Abstract
Regular physical exercise is considered to be an integral component of cancer care strategies. However, the effect of exercise training on tumor microvascular oxygenation, hypoxia, and vascular function, all of which can affect the tumor microenvironment, remains unknown. Using an orthotopic preclinical model of prostate cancer, we tested the hypotheses that, after exercise training, in the tumor, there would be an enhanced microvascular Po2, increased number of patent vessels, and reduced hypoxia. We also investigated tumor resistance artery contractile properties. Dunning R-3327 AT-1 tumor cells (104) were injected into the ventral prostate of 4–5-mo-old male Copenhagen or Nude rats, which were randomly assigned to tumor-bearing exercise trained (TB-Ex trained; n = 15; treadmill exercise for 5–7 wk) or sedentary groups (TB-Sedentary; n = 12). Phosphorescence quenching was used to measure tumor microvascular Po2, and Hoechst-33342 and EF-5 were used to measure patent vessels and tumor hypoxia, respectively. Tumor resistance artery function was assessed in vitro using the isolated microvessel technique. Compared with sedentary counterparts, tumor microvascular Po2 increased ∼100% after exercise training (TB-Sedentary, 6.0 ± 0.3 vs. TB-Ex Trained, 12.2 ± 1.0 mmHg, P < 0.05). Exercise training did not affect the number of patent vessels but did significantly reduce tumor hypoxia in the conscious, resting condition from 39 ± 12% of the tumor area in TB-Sedentary to 4 ± 1% in TB-Ex Trained. Exercise training did not affect vessel contractile function. These results demonstrate that after exercise training, there is a large increase in the driving force of O2 from the tumor microcirculation, which likely contributes to the considerable reduction in tumor hypoxia. These results suggest that exercise training can modulate the microenvironment of the tumor, such that a sustained reduction in tumor hypoxia occurs, which may lead to a less aggressive phenotype and improve patient prognosis.
Keywords: exercise, hypoxia, oxygenation, tumor, vasoconstriction
cancer cells are considered to acquire a defect in the circuits that govern normal cell homeostasis, thereby promoting the progressive transformation of normal cells into malignant derivatives (17). Thus, cancer is a disease involving temporal dynamic changes in vascularization and proliferative traits, resulting in the alteration of a normal physiological environment into a tumor microenvironment (17, 44). A compilation of available data suggests that the lack of responsiveness of neoplasms is influenced by physiological factors (e.g., tumor hypoxia) created, in part, by inadequate vascular networks that impact tumor blood flow, oxygen and nutrient supply, metabolic state, and pH distribution (17, 58). Hypoxia is a common feature of prostate cancer and has been demonstrated in tumors using a variety of measurements, including microelectrodes, hypoxic markers, and hypoxia-associated molecules (4, 35, 59). Within prostate tumors, hypoxia is associated with local recurrence, as well as early biochemical relapse after traditional therapeutic interventions (36). Given that prostate cancer is the second leading cause of cancer-related death in the United States, with an estimated ∼240,000 cases that will be diagnosed in 2013 (42), interventions that can combat tumor hypoxia are of high clinical relevance.
Regular physical exercise lowers the morbidity and mortality of many disease states and is commonly prescribed as an integral component of cancer patient care strategies to combat secondary complications from treatment, such as fatigue (2, 14). Despite prescription of exercise to both cancer patients and survivors, the effect of aerobic exercise training on prostate tumor oxygenation is largely unknown. For example, the elegant studies of Jones et al. (23) have demonstrated an upregulation of hypoxia-inducible factor 1α after exercise training in murine orthotopic prostate tumors, suggesting exercise may exacerbate tumor hypoxia, although no direct measures of tumor oxygenation were performed. Given tumor hypoxia has been indicated as an independent predictor for disease progression (45), it is crucial to understand whether exercise training can influence tumor oxygenation. Recently, we have demonstrated that during an acute bout of exercise, blood flow within the orthotopic prostate tumors of rats increases ∼200% when transitioning from rest to the steady state of exercise (32). In healthy tissues, changes in blood flow in response to exercise are dictated by changes in vascular resistance that redistribute cardiac output to tissues based on metabolic requirements (40). The increase in tumor blood flow during acute exercise (32) suggests an inability of the tumor vasculature to vasoconstrict and increase vascular resistance, which may result in a physiological opportunity to use exercise as a means to enhance tumor blood flow and oxygenation. What is unknown is how chronic training affects prostate tumor vascular function, as well as tumor oxygenation.
In tissues exhibiting impaired vascular function (i.e., spinotrapezius muscle with old age), exercise training increases the basal levels of microvascular oxygenation (31) [even though this muscle is not recruited during exercise (41)]. An elevation in microvascular Po2 would facilitate blood-to-tissue O2 flux under conditions of reduced intracellular Po2, such as those observed in contracting muscle (46) or within prostate tumors (59). In this regard, if a similar adaptation occurs within the microvasculature of the tumor after training, there may be a reduction in tumor hypoxia that could be of high clinical relevance. Therefore, the purpose of this investigation was to test the hypotheses that chronic endurance training will 1) improve tumor microvascular oxygenation and 2) diminish tumor hypoxia compared with sedentary counterparts. On the basis of the findings of an improved tumor oxygenation after training from those studies, we investigated two potential mechanisms that could contribute to changes in tumor oxygenation after exercise training, including 1) patent vessels (i.e., vessels that support flow) within the tumor in the conscious, resting condition, and 2) tumor resistance artery contractile function in vitro. With respect to the latter, there is a diminished pharmacological vasoconstriction in tumors (5), which may affect blood flow distribution within a tumor [vs. dysfunctional vasodilatory capacity (54) that may limit whole tumor perfusion]. On the basis of previous findings that exercise training upregulates contractile properties of resistance arteries in nonactive tissue during exercise (29), we also hypothesized that exercise training would enhance contractile responsiveness, and improve hemodynamic control, of the tumor vasculature. The results from these studies are important in determining the effect of exercise training on tumor oxygenation and potential mechanisms that may contribute to changes in tumor hypoxia.
METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC., rev. 1995). Two groups of male rats were used in the current study. In the first set of studies (i.e., microvascular Po2 and vasomotor function) Copenhagen rats (n = 20, COP/CrCrl; Charles River, Wilmington, MA) were investigated at ∼5 mo of age. Subsequently, because of an unexpected lack of availability of Copenhagen rats, the second set of studies (i.e., tumor hypoxia and patent vessels) were performed in National Institutes of Health (NIH) Nude rats (n = 12; NTac:NIH-Whn; National Cancer Institute, Frederick, MD) at ∼6 mo of age. The parental tumor from which the cell line is derived is the original Dunning R-3327 (11). Rats were housed at 23°C, maintained on a 12:12-h light-dark cycle and provided rat chow and water ad libitum.
Orthotopic Model of Prostate Cancer
The Dunning R3327-AT-1 (AT-1) rat prostate adenocarcinoma cell line (ECACC; Sigma Aldrich, St. Louis, MO) was utilized in this study for both groups of rats. This cell line is a well-established model of prostate cancer (25) with a low metastatic potential, fast growth rate, and characteristics similar to progressive human prostate cancers (21). AT-1 cells were cultured in RPMI 1640 medium (supplemented with 2 mM glutamine, 250 nM dexamethasone, 10% FBS, and 1% penicillin/streptomyocin; Sigma Aldrich) and maintained in a humidified incubator at 5% CO2. At ∼80–90% confluence, viable cells were counted, and a tumor cell stock solution was prepared with physiological saline solution (PSS) and separated into aliquots of 0.1 ml containing ∼104 AT-1 cells each.
