Abstract
Pulmonary muscle weakness is common in ambulatory people with multiple sclerosis (MS) and may lead to deficits in mobility function. The purpose of this study was to examine the effect of a 10-week home-based exercise program using an inspiratory muscle threshold trainer (IMT) on the results of four lower-extremity physical performance tests in people with MS. The study design was a two-group (experimental-control), pretest-posttest study. Outcome measures consisted of pulmonary function measures including maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and maximal voluntary ventilation (MVV), and the following lower-extremity physical performance measures: the 6-Minute Walk (6MW) distance, gait velocity (GV), the Sit-to-Stand Test (SST), the Functional Stair Test (FST), and a balance test (BAL). A total of 46 ambulatory participants (Expanded Disability Status Scale [EDSS] score, 2.0–6.5) with MS were randomly assigned to an intervention group (mean EDSS score, 4.1) that received 10 weeks of home-based inspiratory muscle training or a nontreatment control group (mean EDSS score, 3.2). Of the original 46 participants, 20 intervention group participants and 19 control group participants completed the study. Compared with the control group, the intervention group made significantly greater gains in inspiratory muscle strength (P = .003) and timed balance scores (P = .008). A nonsignificant improvement in 6MW distance (P = .086) was also noted in the IMT-trained group as compared with the control group. This is the first study directly linking improvement in respiratory function to improvement in physical performance function in people with mild-to-moderate disability due to MS.
Multiple sclerosis (MS) is a central nervous system disorder that often affects motor pathways, with delayed conduction time resulting in reduced muscle strength and endurance throughout the body, including the muscles involved in ventilation.1–7 Specifically, Garland et al.8 found central motor conduction to the diaphragm muscle to be abnormal in 12 of 15 individuals with mild-to-moderate MS-related disability (Expanded Disability Status Scale [EDSS] score ≤6.5). Respiratory muscle weakness and fatigue result in impaired ventilation. Impaired ventilation is recognized as a major cause of morbidity and mortality in individuals with advanced MS (EDSS score ≥6.5).1–7,9–14 The National Multiple Sclerosis Society (NMSS) added respiration/breathing problems to its list of less common symptoms of MS in 200815 and issued a clinical bulletin on pulmonary function and rehabilitation in MS in 2007,16 reflecting the recent recognition of respiratory impairment as a symptom of MS.
Recent studies have demonstrated that pulmonary muscle weakness is present not only in people with advanced MS, but also in ambulatory individuals with MS who have minimal disability.7,9,10 Resistive inspiratory9,17 and expiratory1,10,11 muscle training conducted in people with MS who have mild to severe disability increases the strength of muscles involved in inspiration and forced expiration. While these studies reported increased pulmonary muscle function (strength and endurance) following resistive pulmonary muscle training, very limited data have been published on physical function following this type of training.
Gosselink et al.1 conducted expiratory muscle training for 3 months in individuals with MS and an EDSS score of 6.5 or more, which resulted in increased cough efficiency as measured by the pulmonary index. The pulmonary index is an index of clinical signs, including maximum number achieved in verbally counting on a single exhalation, ratings of cough weakness, and difficulty clearing secretions.18 No other physical function data were provided. Klefbeck and Nedjad17 used inspiratory muscle training for 10 weeks in people with advanced MS (EDSS score >6.5) and found no improvement in fatigue as measured by the Fatigue Severity Scale (FSS) or in subjective perception of physical endurance. The Klefbeck and Nedjad17 study had only seven participants in the training group, however, and no power analysis was presented, which raises questions about their nonsignificant results. Chiara et al.19 conducted an 8-week expiratory muscle training study involving 17 individuals with MS (EDSS score <6.5) and found no change in sustained vowel prolongation or words per minute measured from connected speech, or voice-related quality of life. Again, no power analysis of the nonsignificant results was reported. None of these studies directly measured lower-extremity physical function to identify changes secondary to resistive pulmonary muscle training.
Impaired respiratory muscle activation may lead to a reduction in the core stability that is necessary for normal balance and mobility. The link between respiratory function and trunk muscle strength is demonstrated in the normal population by the coordinated timing of trunk muscle and respiratory muscle activation during limb movement.20–22 Also, in normal individuals the diaphragm receives feed-forward input prior to some limb movement.21 The link between respiratory function and mobility function in patient populations has not been substantially researched. Individuals with chronic obstructive pulmonary disease (COPD) exhibited reduced mediolateral stability compared with normal control participants, and mediolateral stability in the COPD group was further reduced following an upper-extremity exercise.23 Garland et al.8 reported central motor conduction delays to the diaphragm in mildly to moderately disabled individuals with MS. No research is available, however, directly linking respiratory function in MS and mobility measures of lower-extremity physical performance, such as balance, stair-climbing, sit-to-stand activities, and ambulation.
Reliable and valid measures of lower-extremity mobility that are responsive to change with intervention are necessary to study the effects of respiratory muscle training on lower-extremity mobility. Examination of key factors in maintaining lower-extremity mobility and avoiding disability in other populations was useful to guide selection of tasks to include in this study. Several large studies of community-dwelling older adults have demonstrated that physical performance on selected tasks is important for maintaining functional walking distance (at least a quarter-mile). In the prospective Women's Health and Aging Study (WHAS) of women aged 65 years and older, balance and knee extension strength were independent predictors of severe walking disability.24,25 In patients with hemiparesis secondary to cerebrovascular accident, time to ascend four stairs was highly correlated with knee extensor strength, indicating that stair-climbing may be a reasonable functional test of knee extensor strength.26 In the Established Populations for the Epidemiologic Study of the Elderly (EPESE), gait speed alone or combined with a repeated sit-to-stand test and balance test was highly predictive of mobility disability and hospitalization rates.27–29 In the Third National Health and Nutrition Examination Survey (NHANES III) of individuals aged 60 years and older, gait speed, repeated sit-to-stand ability, and peak expiratory flow were identified as factors predictive of mobility-related limitations.30
This study examined the effect of a 10-week home-based exercise program using an inspiratory muscle threshold trainer (IMT) on four lower-extremity physical performance tests in people with MS. Our primary hypothesis was that the intervention group would increase their physical performance test scores significantly more than the control group following the inspiratory muscle training.
