.1042/bj1010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin I. V., Kazanskaya N. F., Klyosov A. A. Determination of the individual rate constants of alpha-chymotrypsin-catalyzed hydrolysis with the added nucleophilic agent, 1,4-butanediol. FEBS Lett. 1971 Jun 10;15(2):121–124. doi: 10.1016/0014-5793(71)80037-6. [DOI] [PubMed] [Google Scholar]
- Bernhard S. A., Gutfreund H. The optical detection of transients in trypsin- and chymotrypsin-catalyzed reactions. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1238–1243. doi: 10.1073/pnas.53.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard S. A., Lee B. F., Tashjian Z. H. On the interaction of the active side of alpha-chymotrypsin with chromophores: proflavin binding and enzyme conformation during catalysis. J Mol Biol. 1966 Jul;18(3):405–420. doi: 10.1016/s0022-2836(66)80033-5. [DOI] [PubMed] [Google Scholar]
- Brandt K. G., Himoe A., Hess G. P. Investigations of the chymotrypsin-catalyzed hydrolysis of specific substrates. 3. Determination of individual rate constants and enzyme-substrate binding constants for specific amide and ester substrates. J Biol Chem. 1967 Sep 10;242(17):3973–3982. [PubMed] [Google Scholar]
- CLEMENT G. E., BENDER M. L. THE EFFECT OF APROTIC DIPOLAR ORGANIC SOLVENTS ON THE KINETICS OF ALPHA-CHYMOTRYPSIN-CATALYZED HYDROLYSES. Biochemistry. 1963 Jul-Aug;2:836–843. doi: 10.1021/bi00904a036. [DOI] [PubMed] [Google Scholar]
- Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1969 Jun 18;91(13):3639–3645. doi: 10.1021/ja01041a037. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Fastrez J., Fersht A. R. Mechanism of chymotrypsin. Structure, reactivity, and nonproductive binding relationships. Biochemistry. 1973 Mar 13;12(6):1067–1074. doi: 10.1021/bi00730a008. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Mechanism of the -chymotrypsin-catalyzed hydrolysis of specific amide substrates. J Am Chem Soc. 1972 Jan 12;94(1):293–295. doi: 10.1021/ja00756a061. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Requena Y. Equilibrium and rate constants for the interconversion of two conformations of -chymotrypsin. The existence of a catalytically inactive conformation at neutral p H. J Mol Biol. 1971 Sep 14;60(2):279–290. doi: 10.1016/0022-2836(71)90294-4. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Requena Y. Mechanism of the -chymotrypsin-catalyzed hydrolysis of amides. pH dependence of k c and K m . Kinetic detection of an intermediate. J Am Chem Soc. 1971 Dec 15;93(25):7079–7087. doi: 10.1021/ja00754a066. [DOI] [PubMed] [Google Scholar]
- Frankfater A., Kézdy F. J. Kinetics of hydrolysis of p-nitrophenyl thiolacetate by chymotrypsin. J Am Chem Soc. 1971 Aug 11;93(16):4039–4043. doi: 10.1021/ja00745a036. [DOI] [PubMed] [Google Scholar]
- Hague D. N., Henshaw J. S., John V. A., Pooley M. J., Chock P. B. Dimerization of Biebrich scarlet and the monomer-alha-chymorypsin interaction. Nature. 1971 Jan 15;229(5281):190–191. doi: 10.1038/229190a0. [DOI] [PubMed] [Google Scholar]
- Havsteen B. H. The kinetics of the two-step interaction of chymotrypsin with proflavin. J Biol Chem. 1967 Feb 25;242(4):769–771. [PubMed] [Google Scholar]
- Himoe A., Brandt K. G., DeSa R. J., Hess G. P. Investigations of the chymotrypsin-catalyzed hydrolysis of specific substrates. IV. Pre-steady state kinetic approaches to the investigation of the catalytic hydrolysis of esters. J Biol Chem. 1969 Jul 10;244(13):3483–3493. [PubMed] [Google Scholar]
- Inagami T., Patchornik A., York S. S. Participation of an acidic group in the chymotrypsin catalysis. J Biochem. 1969 May;65(5):809–819. doi: 10.1093/oxfordjournals.jbchem.a129081. [DOI] [PubMed] [Google Scholar]
- Ingles D. W., Knowles J. R. The alpha-chymotryptic ydrolysis of glycine esters. Biochem J. 1966 May;99(2):275–282. doi: 10.1042/bj0990275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Lucas E. C., Caplow M., Bush K. J. Chymotrypsin catalysis. Evidence for a new intermediate. 3. J Am Chem Soc. 1973 Apr 18;95(8):2670–2673. doi: 10.1021/ja00789a043. [DOI] [PubMed] [Google Scholar]
- Lucas E. C., Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1972 Feb 9;94(3):960–963. doi: 10.1021/ja00758a039. [DOI] [PubMed] [Google Scholar]
- Marini J. L., Caplow M. Substrate-induced pK perturbations with chymotrypsin. J Am Chem Soc. 1971 Oct 20;93(12):5560–5566. doi: 10.1021/ja00750a040. [DOI] [PubMed] [Google Scholar]
- McConn J., Ku E., Himoe A., Brandt K. G., Hess G. P. Investigations of the chymotrypsin-catalyzed hydrolysis of specific substrates. V. Determination of pre-steady state kinetic parameters for specific substrate esters by stopped flow techniques. J Biol Chem. 1971 May 10;246(9):2918–2925. [PubMed] [Google Scholar]
- Philipp M., Pollack R. M., Bender M. L. Influence of Leaving-Group Electronic Effect on alpha-Chymotrypsin: Catalytic Constants of Specific Substrates. Proc Natl Acad Sci U S A. 1973 Feb;70(2):517–520. doi: 10.1073/pnas.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár L. Symmetry and asymmetry in the mechanisms of hydrolysis by serine proteases and their thiol analogues. Acta Biochim Biophys Acad Sci Hung. 1972;7(4):319–334. [PubMed] [Google Scholar]
- Smallcombe S. H., Ault B., Richards J. H. Magnetic resonance studies of protein-small molecule interactions. Dynamics of binding between N-trifluoroacetyl-D-tryptophan and -chymotrypsin. J Am Chem Soc. 1972 Jun 28;94(13):4585–4590. doi: 10.1021/ja00768a029. [DOI] [PubMed] [Google Scholar]