Under anesthesia (isoflurane; 2%/O2 balance), the bladder and prostate complex was exposed and isolated through a small abdominal incision (<2 cm) lateral to the midline of the abdomen. Using sterile insulin syringes (26 G), we injected 0.1 ml of cell stock solution (or 0.1 ml of PSS for vehicle control rats) into the ventral lobe of the prostate. Following the injection, closure of the abdominal wall (3–0, polyglycolic acid coated; DemeTECH, Miami Lakes, FL) and overlying skin/fascia (3–0 nylon monofilament; DemeTECH) incisions were performed. All procedures were performed under aseptic conditions. Postoperative monitoring of the animals was performed daily until animals were placed in either sedentary or exercise-trained groups ∼7–10 days postinjection.
Exercise Training Protocol
Animals were randomly assigned to a tumor-bearing sedentary (TB-Sedentary, Copenhagen: n = 6; Nude: n = 6) group, tumor-bearing endurance-trained (TB-Ex Trained, Copenhagen: n = 9; Nude, n = 6) group, or a vehicle control (Control, Copenhagen: n = 5). Within each strain of rats, all animals were studied at the same time point after injection of the tumor cells or vehicle. Endurance-trained animals performed treadmill running 5 days/wk for 60 min/day at 15 m/min (15° incline), for 7 wk in the Copenhagen rats, and 5 wk in the Nude rats. The time frame of exercise training was reduced in the Nude rats by 2 wk to ensure tumor mass did not approach institutional size limits within the rat. Specifically, the first studies were performed in the Copenhagen rats with the cells implanted for a total of ∼10 wk (7–10 days postsurgery recovery, + 1 wk acclimation, + 7 wk training or cage confinement), which resulted in tumors approaching, but not exceeding, institutional size limits. The subsequent studies in the Nude rats were shortened by 2 wk to avoid the potential of tumor size constraints. The intensity of exercise corresponds to an intensity of ∼60% of maximal aerobic capacity (i.e., low-to-moderate exercise intensity domain) for animals of similar age and weight (39). We chose a low-intensity exercise vs. a more energetically demanding intensity as 1) this intensity is well tolerated by the animals, and 2) high-intensity exercise has been suggested to enhance tumor metastases (7). Experimental protocols were performed on trained rats 48 h following the last bout of training to avoid any existent postexercise hemodynamic effects and ensure a true resting condition.
Experimental Protocol
Study I. tumor microvascular Po2.
Phosphorescence quenching was used to measure prostatic tumor microvascular Po2 in TB-Sedentary (n = 6) and TB-Ex Trained (n = 9) Copenhagen rats. Specifically, 48 h after the last bout of exercise, rats were anesthetized with pentobarbital sodium (40 mg/kg ip, supplemented as needed), and the right carotid artery was isolated. The artery was cannulated with a fluid-filled catheter (PE-50) to monitor arterial blood pressure and heart rate for the duration of the experiment (Digi-Med BPA model 200). This fluid-filled catheter was used for the administration of additional anesthesia and for the infusion of the phosphorescent probe. Rectal temperature was monitored and maintained at 37–38°C with a heating pad. The prostatic tumor was exposed and a PMOD 5000 Frequency Domain Phosphorimeter probe (Oxygen Enterprises, Philadelphia, PA) was positioned ∼2 mm above the surface of the medial region of the tumor. A light guide contained within the probe focuses excitation light (635 nm) on the medial region of the exposed tumor tissue (∼2.0-mm diameter) with penetration depths up to 4,000 μm (22) with the phosphorescence probe G2, which was used to measure tumor microvascular Po2 vs. G3, which measures tumor interstitial Po2 (63). After the probe was positioned, G2 (3.0 mg/kg; Oxygen Enterprises, Philadelphia, PA) was injected into the circulation, and measurements were immediately initiated. Once the signal was acquired and stabilized (<30 s after the injection of G2), measurements were collected for 1 min to ensure that the signal originated from the microvasculature (vs. longer times that may result in extravasation of the probe) and to calculate an average Po2. Probe accumulation in the interstitial space would result in an increase in signal amplitude over time if the O2 pressure is stable (Wilson DF, personal communication); however, there were no changes in Po2 values nor signal amplitude during the short time frame utilized (<1.5 min total), thus Po2 values reflect those within the vasculature of the tumor vs. the interstitial space. The PMOD 5000 uses a sinusoidal modulation of the excitation light (635 nm) at frequencies between 100 Hz and 20 kHz, which allows phosphorescence lifetime measurements from 10 μs to ∼2.5 ms. In the single-frequency mode, 10 scans (100 ms) were used to acquire the resultant lifetime of the phosphorescence (800 nm) and repeated every 2 s (for review, see Ref. 60). The phosphorescence lifetime was obtained computationally on the basis of the decomposition of data vectors to a linearly independent set of exponentials (61).
Po2 calculations.
The Stern-Volmer relationship allows the calculation of Po2 from a measured phosphorescence lifetime using the following equation (47): Po2 = [(tO/t) − 1]/(kQ·tO), where kQ is the quenching constant (mmHg/s), and to and t are the phosphorescence lifetimes in the absence of O2 and at the ambient O2 pressure, respectively. For G2, in in vitro conditions similar to those found in the blood, kQ is 273 mmHg/s, and to is 251 μs (30). G2 is tightly bound to albumin in the plasma and is negatively charged. These properties, in combination with the measurement period occurring immediately with the injection of G2 into the circulation, ensure that the Po2 measurements emanate from the plasma within the microvasculature rather than the surrounding tumorous tissue. Moreover, Po2 represents the O2 pressure head for diffusive blood-tissue O2 movement. The phosphorescence lifetime is insensitive to probe concentration, excitation light intensity, and absorbance by other chromophores in the tissue (47). The effects of pH and temperature are negligible within the normal physiological range, which was maintained herein (30). Upon completion of the experiment, each rat was euthanized with an overdose of anesthesia (pentobarbital sodium, >100 mg/kg ia), and a thoracotomy was performed to verify cardiac arrest.
Study II. Tumor hypoxia and patent vessels.
Localized tumor hypoxia and patent vessels were determined in male Nude rats (TB-Sedentary: n = 6 and TB-Ex Trained: n = 6). The animals were anesthetized with isoflurane (2%/O2 balance), and a catheter (Braintree Scientific; inner diameter 0.36 mm; outer diameter 0.84 mm) filled with heparinized saline solution (Elkins-Sinn, 100 U/ml) was implanted in the caudal tail artery and externalized. The catheter was used for infusion of hypoxic markers and stains.
Tumor hypoxia and patent vessels were assessed in conscious, resting animals 48 h after the last bout of exercise or in time-matched sedentary animals. Tumor hypoxia was assessed by immunohistochemical identification of the pentafluorinated nitroimidazole derivative EF5, designed and provided by Dr. C. J. Koch (University of Pennsylvania, Philadelphia, PA) (27). A 10-mM solution was prepared by dissolving EF5 in 0.9% saline and was administered intraperitoneally at 30 mg/kg, 1 h prior to death. Vessels actively perfused (i.e., patent vessels) were identified using Hoechst-33342 (Invitrogen, Life Technologies, Grand Island, New York), which provides optimal visualization of tumor vasculature by staining cells immediately adjacent to perfused blood vessels (55) and is an established technique for determining functional tumor vasculature (50). Hoechst-33342 was dissolved in dH2O and administered 1 min prior to death via the caudal artery at a dose of 40 mg/kg. Animals were euthanized with pentobarbital sodium (>100 mg/kg ia), and tumors were excised, weighed, coated with OCT compound (Sakura Finetek, Torrance, CA), submerged in isopentane, and frozen in liquid nitrogen. The excised tissue was cut into 10-μm sections, obtained from three different depths between the periphery, and the equatorial plane, and placed on poly-l-lysine-coated glass slides for staining and imaging.