Methods
Research Design
The study design was a two-group (experimental-control), single-blinded randomized pretest-posttest study.
Participants
A total of 46 adult ambulatory (with or without an assistive device) individuals with clinically diagnosed MS were recruited through local MS support group meetings and a television news interview. Individuals with acute respiratory infection diagnosed by a physician or oral temperature greater than 100°F, unstable cardio-pulmonary or musculoskeletal conditions unrelated to MS affecting performance, and/or a history of smoking in the past year were excluded from participation in the study.
Participants were randomly placed into a home exercise inspiratory muscle training intervention group or a nonintervention control group by date of enrollment in the study. The study was approved by the institutional review board of the University of Michigan–Flint, and informed consent was obtained from all participants prior to enrollment in the study.
MS-Related Impairment/Disability
Two tests were included in this study to classify level of disability and overall response to the exercise program in terms of fatigue. The EDSS, a scale based on ambulatory status and a standard neurologic examination for people with MS, was included to assess overall disability level and functional mobility. The EDSS was specifically included in this study because of the heavy weighting of ambulatory ability in calculating EDSS scores. Higher scores indicate greater disability.31 As part of the neurologic examination for the EDSS, strength was measured using manual muscle tests to determine normal versus diminished strength, and muscle tone was measured using the scale in the Neurologic Assessment of the Kurtzke Functional Systems.31 Lower-extremity strength was used as an indirect measure of trunk strength for the purposes of this study, as trunk strength was not measured directly. The FSS, a nine-item questionnaire about how fatigue affects a person's life, was included as an overall indication of whether fatigue levels changed as a result of the exercise intervention. Higher values on the scale indicate greater fatigue.32
Pulmonary Function Tests
Pulmonary function tests (PFTs) including maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and maximal voluntary ventilation (MVV), as well as standard spirometry tests, were measured with the VMax metabolic cart and test protocols from Sensor Medics Corporation (Yorba Linda, CA). Pulmonary function tests were performed by the same investigator for pretests and posttests. The investigator completed pilot tests to verify the accuracy of data acquisition during pulmonary tests. The metabolic cart was calibrated prior to each testing session according to the American Thoracic Society and European Respiratory Society Statement on Respiratory Muscle Testing.33 The VMax pulmonary test software has routine measures of consistency and accuracy embedded in the test protocol. The investigator was blinded as to group placement of participants. All tests were performed in the seated position with both feet flat on the floor. A rubber mouthpiece and nose clips were provided to each participant to be used throughout testing. To ensure reproducibility, each test was performed a minimum of two times and until values were within 10% of each other with the best trial accepted. Verbal instructions were provided, followed by a videotaped demonstration of each test procedure. Full protocols are described in Fry et al.9
Mobility Outcome Measures
The mobility tests conducted in this research meet the criteria for outcome measures established by the NMSS and were selected to reflect physical tasks people perform repeatedly during any given day.34 Balance (BAL) was tested because it is a fundamental skill necessary to support the Functional Stair Test (FST), Sit-to-Stand Test (SST), and 6-Minute Walk (6MW) test, as well as many other mobility activities.24,35–37 The FST and SST were included as functional tests of power and strength of the lower extremities.26,38–40 The 6MW test was included as a functional measure of endurance.41–44
Balance testing involved three timed trials with the participant standing on his or her self-defined “best leg.” If the person was unable to maintain single-limb stance for more than 3 seconds, then tandem balance was tested with the “best leg” in back. Trials were timed for up to 30 seconds. In ambulatory individuals with MS, the test-retest reliability intraclass correlation coefficient (ICC) for the single-limb stance test is 0.95 and for the tandem stance test is 0.63.45 To allow analysis of the single-limb and tandem stance data in one variable, the BAL score was determined using an ordinal scale for balance (Table 1).
Table 1.
Ordinal scale for balance
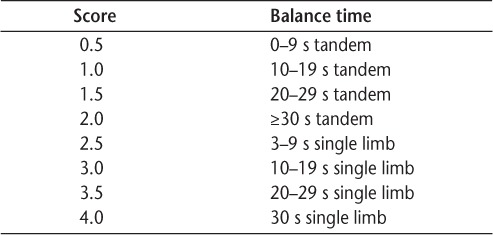
In the FST, participants completed three timed trials in which they were asked to ascend four steps, turn, and descend four steps, using a handrail as necessary. In ambulatory individuals with MS, the test-retest reliability ICC for the FST is 0.97.45
In the SST, participants were instructed to rise from a chair to a full standing position and return to sitting six times in three separate timed trials. In ambulatory individuals with MS, the test-retest reliability ICC for the SST is 0.94.45
For the 6MW test, participants were instructed to walk as quickly and safely as possible for 6 minutes. They were allowed to use an assistive device and to rest when needed. In ambulatory individuals with MS, the test-retest reliability ICC for the 6MW test is 0.96.45 The gait velocity (GV; meters/second) was calculated from the 6MW test distance (meters).
