Abstract
Multiple sclerosis (MS) is most prevalent in women of childbearing age. It is well established that the relapse rate decreases during pregnancy but increases significantly during the first postpartum trimester. The objective of this retrospective study was to evaluate the effectiveness of the administration of 1 g of intravenous methylprednisolone (IVMP) after delivery in the prevention of MS relapses. The study involved 47 women with one or more documented pregnancies; each pregnancy was treated as a separate case. There were 50 cases with relapsing-remitting MS and 2 with secondary progressive MS. The cases were divided into two groups: the IVMP group (those who received 1 g of IVMP after delivery) and the no-IVMP group (those who did not receive IVMP after delivery). There were 39 cases in the IVMP group and 13 in the no-IVMP group. During the first postpartum trimester, relapses occurred in 17.9% of the IVMP group, compared with 46.2% of the no-IVMP group (P = .0448). The difference in relapse percentage between the two groups during the second and third postpartum trimesters was not statistically significant. Our study shows a statistically significant benefit of postpartum IVMP administration in reducing MS relapses.
Multiple sclerosis (MS) is most prevalent among women of childbearing age.1 Recent studies have shown that pregnant MS patients have the same risk of pregnancy complications as the general population.2 The influence of pregnancy on the course of disease in MS has also been studied over the years.
According to the Pregnancy in Multiple Sclerosis (PRIMS) trial, the mean ± SD number of relapses was 0.7 ± 0.9 per woman per year in the year before pregnancy; it was 0.5 ± 1.3 during the first trimester of pregnancy, 0.6 ± 1.6 during the second trimester, and 0.2 ± 1.0 during the third trimester. It increased to 1.2 ± 2.0 during the first 3 months postpartum and then returned to the pre-pregnancy rate.3 After a 2-year follow-up analysis, the researchers described clinical factors that might predict the likelihood of relapse in the 3 months after delivery: an increased relapse rate in the pre-pregnancy year, an increased relapse rate during pregnancy, and a higher Expanded Disability Status Scale (EDSS) score at pregnancy onset.4 The reason for the increased postpartum activity is not yet clear, but many factors have been considered, including the decrease in estrogen levels and the reduced activity of some immune-inhibitory molecules.5
Previous studies suggested a reduction in the postpartum relapse rate for MS patients after exclusive breastfeeding, 6,7 but this tendency was not shown in a more recent study.8 Breastfeeding was also not shown to affect postpartum relapse activity in PRIMS.4
Data published by Oliveri et al.9 showed that intravenous methylprednisolone (IVMP) reduced magnetic resonance imaging (MRI) activity, defined as the number of contrast-enhancing lesions over a 2-month period. Therefore, we hypothesized that a dose of IVMP in the immediate postpartum period might reduce the risk of relapse. In our MS center, we suggest to our pregnant patients and their obstetricians the administration of 1 g of IVMP just after delivery with the goal of reducing the risk of postpartum relapses. We reviewed the outcomes for the pregnant patients seen most recently in our MS center. The objective of the study was to compare the percentage of MS patients having relapses between those treated with 1 g of IVMP after delivery and those who did not receive IVMP after delivery.