Image acquisition and immunohistochemistry.
Sections were imaged with a fluorescent microscope (Zeiss AXIO Observer A1, Oberkochen, Germany) equipped with a 100 W mercury short-arc lamp, 6× HD illumination, excitation light source (X-Cite Series 120-Q; Lumen Dynamics, Mississauga, Ontario, Canada), and mounted with an AxioCam ERc5s camera (Zeiss) connected to a Dell OptiPlex 3010. Images were acquired through a Zeiss neofluar 5× objective and captured and digitized using ZEN 2011 software (Zeiss).
Immediately after sectioning, slides were imaged for Hoechst-33342 (420-nm barrier filter) prior to EF5 staining (×10 magnification). Quantification of Hoechst-33342-stained vessels was determined using the Chalkley-point array for random sample analysis (18), and perfused vessels were identified by the surrounding halo of the fluorescent Hoechst-33342-labeled cells, as previously described (48, 49). A 25-point Chalkley grid was positioned randomly over a field of view, and the number of points falling within the fluorescent halo was scored as positive (48, 49). The mean vessel density was determined from 30 random fields across a minimum of 10 sections per tumor.
Slides were then fixed in acetone (5 min) and allowed to air dry. Sections were blocked overnight with 5% goat serum in PBS containing 0.3% Tween-20 (ttPBS). Slides were rinsed with PBS and stained with the Cy-3-conjugated monoclonal antibody ELK-351 (specific EF5 adduct, 75 mg/ml) for 6 h. Sections were rinsed twice with PBS and ttPBS and stored in 1% paraformaldehyde until image acquisition [Rhodamine-(FITC) excitation filter].
To determine the area of EF5-bound, section-by-section images were analyzed using public domain NIH Image software (ImageJ, developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/ij) (64). Briefly, images were converted to a binary format, and pixels with a higher intensity than the threshold were treated as an area with positive uptake of EF5. Regional hypoxia was quantified by dividing the EF5-bound area by the total viable tissue area.
Study III. isolated microvessel preparation.
In the Copenhagen rats from study I, vasoconstrictor responsiveness resistance arteries of the prostate from the healthy vehicle control group (Control; n = 5) and prostatic tumor of the tumor-bearing group (TB-Sedentary: n = 6; TB-Ex Trained: n = 6) were investigated in vitro using the isolated microvessel technique. Immediately after Po2 measurements, the prostate tumor tissue was carefully excised and placed in cold (4°C) PSS containing the following (mM): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer, and 1 g/100 ml BSA at pH 7.4. Resistance arteries [∼150 to 200 μm intraluminal diameter, which provide a substantial portion of vascular resistance; (6)] from the prostatic tumor (defined as the first branch of the prostatic feed artery perforating the tumor) were isolated with the aid of a dissecting microscope (Olympus SVH12), cleared of surrounding tissue, and placed in Lucite chambers containing MOPS-buffered PSS equilibrated to room air. For vehicle control animals, the bladder-prostate complex was removed and placed in cold PSS, as described above. The bladder was reflected, and resistance arteries (similar in diameter as above), perforating the glandular tissue of the anterior-lateral portion of the ventral lobe of the prostate, were isolated and dissected. The arteries were cannulated on both ends to glass micropipettes and secured with ophthalmic nylon suture (Alcon 11–0). After cannulation, the chambers were transferred to the stage of an inverted microscope (Olympus IX70), equipped with a video camera (Panasonic BP310) and video caliper (Colorado Video) for recording luminal diameter. Intraluminal pressure was set at 90 cmH2O with two independent hydrostatic pressure reservoirs; this intraluminal pressure is equivalent to that measured in vivo in vessels of similar size (24, 33). Leaks were detected by pressurizing the vessel and determining whether vessel diameter was maintained. Vessels that exhibited leaks were discarded. Vessels free of leaks were warmed to 37°C and allowed to develop spontaneous tone during a 60-min equilibration period.
To evaluate vasoconstrictor responsiveness, two series of experiments were performed within the same animal. One artery was exposed to 1) cumulative additions of the α-adrenoreceptor agonists norepinephrine (NE: 10−9 to 10−4 M) and phenylephrine (PE: 10−9 to 10−4 M), or 2) myogenic response to increases in intraluminal pressure from 0 to 135 cmH2O in 15-cmH2O increments (37). All pressure changes occurred in the absence of intraluminal flow. Diameter was continuously recorded for 5 min at each dose of NE, PE, or change in pressure. The passive pressure-diameter relation was then determined after the vessels were incubated at 37°C for 60 min in Ca2+-free PSS containing 100 μM SNP. The same protocol, as described for the active myogenic response, was used to determine passive vessel characteristics. Intraluminal diameter was measured in response to agonists and expressed as a percentage of vasoconstrictor response, according to the following: vasoconstriction (% maximal response) = (Db − Ds)/Db·100, where Ds is the steady-state inner diameter recorded after addition of agonist, Db is the initial baseline inner diameter before the first addition of a pharmacological agonist, and Dm is the maximal intraluminal diameter obtained in Ca2+-free PSS. Spontaneous tone was expressed as a percentage of maximal diameter as follows: Spontaneous tone (%) = (Dmax − Db)/Dmax·100.
Comparison of data as a percentage of the maximal response normalizes for potential differences in maximal diameter or spontaneous tone among vessels. There are no data, to our knowledge, regarding spontaneous tone in tumor vessels in vitro. To be able to analyze differences in constrictor responsiveness, a minimum of 10% steady tone (unchanging for at least 15 min) was required prior to the addition of pharmacological or pressure stimuli in all vessels.
Active myogenic responses after sequential intraluminal pressure changes were normalized according to the following formula: normalized diameter = (IDSS/ID90), where IDSS is the steady-state diameter measured after each step-wise change in intraluminal pressure, and ID90 is the diameter measured at 90 cmH2O during the passive pressure response, which reflects the maximal diameter for vessels, in which active and passive pressure-responses were determined. Diameter is normalized to account for possible differences in vessel size between groups.
Muscle Oxidative Capacity
The mitochondrial enzyme and marker of muscle oxidative potential, citrate synthase, was measured in duplicate from soleus muscle homogenates, according to the method of Srere (51) to determine the efficacy of the training protocol in both strains. Citrate synthase activity was measured spectrophotometrically using a Spectramax M5 microplate (Molecular Devices, Sunnyvale, CA) in 300-μl aliquots at 30°C. Citrate synthase activity is expressed as micromoles per minute per gram wet weight.
Data Analysis
One-way ANOVA was used to determine differences in body mass, tissue mass, perfused vessel density, mean Po2, Po2 variance, regions of hypoxia between groups and soleus muscle citrate synthase activity. A two-way ANOVA was used to compare group differences in dose-response and pressure-diameter curves. All values are presented as means ± SE. P ≤ 0.05 was required for significance.
RESULTS
Animal and Tissue Characteristics
Left ventricular weight-to-body weight ratio was significantly greater in both Copenhagen and Nude TB-Ex Trained rats, compared with their sedentary counterparts (Table 1). Soleus muscle citrate synthase activity was significantly greater in both trained groups compared with sedentary counterparts, validating the efficacy of the exercise training protocol (Table 1). There were no differences in tumor weights after exercise training in the Copenhagen rats, whereas there was a increase in tumor weight after exercise training in Nude rats compared with sedentary animals (Table 1). There were no significant differences in vessel maximal diameter, spontaneous tone, or wall thickness between groups. However, TB-Ex Trained Copenhagen rats showed a trend (P = 0.07) for a reduced maximal diameter vs. the other two groups (Table 1).
Table 1.