The 15-point (6–20) original category rating of perceived exertion (RPE) Borg scale was used to subjectively rate perceived exertion following each trial for each of the four mobility tests as an indication of impending fatigue.46,47 A full description of the testing procedures for each mobility test is reported in Fry and Pfalzer.45
Pulmonary Home Exercise Intervention
Experimental participants completed a 10-week exercise trial using a Threshold Inspiratory Muscle Trainer (IMT) device (Respironics Health Scan, Inc, Cedar Grove, NJ). The home IMT resistive exercise intervention consisted of three sets of 15 repetitions based on the previously published protocols of Klefbeck and Nedjad17 and Smeltzer et al.11 Exercise was performed for approximately 10 to 15 minutes daily for 10 weeks. Exercise adherence was recorded by the participants in their exercise logs. During the training and/or control period, participants were asked to maintain their normal level of physical activity. No further exercise program or physical therapy was provided. A full description of the intervention protocol is provided in Fry et al.9
Study Procedures
Participants completed a Health Intake Questionnaire that included demographic information, general health, and MS-related information to determine whether they had any of the exclusion criteria for the study. Depression and fall history within the past year were also noted. Investigators reviewed the participants' health information and participants answered all questions prior to testing. Resting vital signs including blood pressure, oxygen saturation, heart rate, respiratory rate, and oral temperature were assessed prior to activity using the guidelines of the American College of Sports Medicine to ensure participant safety.46 Exercise participation was determined to be safe for all individuals enrolled.
Each participant's height (inches) and weight (pounds) were determined using a standard medical scale. Body-mass index (BMI) was calculated by converting inches to meters and pounds to kilograms, and dividing the weight by the square of the height (kg/m2). Participants with a BMI of less than 20 were classified as underweight, and those with BMI scores above 30 were classified as obese. The FSS32 and Kurtzke functional systems test31 were administered and the EDSS level established at the pretest session. All PFT and spirometry measures were taken on day 1 of testing. The mobility tests were administered on day 2 of testing in the following order for each participant: BAL, FST, SST, 6MW. Participants were provided rest periods of 1 to 5 minutes between tests and between trials as needed. Safety precautions during the physical performance tests included gait belts worn by all participants for all tests, guarding (as needed), and manual assistance if a fall was imminent. One participant fell on the 6MW. The participant was assessed according to university protocol and determined to be uninjured. The 6MW for this participant was repeated after a rest period. The RPE was solicited from participants at the completion of each trial of each test. The IMT training commenced following testing on day 2. All participants were tested on PFT, spirometry, and mobility measures after 10 weeks of training or the nontreatment control period.
Statistical Analysis
Data analysis was performed using SPSS, version 17.0 (SPSS, Chicago, IL). Descriptive statistics were calculated for all participant characteristics, PFTs, spirometry, and mobility tests. Black and Hyatt's48 equations were used for calculating percent predicted values of MIP and MEP. Based on recommendations of Fry and Pfalzer,45 best trial rather than mean data were used for analysis of the balance tests. Baseline data were compared between groups with a one-way analysis of variance (ANOVA) for interval scale outcome measures and with a nonparametric Mann-Whitney test for the EDSS scores (P < .05) to determine the effectiveness of the participant randomization process. Prior to conducting the ANOVA, the assumptions for the ANOVA were tested and met, as all variables had homogeneity of variance and demonstrated a normal distribution (P < .05).
To determine the effect of the 10-week IMT home exercise intervention on individual lower-extremity physical performance measures (SST, FST, 6MW, BAL) by group, we conducted a two-way, repeated-measures ANOVA (type IV full factorial model with simple contrast with α level of ≤.05) of percent of predicted MIP and lower-extremity physical performance measures by group (intervention vs. control).49 To determine the relationship between the EDSS and the various physical performance measures, Pearson product moment correlation (FST, SST, 6MW) and Spearman rank correlation (BAL) coefficients were calculated.
Results
Of the original 46 participants, 2 dropped out because of medical illness unrelated to their MS, and 3 dropped out because they did not wish to continue the study. Data from one participant were removed because the participant was an extreme outlier on her physical performance measures, although the PFT results were within the same range as those of other participants. Data from another participant were removed because the participant declined to complete all PFTs. Thus, 20 participants remained in the intervention group (dropout rate, 13%) and 19 participants remained in the control group (dropout rate, 17%). Adherence to the IMT training protocol ranged from 76.25% to 83.50%, with an average adherence across participants of 81% for the duration of the study.
Participant Characteristics
Of the 39 participants whose data were analyzed, 31 were female (18 in the intervention group and 13 in the control group) and 8 were male (2 in the intervention group and 6 in the control group). No statistically significant differences were found between the two groups at baseline on participant characteristics and indicators of MS impairment and disability (eg, age, BMI, EDSS score, total FSS score, number of comorbidities) (Tables 2 and 3), and pulmonary function measures, indicating adequate randomization of participants into groups, with the exception of MIP (F = 5.753, P = .021) and forced expiratory flow at 25% to 75% (FEF25%–75%) (F = 9.650, P = .003), with the intervention group showing significantly lower mean values than the control group (Table 4).
Table 2.
Participant characteristics
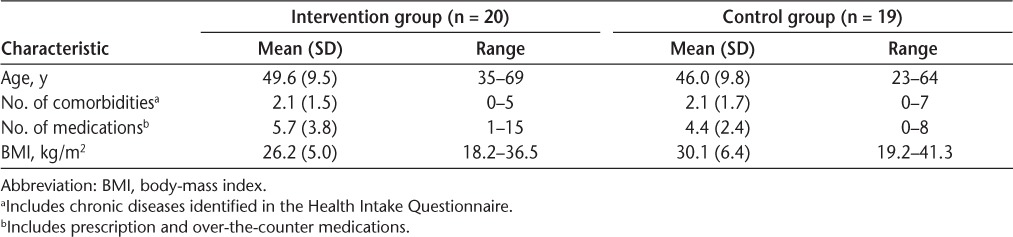
Table 3.