Methods
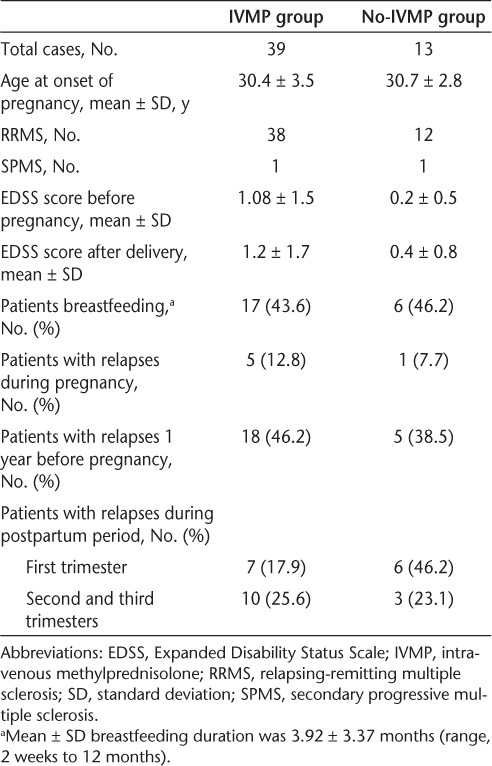
This was a retrospective study involving 47 patients, 5 of whom had two documented pregnancies; each pregnancy was considered a single case. There were 50 relapsing-remitting MS (RRMS) cases and 2 secondary progressive MS (SPMS) cases from 2001 to 2009. We included only those cases for which we were able to collect most of the database information before, during, and after pregnancy. Data obtained from medical records of patients in the Maxine Mesinger Multiple Sclerosis Comprehensive Care Center included MS type; number of relapses before, during, and after pregnancy (first, second, and third postpartum trimesters); MS treatment history; breastfeeding duration; postpartum IV steroid use; and EDSS scores before and after pregnancy (Table 1). Cases were divided into two groups: the IVMP group (those who received 1 g of IVMP after delivery) and the no-IVMP group (those who did not receive IVMP after delivery). The steroids were administered by the obstetrics team within the first 6 hours after delivery. An MS relapse was defined as appearance, reappearance, or worsening of symptoms of neurologic dysfunction lasting at least 24 hours that could not be explained by a current infection or other causes.
Table 1.
Demographics of study participants
The study was approved by the Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals.
Results
This is an ongoing study, with 52 cases analyzed to date. There were 39 cases in the IVMP group and 13 cases in the no-IVMP group. During the first postpartum trimester, relapses were seen in 17.9% of the IVMP cases, compared with 46.2% of the no-IVMP cases (P = .0448).
During the second and third postpartum trimesters, ten IVMP cases had a relapse (25.6%), compared with three no-IVMP cases (23.1%) (P = .8323). The EDSS score difference between the two groups was not statistically significant, either before pregnancy (P = .154) or after delivery (P = .204). There was a moderate disease progression in each group. In terms of percentage of patients with relapses 1 year before pregnancy, no statistically significant difference was observed between the two groups (P = .626). During pregnancy, six patients had at least one relapse. Five patients in the IVMP group had a relapse, with one of them having two relapses. Only one patient in the no-IVMP group had a relapse (P = .550). There were five patients who had two pregnancies each. Two of these patients did not receive IV steroids with either pregnancy. One of these two had a relapse during the first trimester of the first pregnancy.
In the IVMP group, 43.6% of the cases breastfed, 25.6% did not breastfeed, and in 30.8% of cases it was not recorded. In the no-IVMP group, 46.2% of cases breastfed, 30.7% did not breastfeed, and in 23.2% of cases it was not recorded. The mean ± SD duration of breastfeeding was 3.92 ± 3.37 months, with a minimum duration of 2 weeks and a maximum of 12 months. A total of 89.7% of the IVMP group were on a diseasemodifying therapy (DMT) prior to pregnancy, compared with 69.2% in the no-IVMP group (P = .078). No major side effects were reported after IVMP infusion.
Discussion
The prevalence of MS relapse increases during the first postpartum trimester. Previous trials have used IV immunoglobulin (IVIG) to reduce this risk,10,11 and another trial, POPART'MUS, is using progestin and estradiol with the same goal.12 Another study had already shown a beneficial effect of postpartum IV steroids in relapse rate reduction in a small group, but with monthly infusion for 6 months.13
In our study we found a significant reduction in the percentage of patients having relapses during the first postpartum trimester in those who received postpartum IV steroids compared with those who did not receive them. The difference between the two groups in the percentage of patients having relapses was not significant during the second and third postpartum trimesters. There was no significant difference between the two groups in terms of the percentage of patients who breastfed, a factor that has also been implicated in reduction of relapse risk.6–8 The advantages of the use of IVMP over the use of IVIG would be better tolerance, fewer side effects, and significantly lower cost.
This retrospective study had a number of important limitations. Although we tried to include all pregnant MS patients during the chosen time period, we were unable to include some because of unavailable information. A prospectively studied cohort currently being collected will help address this shortcoming. We did not attempt to randomize patients into the two groups. There are important reasons why a woman may have elected not to receive IVMP after delivery, including previous side effects, disease course, concerns regarding her infant, and so on. However, no statistically significant differences were noted in pre-pregnancy relapse percentage, pregnancy relapse percentage, or DMT usage to suggest a strong bias in this regard. Only two patients with SPMS who were still having relapses were included, one in each group.