Animal, soleus muscle citrate synthase activity, tissue, and arteriolar characteristics
| Copenhagen Rats |
Nude Rats |
||||
|---|---|---|---|---|---|
| TBSED | TBExT | Control | TBSED | TBExT | |
| Animal characteristics | |||||
| n | 6 | 9 | 5 | 6 | 6 |
| Body weight (BW), g | 276 ± 11 | 258 ± 9 | 304 ± 12‡ | 359 ± 6 | 323 ± 8* |
| Tumor weight (TW), g | 16 ± 5 | 19 ± 3 | 1.1 ± 0.2 | 1.8 ± 0.2* | |
| TW/BW, % | 6 ± 2 | 7 ± 1 | 0.32 ± 0.05 | 0.54 ± 0.05* | |
| Heart weight (HW), g | 0.57 ± 0.05 | 0.60 ± 0.03 | 0.76 ± 0.04*‡ | 0.97 ± 0.01 | 0.95 ± 0.06 |
| HW/BW, % | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.02*‡ | 0.27 ± 0.01 | 0.29 ± 0.02 |
| Left ventricle weight (LV), g | 0.18 ± 0.02 | 0.24 ± 0.01* | 0.23 ± 0.01* | 0.32 ± 0.01 | 0.38 ± 0.03 |
| LV/HW, % | 31 ± 1‡ | 40 ± 1 | 31 ± 1‡ | 33 ± 1 | 40 ± 1* |
| Soleus citrate synthase activity, μmol·min−1·g wet wt−1 | 16.9 ± 1.6 | 23.1 ± 2.1* | 17.1 ± 1.3 | 15.9 ± 1.4 | 22.6 ± 1.5* |
| Vessel characteristics | |||||
| Maximal diameter, μm | 190 ± 23 | 136 ± 6¥ | 172 ± 15 | ||
| Spontaneous tone, % | 11 ± 3 | 11 ± 3 | 21 ± 4 | ||
| Wall thickness, μm | 22 ± 1 | 19 ± 1 | 21 ± 1 | ||
Values are expressed as means ± SE; n, number of rats.TBSED, tumor-bearing, sedentary; TBExt, tumor-bearing, exercise-trained. Maximal diameter was determined in the absence of extracellular Ca2+ in resistance arteries in Copenhagen rats. Spontaneous tone is expressed as a relative percentage of tone established following pressurization at 90 cmH2O.
P ≤ 0.05 vs. TBSED within same group,
P ≤ 0.05 vs. TBExT within same group,
P = 0.07 vs. TBSED and Control.
Tumor Microvascular Po2
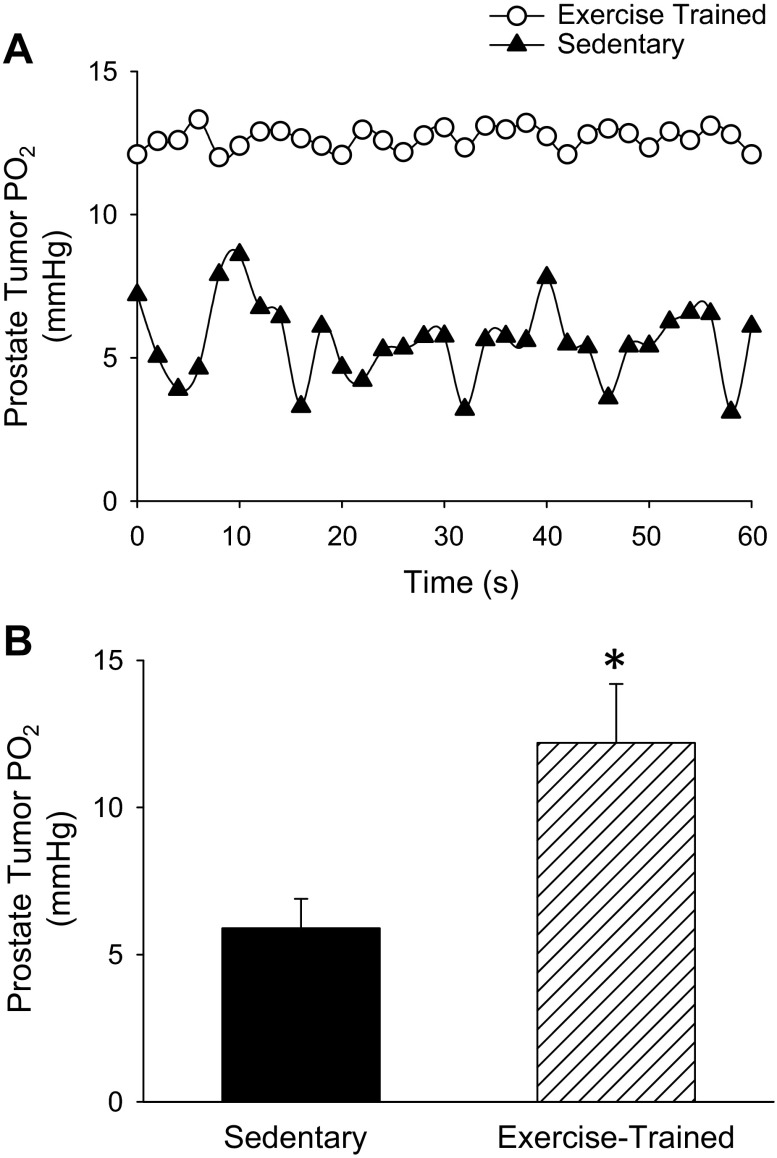
Representative tumor microvascular Po2 profiles over time for sedentary and exercise-trained groups are illustrated in Fig. 1A. There was considerably more variance around the mean in tumor microvascular Po2 values across the measurement period in the sedentary (2.1 ± 0.3 mmHg) compared with that from the exercise-trained animals (0.25 ± 0.09 mmHg; P ≤ 0.05) (Fig. 1A). The average microvascular Po2 in the prostate tumor of the sedentary rats was similar to the average Po2 found in prostate tumors from a compilation of data from different studies [i.e., ∼ 6 mmHg; (59)]. Endurance training significantly improved microvascular Po2 within the prostate tumor of rats compared with their sedentary counterparts. The average Po2 values of TB-Ex Trained were double that of the TB-Sedentary (12.2 ± 1.0 mmHg vs. 6.0 ± 0.3 mmHg, respectively; P ≤ 0.05, Fig. 1B).
Fig. 1.
A: representative prostate tumor microvascular partial pressure of oxygen (Po2) profile over time from an exercise-trained and sedentary rat. B: average prostate tumor microvascular Po2 group values for tumor-bearing sedentary (n = 6) and exercise-trained (n = 9) Copenhagen rats. *P < 0.05 between groups.