Baseline indicators of MS impairment and disability
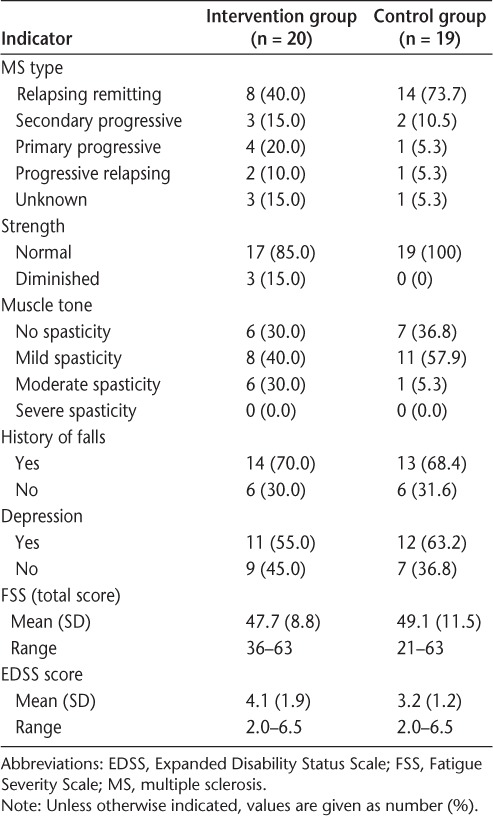
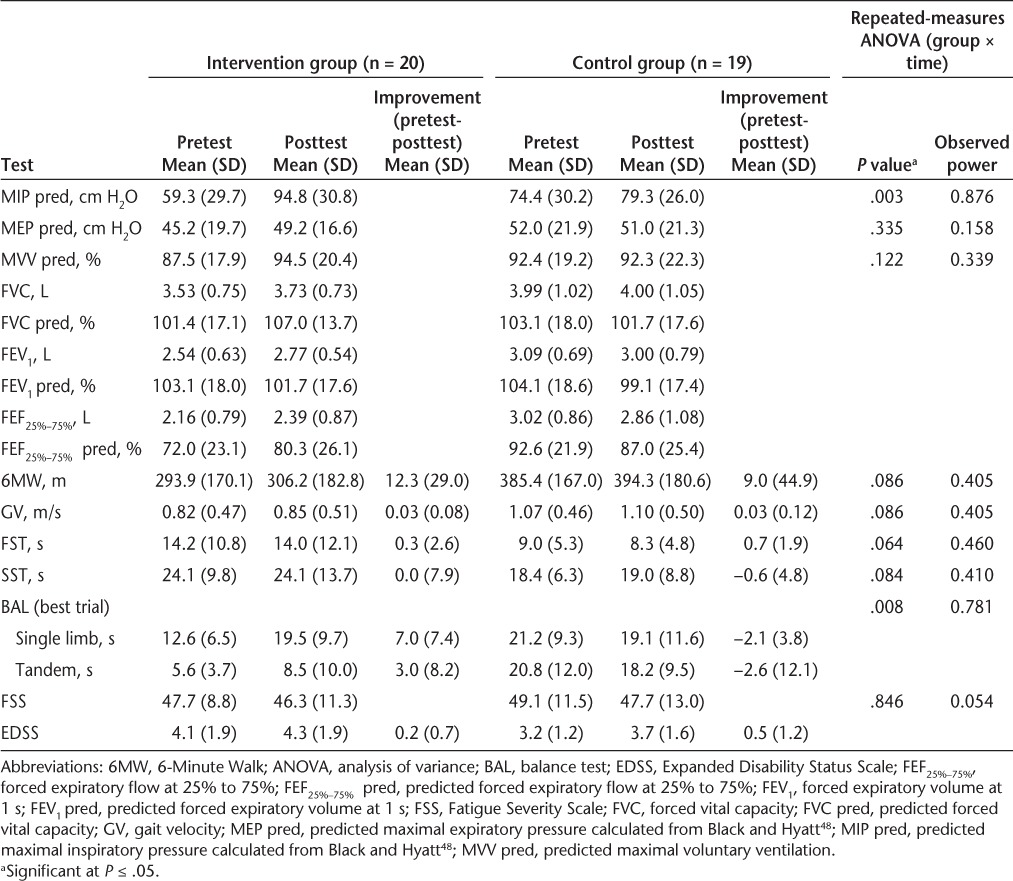
Table 4.
Descriptive statistics and two-way repeated-measures ANOVA of physical performance test measures and pulmonary strength and endurance
MS-Related Impairment/Disability
Participants' EDSS scores ranged from 2.0 to 6.5, indicating mild-to-moderate disease severity and disability, with all participants still ambulatory (Table 3). Strength was within normal limits in all but three intervention participants and in all control participants. Muscle tone varied from normal to moderately increased in both groups, with no one exhibiting severe spasticity. Fatigue levels measured with the FSS were comparable in the two groups, with participants in each group achieving the top score of 63, indicating a possible ceiling effect for this test. Depression and history of falls were roughly equivalent in the two groups, with a history of depression reported in 55% to 63% of the participants and a history of falls in the past year reported in 68% to 70% of participants (Table 3).
Pulmonary Function Tests
Maximal inspiratory pressure increased by 71.4% in the intervention group following training, compared with a 6.4% decrease in the control group (P < .003). Maximal expiratory pressure (21.0% gain for the intervention group and 4.9% gain for the control group, P = .335) and MVV (9.0% gain for the intervention group and 0.2% decrease for the control group, P = .122) improved following training, but not significantly. Additional pulmonary data are reported in Table 4. Comparison using a two-way (group × time) repeated-measures ANOVA revealed that the intervention group exhibited significantly greater improvement (P = .003; observed power, 0.876) in percent predicted MIP than the control group (Table 4). Percent predicted MEP and MVV were not significantly different between groups at P ≤ .05. Nonspecific or generalized improvements in expiratory pulmonary function (forced expiratory volume at 1 second [FEV1], forced vital capacity [FVC], and FEF25%–75%) were observed for the intervention group, while the control group exhibited either no change or diminished pulmonary function values.9
Mobility Outcomes
Descriptive results of the physical performance measures are shown in Tables 4 and 5. Performance on the physical performance tests is presented graphically in Figure 1, with data for participants with mild and with moderate disability shown separately within each group.