The goal of this study was to investigate the use of postpartum steroids to reduce MS relapses. We did not collect information regarding pregnancy complications, mode of delivery, or other obstetrical outcomes. Future studies will include this important information.
In summary, this is the largest published study of the use of IVMP after delivery to reduce the risk of postpartum relapse in women with MS. The results of this uncontrolled, nonrandomized study suggest that this practice may be beneficial. Future studies should include randomization and control intervention to further evaluate this practice. Our current results support prior reports. The suggestion of Oliveri et al.9 that a single dose of IVMP protects against relapse for an average period of 2 months may explain the apparent lack of continued protection during the second and third postpartum trimesters. We may consider use of a second steroid infusion immediately after the third postpartum month to lengthen this protection.
PracticePoints.
In women with MS, the risk of relapse decreases during pregnancy but increases significantly during the first postpartum trimester.
The prophylactic use of intravenous methylprednisolone (IVMP; 1 g immediately after delivery) was associated with a statistically significant reduction in the percentage of patients having relapses during the first postpartum trimester.
The difference in percentage of patients having relapses between those who did and did not receive IVMP after delivery over the second and third postpartum trimesters was not statistically significant.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Kantarcia O, Wingerchuk D. Epidemiology and natural history of multiple sclerosis: new insights. Curr Opin Neurol. 2006;19:248–254. doi: 10.1097/01.wco.0000227033.47458.82. [DOI] [PubMed] [Google Scholar]
- 2.Jalkanen A, Alanen A, Airas L. Pregnancy outcome in women with multiple sclerosis: results from a prospective nationwide study in Finland. Mult Scler. 2010;16:950–955. doi: 10.1177/1352458510372629. Finnish Multiple Sclerosis and Pregnancy Study Group. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. Pregnancy in Multiple Sclerosis Group. [DOI] [PubMed] [Google Scholar]
- 4.Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of postpartum relapse. Brain. 2004;127:1353–1360. doi: 10.1093/brain/awh152. Pregnancy in Multiple Sclerosis Group. [DOI] [PubMed] [Google Scholar]
- 5.Airas L, Nikula T, Huang YH, Lahesmaa R, Wiendl H. Postpartum-activation of multiple sclerosis is associated with down-regulation of tolerogenic HLA-G. J Neuroimmunol. 2007;187:205–211. doi: 10.1016/j.jneuroim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Hellwig K, Gold R. Breastfeeding and multiple sclerosis in a German cohort. Mult Scler. 2008;5:718. doi: 10.1177/1352458507087847. [DOI] [PubMed] [Google Scholar]
- 7.Langer-Gould A, Huang S, Gupta R, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. 2009;66:958–963. doi: 10.1001/archneurol.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Airas L, Jalkanen A, Alanen A, Pirttilä T, Marttila RJ. Breast-feeding, postpartum and pregnancy disease activity in multiple sclerosis. Neurology. 2010;75:474–476. doi: 10.1212/WNL.0b013e3181eb5860. [DOI] [PubMed] [Google Scholar]
- 9.Oliveri RL, Valentino P, Russo C, et al. Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology. 1998;50:1833–1836. doi: 10.1212/wnl.50.6.1833. [DOI] [PubMed] [Google Scholar]
- 10.Achiron A, Rostein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996;243:25–28. doi: 10.1007/BF00878527. [DOI] [PubMed] [Google Scholar]
- 11.Haas J. High dose IVIG in the postpartum period for the prevention of exacerbation in MS. Mult Scler. 2000;6:18–20. [PubMed] [Google Scholar]
- 12.Vukusic S, Ionescu I, El-Etr M, et al. The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART'MUS) trial: rationale, objectives and state of advancement. J Neurol Sci. 2009;286:114–118. doi: 10.1016/j.jns.2009.08.056. Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis Study Group. [DOI] [PubMed] [Google Scholar]
- 13.De Seze J, Chapelotte M, Delalande S, Ferriby D, Stojkovic T, Vermersch P. Intravenous corticosteroids in the postpartum period for reduction of acute exacerbations in multiple sclerosis. Mult Scler. 2004;10:596. doi: 10.1191/1352458504ms1079sr. [DOI] [PubMed] [Google Scholar]