Hypoxia and Patent Tumor Blood Vessels
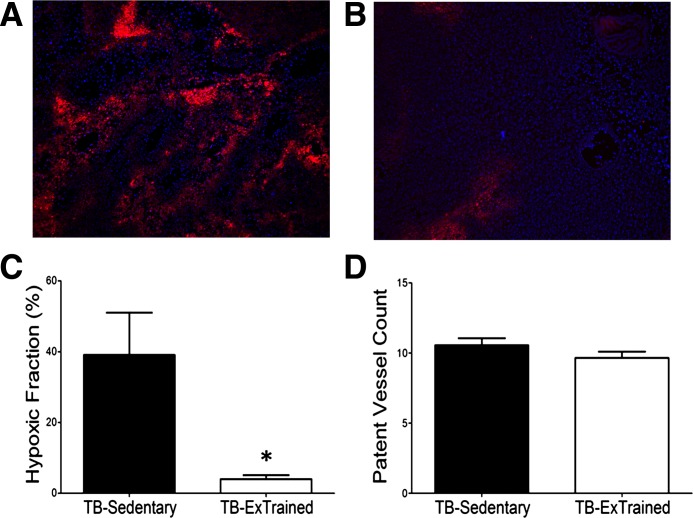
Representative images of bound EF5 (red) and perfused vessels as indicated by Hoechst 33342 (blue) in a prostate tumor from TB-Sedentary and TB-Ex Trained rats are shown in Fig. 2, A and B, respectively. Following endurance training, the average spatial distribution of hypoxia, as demonstrated by the presence of bound EF5, in TB-Sedentary and TB-Ex Trained rats is illustrated in Fig. 2C. The fraction of EF5-bound cells decreased significantly in the TB-Ex Trained (4 ± 1%) compared with TB-Sedentary (39 ± 12%, P ≤ 0.05; Fig. 2C) rats, demonstrating a reduction in tumor hypoxia after endurance training in the conscious resting condition. However, the average number of patent vessels per field (minimum of 30 random fields per tumor) in the prostatic tumor did not differ in the TB-Ex Trained compared with that measured in the TB-Sedentary animals (10 ± 1 vs. 11 ± 1 vessels per field, respectively; Fig. 2D).
Fig. 2.
Representative fluorescence photomicrograph of a prostate tumor cross section following intraperitoneal injection of EF5 (red; 30 mg/kg) and intra-arterial injection of Hoechst-33342 (blue; 40 mg/kg) in sedentary (A; n = 6) and exercise-trained (B; n = 6) nude rats. Graphical representation of the fraction of tissues bound by EF5 (C) and patent vessel count (D), as marked by Hoechst-33342 fluorescence. The slides show regions of EF-5 (red; hypoxia) and Hoechst 33342 (blue; patent vessels) binding. Dark spots in EF-5 areas represent dead cells that cannot bind to EF-5, and dark areas within the Hoechst 33342 areas reflect small holes resulting from snap freezing. *P ≤ 0.05 between groups.
Tumor Resistance Vessel Contractile Responsiveness
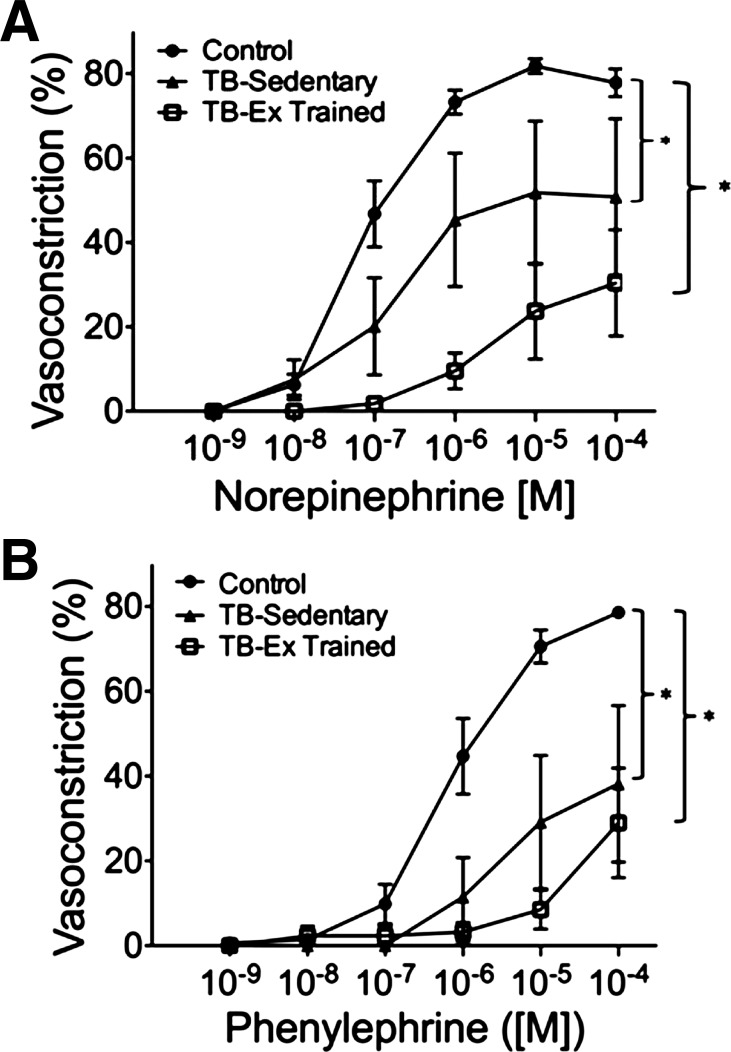
In response to the α-adrenoreceptor agonists norepinephrine and phenylephrine, there was a concentration-dependent vasoconstriction in the control prostate and tumor resistance arteries (TB-Sedentary and TB-Ex Trained) (Fig. 3, A and B). However, the maximal constriction to NE dose was significantly diminished in the TB-Sedentary and TB-Ex Trained arteries vs. that from the healthy prostate (Fig. 3A). The maximal constriction elicited by PE was severely blunted in tumor-bearing vessels (TB-Sedentary; 38 ± 18%, TB-Ex Trained; 29 ± 13%) compared with control arteries (79 ± 1%; P ≤ 0.05, Fig. 3B). There was no difference between tumor artery responsiveness to norepinephrine or phenylephrine.
Fig. 3.
Concentration-response relations to adrenergic receptor agonists norepinephrine (A) and phenylephrine (B) in the control prostate (Control; n = 5) and prostatic tumor arteries from sedentary [tumor-bearing (TB)-Sedentary; n = 6] and exercise-trained (TB-Ex Trained; n = 6) Copenhagen rats. *P ≤ 0.05 vs. Control group.