Table 5.
Pretest and posttest ordinal scale results of balance measures
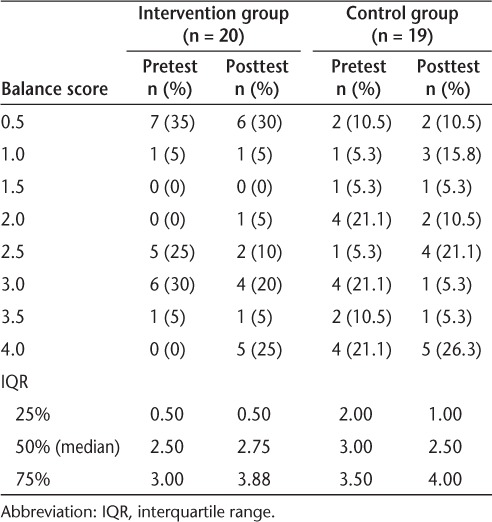
Figure 1.
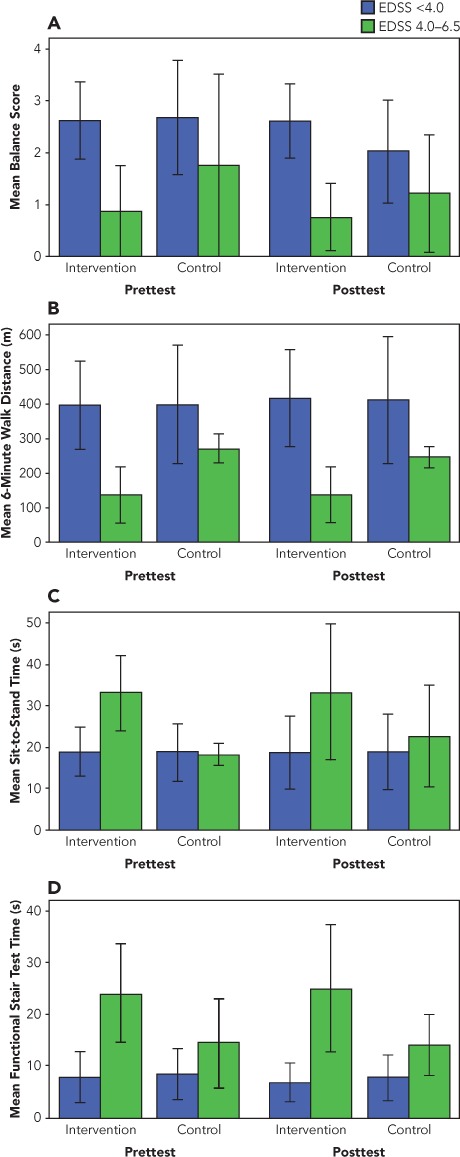
Pretest and posttest results on physical performance measures for the intervention and control groups by level of disability
A, Balance test. B, 6-Minute Walk test. C, Sit-to-Stand Test. D, Functional Stair Test. Error bars indicate ±1 SD.
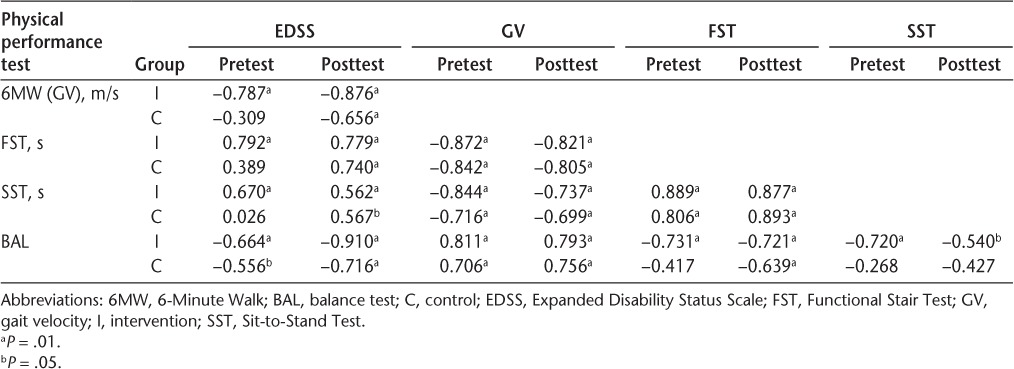
Pearson product moment correlations and Spearman rank correlations (rho) between the individual physical performance measures (6MW, GV, FST, SST, BAL) and the EDSS score for the intervention and control groups before and after the intervention are reported in Table 6. These individual performance measures are moderately to highly associated with each other (r = 0.5–0.075 and r > 0.75, respectively).50 The correlations should be interpreted in light of whether a high or a low score indicates better performance. Higher scores on the 6MW and BAL indicated better performance (greater distance walked or time balanced). Lower scores on the FST, SST, and RPE indicated better performance (faster performance on the tests). For example, in Table 6, the intervention group's –0.872 (pretest) coefficient for the 6MW and FST correlation indicates that those participants who walked greater distances tended to ascend and descend the stairs more quickly. Spearman rank correlation (rho) coefficients between the BAL and other physical performance tests and their associated RPE scores were relatively high for the BAL (r = –0.742, P = .000) and FST (r = 0.730, P = .016) tests compared with the SST (r = 0.487, P = .002) and the 6MW (r = –0.225, P = .340) tests.
Table 6.
Pearson product moment correlation and Spearman rank correlation (rho) coefficients (probability) between EDSS and physical performance tests using best trial data and scales
Results of the two-way (group × time) repeated-measures ANOVAs of the individual mobility factors are shown in Table 4. Balance improved significantly in the intervention group (80.6%) compared with the control group (6.2%). A nonsignificant improvement in the IMT-trained group in 6MW distance (P = .086) was noted. The control group had a decline in performance on the SST, while the intervention group maintained its function (P = .084). On the FST, there was essentially no change in either group following the intervention period (P = .064). In addition, no significant change was noted in FSS score for either group.
Discussion
Inspiratory muscle training effectively increased MIP values by 71.4% in the experimental participants. While improvements in MEP and MVV were also present in the intervention group, they were not significant (P = .335 and P = .122, respectively). The IMT-trained participants experienced a significant improvement in standing balance compared with the nontreatment group (P = .008). This improvement was achieved in both participants who were able to achieve single-limb stance and those whose balance was tested in a tandem-stance position. The greatest balance improvement occurred in intervention group participants with mild disability (lower EDSS scores) (Figure 1). A nonsignificant improvement in the IMT-trained group as compared with the control group in 6MW distance (P = .086; power, 0.405) was noted, with greater improvement in those with mild disability. Thus, the greatest improvements in balance and gait were made by participants with mild disability (EDSS score <4.0) who trained with the IMT. This is the first report documenting improvement in functional mobility as a result of respiratory muscle training in people with mild-to-moderate disability related to MS (EDSS score ≤6.5). A direct measure of fatigue was not used in this study; however, scores on the FSS, a measure of fatigue over the period of 1 week, remained relatively stable from before to after the intervention, suggesting that the improvement in functional mobility may have occurred without any increase in general fatigue levels.
The impact of inspiratory muscle training on the results of the SST and FST was less clear. The intervention group maintained SST speed, while the moderately disabled participants in the control group exhibited a nonsignificant reduction in performance on this task (P = .084; power, 0.410). On the FST, there was no real change in performance for either group (P = .064; power, 0.460). While there was a moderate effect size for these tests, the power was not sufficient to show significant changes in the SST and FST. Future studies should use a larger sample size to adequately power the study.
No previously published studies of people with MS examined respiratory muscle training and functional mobility. However, several studies reported on the relationship between respiratory function and functional mobility. Mutluay et al.4 determined that FVC (r = –0.42), MEP (r = –0.39), and a pulmonary dysfunction index (r = 0.45) significantly correlated with MS disability as measured by the EDSS, a disability scale heavily influenced by ambulation skill level. In healthy normal subjects aged 55 to 75 years, Camarri et al.51 found that FEV1 (r = 0.48) significantly predicted 6MW distance. The work of Lan et al.30 in 5724 adults over age 60 also supports the relationship between respiratory function and functional mobility. They found that gait speed, repeated sit-to-stand ability, and peak expiratory flow were predictive of mobility-related limitations.
In contrast, the study of Savci et al.7 of 30 people with MS determined that neither respiratory muscle weakness (MIP, r = 0.28; MEP, r = 0.27) nor lung function (FVC, r = 0.36) contributed to 6MW distance in people with MS. Savci et al.,7 however, did report that activities of daily living as measured by the Barthel Index affect 6MW distance (r = 0.81). In a study of 64 ambulatory individuals with MS, Wetzel et al.52 found that MVV (r = 0.32), MIP (r = 0.31), and MEP (r = 0.30) were all significantly correlated with 6MW distance. Participants' scores on the Activities-Specific Balance Confidence Scale, FST, and BAL were more significant and independent predictors of 6MW distance than the pulmonary measures.52
Further research should be conducted to explore the relationship between respiratory function and balance/mobility control. Many of the muscles of both inspiration and expiration are also part of the core muscle group that stabilizes the trunk to enhance balance and mobility function. Abdominal muscles (transversus abdominis, internal and external obliques, and rectus abdominis) assist in both inspiration and expiration during periods of increased ventilatory needs. Contraction of the abdominal muscles, especially the transversus abdominis, tightens the fascia surrounding the abdomen, thus contributing to a stabilizing force in the trunk. Ventilatory muscles such as the diaphragm and the scalene muscles help stabilize the rib cage, providing an additional component of core stability to enhance balance and mobility function.53
An interesting secondary finding of this study is the relationship between EDSS scores and lower-extremity function as measured by the physical performance tests. The average EDSS score of the control group (3.2) was lower than that of the intervention group (4.1). Examination of the correlation coefficients in Table 6 shows lower correlations with EDSS scores in the control group than in the intervention group. One potential explanation of this finding is that the EDSS is not as sensitive to variance in lower-extremity function at lower EDSS scores.
Limitations of the Study
As in all tests of physical performance, test results may vary depending on participant effort and fatigue during testing. Test instructions were standardized, attempts were made to provide the same amount of encouragement to all participants, and rest periods were permitted upon request throughout testing; however, this remains a potential limitation for both the mobility and the respiratory function tests. No direct measure of core muscle strength was included in this study. The intervention group received weekly phone calls to progress their exercise, while the control group received only one phone call during the control phase. This difference in attention and activity between the two groups may have motivated the intervention group to put forth more effort during the testing session, thereby affecting the posttest outcome data. The heterogeneity of the MS population and high variability of performance across time among people with MS limited the power of some of the statistical analyses. Further limitations of inspiratory muscle training testing are described elsewhere.9 Given the small sample size, we are concerned about the potential for a type II error resulting in failure to detect an important clinical difference, rather than a type I error resulting in failure to reject the null hypothesis. It is important that each variable be assessed for its own clinical change. Therefore, a Bonferroni correction was not performed.54 The generalizability of the findings of this study is limited to community-dwelling, ambulatory individuals with MS.