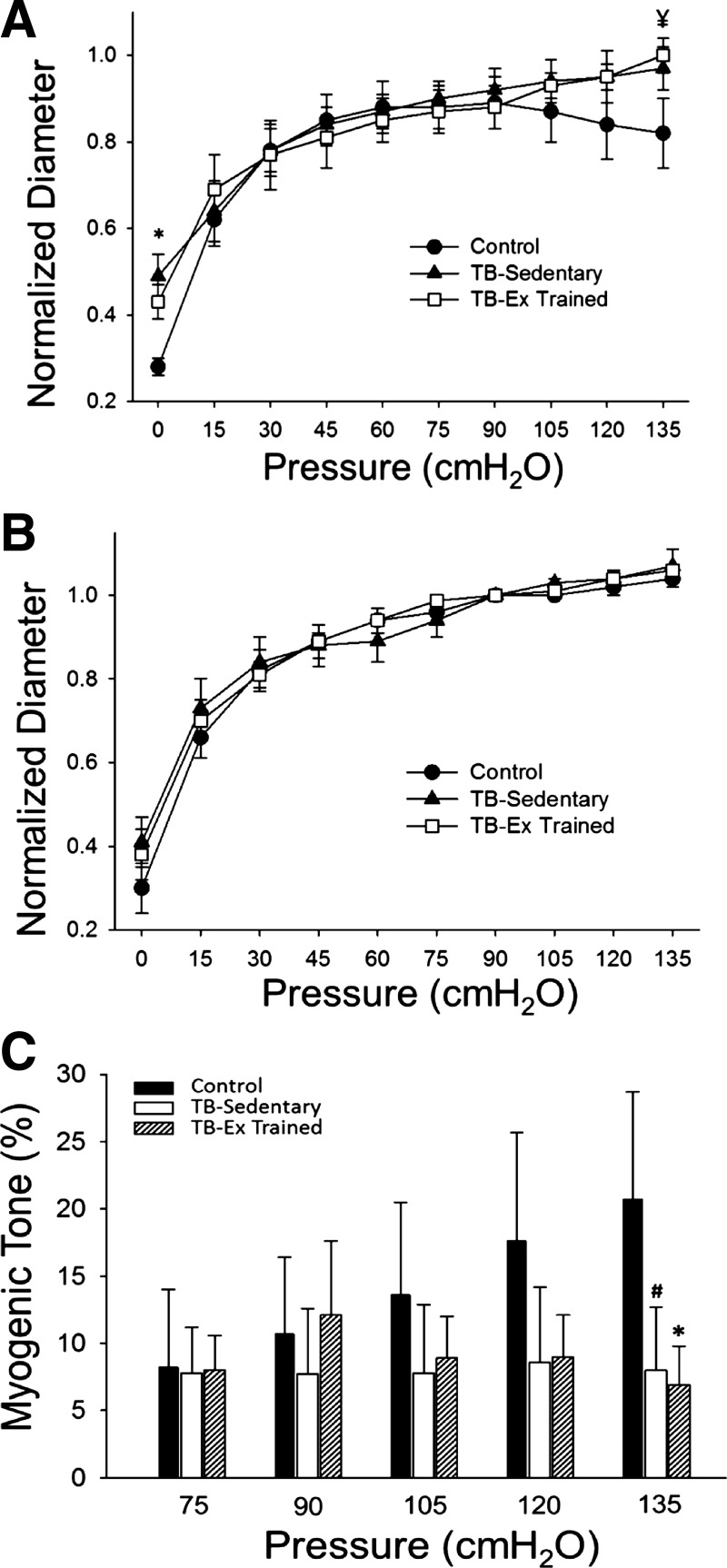
Arteries from both vehicle control and tumor-bearing animals displayed an active myogenic constriction (Fig. 4A). Control prostate arteries demonstrated a significantly reduced intraluminal diameter at 0 cmH2O (P ≤ 0.05) and a trend for reduced diameter at 135 cmH2O (P = 0.08) compared with the tumor arteries (Fig. 4A). In the absence of extracellular calcium, there was no difference in the passive diameter between groups (Fig. 4B). Acute myogenic tone (i.e., the ability to constrict with elevations in intraluminal pressure) was not significantly different between groups except at the highest intraluminal pressure (i.e., 135 cmH2O) at which control prostate arteries developed significantly more tone than the exercise-trained arteries (P ≤ 0.05) and a trend for greater tone development compared with the sedentary tumor vessels (P = 0.10) (Fig. 4C).
Fig. 4.
Active (A) and passive (B) diameter responses to increasing intraluminal pressure in prostate arteries (Control; n = 5) and prostatic tumor arteries from sedentary (TB-Sedentary; n = 6) and exercise-trained (TB-Ex Trained; n = 6) Copenhagen rats. C: resting myogenic tone across the physiological range of pressures to which arteries are subjected. All values are normalized to diameter at 90 cmH2O, and myogenic tone is expressed as a percent change in diameter relative to passive myogenic tone. *P ≤ 0.05 Control vs. TB-Ex Trained. ¥P = 0.08 Control vs. TB-Ex trained. #P = 0.10 Control vs. TB-Ex Trained.
DISCUSSION
The purpose of this present study was to determine the effects of chronic endurance training on orthotopic prostate tumor oxygenation. To our knowledge, this is the first study to demonstrate directly that exercise training increases tumor microvascular oxygenation and significantly reduces tumor hypoxia. On the basis of these changes in tumor oxygenation, we investigated two potential contributing mechanisms, including the number of perfused vessels in the tumor in the conscious resting condition, and vasoconstrictor responsiveness of isolated tumor resistance arteries. Contrary to our hypothesis, improvements in tumor oxygenation occurred without any observable change in patent vessel density, although intravascular hemodynamics (e.g., red blood cell flux through the vessel) could not be determined and may contribute to the enhanced oxygenation. Furthermore, there were no changes in the contractile responsiveness of the tumor vasculature to the constrictor stimuli tested in this study. Despite the increase in oxygenation, our results do not suggest any substantial effect of exercise on tumor growth in the orthotopic model, as only one group had a small increase in tumor size after training, whereas the group that was trained 2 wk longer had no difference in tumor weight (Table 1). Indeed, several studies have investigated the effects of different types of exercise on tumor progression with conflicting results (12, 23, 65), likely because of differences in animals, exercise modality, and/or implantation site. Whether the change in tumor oxygenation observed herein influenced the phenotype of the tumor (e.g., aggressiveness) remains to be determined. However, findings from several studies support a direct link between metastatic disease and hypoxia (for review, see Ref. 20) and, considering the large reduction in tumor hypoxia (Figs. 1B and 2C), a shift to a less aggressive phenotype is likely after training. Collectively, these data are important to elucidate potential alternative mechanisms to combat tumor hypoxia (e.g., with aerobic exercise training).
Tumor Hypoxia and Oxygenation
There is substantial evidence for the prevalence of hypoxic regions within the solid tumor due to limitations in perfusion (acute) and diffusion (chronic) capacity associated with the chaotic vasculature (57). The maximum diffusion distance for oxygen into the tissue from a capillary bed is limited to ∼100–200 μm, according to the model developed originally for skeletal muscle by August Krogh in 1922 (28), and later applied to tumors by Thomlinson and Gray (52). Their observations were that the distance between perfused vessels and the perimeter of necrotic tissue was at a relatively constant distance of 100–150 μm, suggesting necrosis is due to a lack of diffusive oxygen (52). Furthermore, the tumor microenvironment readily promotes hypoxia, which is of particular prevalence in advanced disease and is indicative of a more clinically aggressive tumor phenotype (34) defined by genetic instability (15), increased metastatic potential (3), and poor patient prognosis (19). Therefore, approaches designed to alleviate tumor hypoxia are of high clinical benefit.
In the current study, the average microvascular Po2 in the tumors of sedentary animals averaged ∼6 mmHg (Fig. 1B), which would mandate a lower intratumoral Po2 for a diffusion gradient of O2 to exist and would lead to severe hypoxia as demonstrated in Fig. 2A. Further, the large oscillations in temporal profile of microvascular Po2 and low values achieved (∼3 mmHg; for example, see Fig. 1A) in the tumor of the sedentary animals would likely create conditions of intermittent hypoxia, which would enhance reactive oxygen species generation (10) that is associated with tumorigenesis (8). However, after exercise training, there was an increase in the average tumor microvascular Po2 (Fig. 1B), as well as a stabilization of the temporal profile of microvascular Po2 (Fig. 1A), suggesting an improved ability to temporally regulate O2 delivery-to-demand after training. Furthermore, there was a substantial attenuation in tumor hypoxia after exercise training (Fig. 2C), as determined under conscious, resting conditions. Interestingly, this reduction in tumor hypoxia occurred in the absence of any increase in patent vessels after training (that would suggest a more homogeneous perfusion), which is opposite of what is observed in the mouse model (23), albeit different training stimuli were used. This was surprising as we hypothesized training would increase the perfused vessels and this would, in turn, contribute to an enhanced microvascular Po2. The reasons for this discrepancy (i.e., increased oxygenation despite no difference in functional vessels) is unknown, but potential mechanisms may include 1) a greater blood flow through functional vessels, 2) an increase in vessel number in hypoxic regions that may be masked in the analysis of whole tissue number, or 3) an increase in capillary tube hematocrit (i.e., red blood cell volume fraction) after exercise training. Importantly, microcirculatory hemodynamics work in concert with capillary Po2 to facilitate O2 flux. Whereas the capillary-to-tissue Po2 gradient largely determines O2 flux, the diffusion characteristics of the tissue (DO2) also facilitate O2 transfer (62). These diffusion characteristics are determined, in part, by the number of red blood cells adjacent to the surrounding tissue [i.e., primarily determined by capillary tube hematocrit (26)] at a given time (13, 16). Although it is apparent that there are patent vessels near the hypoxic regions in the tumor in the Sedentary group (Fig. 2A), the intravascular hemodynamics (e.g., red blood cell flux, velocity, etc.) cannot be determined by the Hoechst staining procedure. Therefore, an increase in capillary tube hematocrit in a tumor vessel after exercise training would facilitate O2 transfer and may contribute to the reduced tumor hypoxia, although intravital microscopic methods are needed to determine such hemodynamic measures.