Clinical Implications
The results of this study indicate that inspiratory muscle training positively affects balance (BAL) and perhaps gait endurance (6MW) and thus may be considered as an adjunct for mobility training in people with MS. We postulate that the mechanism linking improved respiratory function with improved BAL and 6MW scores is improvement in core stability of the trunk. A thorough search of the literature revealed no studies directly linking core muscle strength and respiratory function. However, reduced respiratory function and/or reduced diaphragm strength has been noted in populations in which reduced core muscle strength is highly likely, such as healthy elderly people55 and people with Duchenne muscular dystrophy.56 Coordinated timing of trunk and respiratory muscle activation during limb movement has been previously demonstrated in normal subjects.20–22 No research is available, however, directly linking respiratory function in MS with core muscle strength and mobility functions such as balance, stair-climbing, sit-to-stand activities, and ambulation. Further study on the mechanism underlying the improvement in balance and gait is warranted.
Many community-dwelling, ambulatory individuals with MS exhibit reduced respiratory muscle strength and endurance, suggesting that testing of respiratory muscle function should be routinely performed in this population.7,9,10 Use of both inspiratory muscle training9,17 and expiratory muscle training1,10,11 improves respiratory muscle strength in people with MS and should be included in the treatment of people who exhibit respiratory muscle weakness.
Conclusion
A 10-week inspiratory muscle training program with exercise training monitored and progressed via phone contact with the participants resulted in significantly increased inspiratory muscle strength, timed static standing balance scores, and a trend toward increased distance walked on the 6MW test in ambulatory individuals with mild-to-moderate MS.
PracticePoints.
A 10-week daily home training program using an inspiratory muscle trainer significantly improved inspiratory muscle strength in people with mild-to-moderate MS-related disability.
Inspiratory muscle training had a positive impact on standing balance in people with mild-to-moderate MS-related disability.
The Expanded Disability Status Scale (EDSS) as a measure of disability may not correlate well with lower-extremity mobility function in ambulatory people with MS who have mild disability (EDSS score <4.0).
Acknowledgments
We wish to thank Anang Chokshi, DPT, Michelle Wagner, DPT, and Emily Jackson, DPT, who collected and entered data for this project.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This work was partially funded by grants from the Office of Research at the University of Michigan–Flint awarded to Dr. Fry and a grant from the Michigan Physical Therapy Institute for Education and Research awarded to Anang Chokshi, Michelle Wagner, and Emily Jackson.
References
- 1.Gosselink R, Kovacs L, Ketelaer P, Carton H, Decramer M. Respiratory muscle weakness and respiratory muscle training in severely disabled multiple sclerosis patients. Arch Phys Med Rehabil. 2000;81:747–751. doi: 10.1016/s0003-9993(00)90105-9. [DOI] [PubMed] [Google Scholar]
- 2.Gosselink R, Kovacs L, Decramer M. Respiratory muscle involvement in multiple sclerosis. Eur Respir J. 1999;13:449–454. doi: 10.1183/09031936.99.13244999. [DOI] [PubMed] [Google Scholar]
- 3.Tantucci C, Massucci M, Piperno R, Betti L, Grassi V, Sorbini CA. Control of breathing and respiratory muscle strength in patients with multiple sclerosis. Chest. 1994;105:1163–1170. doi: 10.1378/chest.105.4.1163. [DOI] [PubMed] [Google Scholar]
- 4.Mutluay FK, Gurses HN, Saip S. Effects of multiple sclerosis on respiratory functions. Clin Rehabil. 2005;19:426–432. doi: 10.1191/0269215505cr782oa. [DOI] [PubMed] [Google Scholar]
- 5.Howard RS, Wiles CM, Hirsch NP, Loh L, Spencer GT, Newsom-Davis J. Respiratory involvement in multiple sclerosis. Brain. 1992;115:479–494. doi: 10.1093/brain/115.2.479. [DOI] [PubMed] [Google Scholar]
- 6.Rasova K, Brandejsky P, Havrdova E, Zalisova M, Rexova P. Spiroergometric and spirometric parameters in patients with multiple sclerosis: are there any links between these parameters and fatigue, depression, neurological impairment, disability, handicap and quality of life in multiple sclerosis? Mult Scler. 2005;11:213–221. doi: 10.1191/1352458505ms1155oa. [DOI] [PubMed] [Google Scholar]
- 7.Savci S, Inal-Ince D, Arikan H, et al. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil Rehabil. 2005;27:1365–1371. doi: 10.1080/09638280500164479. [DOI] [PubMed] [Google Scholar]
- 8.Garland SJ, Lavoie BA, Brown WF. Motor control of the diaphragm in multiple sclerosis. Muscle Nerve. 1996;19:654–656. doi: 10.1002/(SICI)1097-4598(199605)19:5<654::AID-MUS15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Fry DK, Pfalzer LA, Chokshi AR, Wagner MT, Jackson ES. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J Neurol Phys Ther. 2007;31:162–172. doi: 10.1097/NPT.0b013e31815ce136. [DOI] [PubMed] [Google Scholar]
- 10.Chiara T, Martin AD, Davenport PW, Bolser DC. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch Phys Med Rehabil. 2006;87:468–473. doi: 10.1016/j.apmr.2005.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeltzer SC, Lavietes MH, Cook SD. Expiratory training in multiple sclerosis. Arch Phys Med Rehabil. 1996;77:909–912. doi: 10.1016/s0003-9993(96)90281-6. [DOI] [PubMed] [Google Scholar]
- 12.Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26:102–107. doi: 10.1159/000090444. [DOI] [PubMed] [Google Scholar]
- 13.Buyse B, Demedts M, Meekers J, Vandegaer L, Rochette F, Kerkhofs L. Respiratory dysfunction in multiple sclerosis: a prospective analysis of 60 patients. Eur Respir J. 1997;10:139–145. doi: 10.1183/09031936.97.10010139. [DOI] [PubMed] [Google Scholar]
- 14.Smeltzer SC, Skurnick JH, Troiano R, Cook SD, Duran W, Lavietes MH. Respiratory function in multiple sclerosis: utility of clinical assessment of respiratory muscle function. Chest. 1992;101:479–484. doi: 10.1378/chest.101.2.479. [DOI] [PubMed] [Google Scholar]
- 15.National Multiple Sclerosis Society Symptoms. http://www.nation-almssociety.org/about-multiple-sclerosis/symptoms/index.aspx. Accessed March 19, 2009.
- 16.Fry D. Clinical Bulletin: Pulmonary Function and Rehabilitation in MS. National Multiple Sclerosis Society website. http://www.nationalms-society.org/for-professionals/healthcare-professionals/publications/clinical-bulletins/index.aspx. Accessed March 19, 2009.
- 17.Klefbeck B, Nedjad JH. Effect of inspiratory muscle training in patients with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:994–999. doi: 10.1016/s0003-9993(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 18.Medical Research Council. Aids to the Examination of the Peripheral Nervous System. London: Pendragon House; 1976. Memorandum 45. [Google Scholar]
- 19.Chiara T, Martin D, Sapienza C. Expiratory muscle strength training: speech production outcomes in patients with multiple sclerosis. Neurorehabil Neural Repair. 2007;213:239–249. doi: 10.1177/1545968306294737. [DOI] [PubMed] [Google Scholar]
- 20.Hodges PW, Gandevia SC. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Appl Physiol. 2000;89:967–976. doi: 10.1152/jappl.2000.89.3.967. [DOI] [PubMed] [Google Scholar]
- 21.Gandevia SC, Butler JE, Hodges PW, Taylor JL. Balancing acts: respiratory sensations, motor control and human posture. Clin Exp Pharm Physiol. 2002;29:118–121. doi: 10.1046/j.1440-1681.2002.03611.x. [DOI] [PubMed] [Google Scholar]
- 22.Saunders SW, Rath D, Hodges PW. Postural and respiratory activation of the trunk muscle changes with mode and speed of locomotion. Gait Posture. 2004;20:280–290. doi: 10.1016/j.gaitpost.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Smith MD, Chang AT, Seale HE, Walsh JR, Hodges PW. Balance is impaired in people with chronic obstructive pulmonary disease. Gait Posture. 2010;31:456–460. doi: 10.1016/j.gaitpost.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Rantanen T, Guralnik J, Ferrucci L, Leveille S, Fried L. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999;54:M172–176. doi: 10.1093/gerona/54.4.m172. [DOI] [PubMed] [Google Scholar]
- 25.Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49:21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83:49–57. [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 29.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 30.Lan T-Y, Melzer D, Tom B, Guralnik J. Performance tests and disability: developing an objective index of mobility-related limitation in older populations. J Gerontol A Biol Sci Med Sci. 2002;57:M294–301. doi: 10.1093/gerona/57.5.m294. [DOI] [PubMed] [Google Scholar]
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 32.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 33.American Thoracic Society/European Respiratory Society. Standardisation of lung function testing: standardisation of spirometry. Eur Respir J. 2005;26:319–338. [Google Scholar]
- 34.Rudick R, Antel J, Confavreux C, et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol. 1997;42:379–382. doi: 10.1002/ana.410420318. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik J, Ferrucci L, Simonsick E, Salive M, Wallace R. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belgen B, Beninato M, Sullivan P, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87:554–561. doi: 10.1016/j.apmr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Thomas J, Lane J. A pilot study to explore the predictive validity of 4 measures of falls risk in frail elderly patients. Arch Phys Med Rehabil. 2005;86:1636–1640. doi: 10.1016/j.apmr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Csuka M, McCarty D. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 39.Salem G, Wang M, Young J, Marion M, Greendale G. Knee strength and lower- and higher-intensity functional performance in older adults. Med Sci Sports Exerc. 2000;32:1679–1684. doi: 10.1097/00005768-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Jones C, Rikli R, Beam W. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 41.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21:87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20:156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Light KE, Behrman AL, Thigpen MT, Triggs WJ. The 2-minute walk test: a tool for evaluating walking endurance in clients with Parkinson's disease. Neuro Report. 1997;21:136–139. [Google Scholar]
- 44.Dean CM. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin Rehabil. 2001;15:415–421. doi: 10.1191/026921501678310216. [DOI] [PubMed] [Google Scholar]
- 45.Fry D, Pfalzer L. Reliability of four functional tests and rating of perceived exertion in persons with multiple sclerosis. Physiother Can. 2006;58:212–220. [Google Scholar]
- 46.Franklin B, Whaley M, editors. ACSM's Guidelines for Exercise Testing and Prescription. 6th ed. Philadelphia, PA: American College of Sports Medicine; 2000. [Google Scholar]
- 47.Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exc. 1982;14:377–381. [PubMed] [Google Scholar]
- 48.Black L, Hyatt R. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 49.Keppel G, Wickens T. Design and Analysis: A Researcher's Handbook. 4th ed. Upper Saddle River, NJ: Prentice-Hall; 2004. pp. 176–181. [Google Scholar]
- 50.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice-Hall; 2000. p. 65. [Google Scholar]
- 51.Camarri B, Eastwood P, Cecins N, Thompson P, Jenkins S. Six-minute walk distance in healthy subjects aged 55–75 years. Respir Med. 2006;100:658–665. doi: 10.1016/j.rmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Wetzel J, Fry D, Pfalzer L. Predictive factors for six-minute walk distance in persons with mild and moderate disability from multiple sclerosis. Physiother Can. (in press) [DOI] [PMC free article] [PubMed]
- 53.LeVangie PK, Norkin CC. Joint Structure and Function: A Comprehensive Analysis. 4th ed. Philadelphia: FA Davis Company; 2005. pp. 170pp. 201–205. [Google Scholar]
- 54.Perneger T. What's wrong with Bonferroni adjustments? Br Med J. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152:677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- 56.De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB. Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax. 1997;52:472–475. doi: 10.1136/thx.52.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]