Tumor Arterial Contractile Responses
On the basis of an earlier finding that exercise can enhance vasoconstrictor properties of resistance vasculature in tissue that is not recruited (i.e., normally does not increase blood flow during exercise) (31), we hypothesized that exercise training would enhance the contractile responses in the tumor resistance vasculature. Contrarily, we did not observe any improvements in adrenergic or myogenic responsiveness of tumor resistance arteries following endurance training (Figs. 3 and 4). This may also be due to the relative lack of smooth muscle (for review, see Ref. 58) and innervation (1) of the tumor vasculature. However, it should be noted that only α-adrenergic and myogenic contractile responsiveness was investigated in this study, and the lack of change with these constrictors may not be generalized to other constrictors. Given tumor vessels display a diminished ability to produce endothelium-derived relaxing factor (54), it is possible that exercise affected vasodilator capacity in the tumor arteries. For example, systemic resistance artery vasodilation (38), including endothelial-function and nitric oxide production (53), is improved after exercise training. Whether exercise training has any effect on vasodilator capacity or vascular integrity in the tumor vasculature remains to be determined, but these factors are also largely effected by vascular shear stress (43), which would be expected to increase with the elevation in tumor blood flow during exercise (32).
Limitations
In the current study two different strains of rats were used, i.e., Copenhagen and Nude rats, due to the relative unavailability of the former to complete the studies. However, complete data sets were collected in each group of rats for a given experiment (i.e., no combined data from different rat strains). Furthermore, the results in the Copenhagen rats (i.e., enhanced tumor microvascular Po2) after exercise training are similar to that observed in the Nude rats after exercise training (i.e., reduced tumor hypoxia), suggesting a preserved effect of training on tumor oxygenation in both strains. Indeed, studies have used the Copenhagen and Nude rat concurrently as comparable strains to produce locally advanced orthotopic prostate cancer models (e.g., Ref. 56).
The tumor sizes were also significantly greater in the Copenhagen vs. Nude rats possibly due, in part, to the additional 2 wk of training the Copenhagen rats received. Specifically, the Copenhagen rats were exercise-trained for 7 wk to ensure the animals demonstrated an “exercise phenotype” (e.g., increased skeletal muscle oxidative capacity), and the tumor mass from those rats were larger than expected, although no effect on urination or mobility was evident. Despite the tumors in the Copenhagen rats being large, the Po2 values observed (Fig. 1B) were identical to the Po2 values of prostate tumors in a variety of models (∼6 mmHg; Ref. 59). To avoid any potential institutional or physical constraints on tumor size and ensure the tumor sizes were biologically relevant, the exercise training period was reduced to 5 wk in the Nude rats. Despite the shorter exercise training period, there was a significant increase in soleus muscle oxidative capacity, required to confirm the efficacy of the training protocol, in the exercise-trained rats (Table 1).
Given the Nude rats were larger and the tumors smaller at the time of death vs. the Copenhagen rats, there exists the possibility that local hemodynamics were altered between strains due to the differences in tumor load. However, the outcomes (with respect to oxygenation) were remarkably similar between strains after exercise training. Furthermore, the oxidative capacity of the soleus muscle between strains and within a condition (i.e., sedentary or trained) were almost identical as well, suggesting a similar metabolic response to exercise training between strains.
Conclusions
To our knowledge, this is the first study to demonstrate a reduction in hypoxia in a solid orthotopic tumor in a preclinical prostate cancer model after aerobic exercise training. The large increase in microvascular Po2 would increase the driving force for diffusion of O2 into the tumor and provides a putative mechanism for the enhanced tumor oxygenation. In contrast to our hypothesis, there were no significant changes in patent vessels in the tumor, albeit hemodynamic profiles (e.g., red blood cell velocity or flux) within the vessels could not be measured, which could affect transcapillary O2 flux. Furthermore, in contrast to previous findings of enhanced arterial contractile function in nonrecruited tissue after training (9), there were no changes in tumor contractile function after exercise training to the constrictors tested in this study. The reason for this discrepancy is unknown but may be related to the lack of innervation in tumor vessels (1). Clinically, these results suggest exercise-training strategies offer hope to improve tumor oxygenation and potentially mitigate the aggressive tumor phenotypes associated with hypoxic microenvironments (20), although future studies are needed to determine how exercise training may affect this phenotype (e.g., apoptosis of tumor cells). Given the responses to exercise training are complex and are influenced by age and health status, the results from the orthotopic prostate model may not be generalized to other solid or nonsolid tumors, which warrants further investigations.
GRANTS
Support for this study was provided, in part, by National Institute on Aging Grants AG-31317 (to B. J. Behnke), Florida Biomedical Research Program 1BN-02 (to B. J. Behnke), and the Grinter Fellowship (to D. J. McCullough) from the Department of Applied Physiology and Kinesiology at the University of Florida.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.J.M., D.W.S., and B.J.B. conception and design of research; D.J.M., L.M.-D.N., and B.J.B. performed experiments; D.J.M. and B.J.B. analyzed data; D.J.M., D.W.S., and B.J.B. interpreted results of experiments; D.J.M. and B.J.B. prepared figures; D.J.M., D.W.S., and B.J.B. drafted manuscript; D.J.M., L.M.-D.N., D.W.S., and B.J.B. edited and revised manuscript; D.J.M., L.M.-D.N., D.W.S., and B.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Peter Adhihetty for the use of his cell culture laboratory.
REFERENCES
- 1.Ashraf S, Loizidou M, Crowe R, Turmaine M, Taylor I, Burnstock G. Blood vessels in liver metastases from both sarcoma and carcinoma lack perivascular innervation and smooth muscle cells. Clin Exp Metastasis 15: 484–498, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients—a systematic review of randomized controlled trials. Support Care Cancer 20: 221–233, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature 423: 593–595, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Carnell DM, Smith RE, Daley FM, Saunders MI, Bentzen SM, Hoskin PJ. An immunohistochemical assessment of hypoxia in prostate carcinoma using pimonidazole: implications for radioresistance. Int J Radiat Oncol Biol Phys 65: 91–99, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chan RC, Babbs CF, Vetter RJ, Lamar CH. Abnormal response of tumor vasculature to vasoactive drugs. J Natl Cancer Inst 72: 145–150, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Cohen LA, Boylan E, Epstein M, Zang E. Voluntary exercise and experimental mammary cancer. Adv Exp Med Biol 322: 41–59, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol 14: 259–266, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Davis RT, 3rd, Stabley JN, Dominguez JM, 2nd, Ramsey MW, McCullough DJ, Lesniewski LA, Delp MD, Behnke BJ. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. J Appl Physiol 114: 808–815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol Cell Physiol 264: C961–C967, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Dunning WF. Prostate cancer in the rat. Natl Cancer Inst Monogr 12: 351–369, 1963 [PubMed] [Google Scholar]
- 12.Esser KA, Harpole CE, Prins GS, Diamond AM. Physical activity reduces prostate carcinogenesis in a transgenic model. Prostate 69: 1372–1377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res 32: 164–189, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacosa A, Franceschi S, La Vecchia C, Favero A, Andreatta R. Energy intake, overweight, physical exercise and colorectal cancer risk. Eur J Cancer Prev 8 Suppl 1: S53–S60, 1999 [PubMed] [Google Scholar]
- 15.Goliasova T, Denko NC. Hypoxia-inducible factor 1 (HIF1) mediated adaptive responses in the solid tumor. In: Tumor Microenvironment, edited by Siemann DW. New York: John Wiley & Sons, 2011, p. 271–290 [Google Scholar]
- 16.Groebe K, Thews G. Calculated intra- and extracellular Po2 gradients in heavily working red muscle. Am J Physiol Heart Circ Physiol 259: H84–H92, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hansen S, Grabau DA, Sorensen FB, Bak M, Vach W, Rose C. The prognostic value of angiogenesis by Chalkley counting in a confirmatory study design on 836 breast cancer patients. Clin Cancer Res 6: 139–146, 2000 [PubMed] [Google Scholar]
- 19.Helczynska K, Larsson AM, Holmquist Mengelbier L, Bridges E, Fredlund E, Borgquist S, Landberg G, Pahlman S, Jirstrom K. Hypoxia-inducible factor-2α correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res 68: 9212–9220, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hill RP, Chaudary N. Infleunce of hypoxia on metastatic spread. In: Tumor Microenvironment, edited by Siemann DW. New York: John Wiley & Sons, 2011, p. 311–328 [Google Scholar]
- 21.Isaacs JT, Heston WD, Weissman RM, Coffey DS. Animal models of the hormone-sensitive and -insensitive prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and R-3327-AT. Cancer Res 38: 4353–4359, 1978 [PubMed] [Google Scholar]
- 22.Johannes T, Mik EG, Ince C. Dual-wavelength phosphorimetry for determination of cortical and subcortical microvascular oxygenation in rat kidney. J Appl Physiol 100: 1301–1310, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JR, Nagendran J, Flores CT, Betof AS, Nelson ER, Pollak M, Dash RC, Young ME, Freedland SJ. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol 113: 263–272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyner WL, Davis MJ. Pressure profile along the microvascular network and its control. Fed Proc 46: 266–269, 1987 [PubMed] [Google Scholar]
- 25.Kiessling F, Huber PE, Grobholz R, Heilmann M, Meding J, Lichy MP, Fink C, Krix M, Peschke P, Schlemmer HP. Dynamic magnetic resonance tomography and proton magnetic resonance spectroscopy of prostate cancers in rats treated by radiotherapy. Invest Radiol 39: 34–44, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol 237: H481–H490, 1979 [DOI] [PubMed] [Google Scholar]
- 27.Koch CJ, Hahn SM, Rockwell K, Jr, Covey JM, McKenna WG, Evans SM. Pharmacokinetics of EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol 48: 177–187, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Krogh A. The Anatomy and Physiology of Capillaries. New Haven, CT: Yale University Press, 1922 [Google Scholar]
- 29.Lash JM. Exercise training enhances adrenergic constriction and dilation in the rat spinotrapezius muscle. J Appl Physiol 85: 168–174, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lo LW, Vinogradov SA, Koch CJ, Wilson DF. A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol 428: 651–656, 1997 [DOI] [PubMed] [Google Scholar]
- 31.McCullough DJ, Davis RT, 3rd, Dominguez JM, 2nd, Stabley JN, Bruells CS, Behnke BJ. Effects of aging and exercise training on spinotrapezius muscle microvascular Po2 dynamics and vasomotor control. J Appl Physiol 110: 695–704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Acute exercise increases prostate tumor blood flow in rats (Abstract). FASEB J 1138.1136 2012 [Google Scholar]
- 33.Meininger GA, Harris PD, Joshua IG. Distributions of microvascular pressure in skeletal muscle of one-kidney, one clip, two-kidney, one clip, and deoxycorticosterone-salt hypertensive rats. Hypertension 6: 27–34, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging 8: 81–86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milosevic M, Chung P, Parker C, Bristow R, Toi A, Panzarella T, Warde P, Catton C, Menard C, Bayley A, Gospodarowicz M, Hill R. Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Res 67: 6022–6025, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Milosevic M, Warde P, Menard C, Chung P, Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M, Catton C, Hill RP, Bristow R. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res 18: 2108–2114, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol 63: 2269–2277, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Musch TI, Poole DC. Blood flow response to treadmill running in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 271: H2730–H2734, 1996 [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute Prostate cancer Home Page National Institutes of Health. http://www.cancer.gov/cancertopics/types/prostate [Google Scholar]
- 43.Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol 28: 1652–1660, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Parker NM, Siemann DW. The microenvironment in Cancer. In: Tumor Microenvironment. New York: John Wiley & Sons, 2011, p. 1–6 [Google Scholar]
- 45.Pitson G, Fyles A, Milosevic M, Wylie J, Pintilie M, Hill R. Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. Int J Radiat Oncol Biol Phys 51: 699–703, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular Po2 assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol 91: 2679–2685, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241: 1649–1651, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res 12: 4090–4094, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Siemann DW, Rojiani AM. The vascular disrupting agent ZD6126 shows increased antitumor efficacy and enhanced radiation response in large, advanced tumors. Int J Radiat Oncol Biol Phys 62: 846–853, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Smith KA, Hill SA, Begg AC, Denekamp J. Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer 57: 247–253, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srere P. Citrate synthase. In: Methods in Enzymology. 1969, p. 1–11 [Google Scholar]
- 52.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 9: 539–549, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Tozer GM, Prise VE, Bell KM, Dennis MF, Stratford MR, Chaplin DJ. Reduced capacity of tumour blood vessels to produce endothelium-derived relaxing factor: significance for blood flow modification. Br J Cancer 74: 1955–1960, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trotter MJ, Chaplin DJ, Durand RE, Olive PL. The use of fluorescent probes to identify regions of transient perfusion in murine tumors. Int J Radiat Oncol Biol Phys 16: 931–934, 1989 [DOI] [PubMed] [Google Scholar]
- 56.Tumati V, Mathur S, Song K, Hsieh JT, Zhao D, Takahashi M, Dobin T, Gandee L, Solberg TD, Habib AA, Saha D. Development of a locally advanced orthotopic prostate tumor model in rats for assessment of combined modality therapy. Int J Oncol 42: 1613–1619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr 152: 334–342, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49: 6449–6465, 1989 [PubMed] [Google Scholar]
- 59.Vaupel P, Kelleher DK. Blood flow and oxygenation status of prostate cancers. Adv Exp Med Biol 765: 299–305, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Vinogradov SA, Fernandez-Searra MA, Dugan BW, Wilson DF. Frequency domanin instrument for measuring phosphorescence lifetime distributions in heterogenous samples. Rev Sci Instrum 72: 3396–3406, 2001 [Google Scholar]
- 61.Vinogradov SA, Wilson DF. Phosphorescence lifetime analysis with a quadratic programming algorithm for determining quencher distributions in heterogeneous systems. Biophys J 67: 2048–2059, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner PD. Muscle O2 transport and O2-dependent control of metabolism. Med Sci Sports Exerc 27: 47–53, 1995 [PubMed] [Google Scholar]
- 63.Wilson DF, Lee WM, Makonnen S, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space of skeletal muscle and tumors in vivo. Adv Exp Med Biol 614: 53–62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med 47: 989–998, 2006 [PubMed] [Google Scholar]
- 65.Zheng X, Cui XX, Huang MT, Liu Y, Shih WJ, Lin Y, Lu YP, Wagner GC, Conney AH. Inhibitory effect of voluntary running wheel exercise on the growth of human pancreatic Panc-1 and prostate PC-3 xenograft tumors in immunodeficient mice. Oncol Rep 19: 1583–1588, 2008 [PMC free article] [PubMed] [Google Scholar]