Abstract
The objective of this study was to determine whether meditation affects pain and quality of life in people with multiple sclerosis (MS) and peripheral neuropathy (PN). A total of 22 patients (10 with MS, 12 with PN) participated in a weekly meditation class over a 2-month period. A total of 18 controls (7 with MS, 11 with PN) received standard care. Primary outcome assessments were based on the 36-item Short Form Health Status Survey (SF-36) and a visual analogue scale (VAS) for pain at baseline and at 2 months. Secondary outcome measures included the Neuropathy Impairment Score (NIS) for PN patients and the Patient-Determined Disease Steps (PDDS) questionnaire and 5-item Modified Fatigue Impact Scale (MFIS-5) for MS patients. After 2 months, study participants who practiced meditation reported an improvement in pain on the VAS (P = .035 combined group), summed physical health scores on the SF-36 (P = .011 MS, P = .014 PN), summed mental health scores (P = .02 combined group), vitality (P = .005 combined group), and physical role (P = .003 combined group). A significant improvement was also observed for bodily pain (P = .031) in MS patients. In contrast, no significant differences before and after the intervention were observed for controls. Regarding the secondary measure of fatigue, improved scores for the cognitive and psychosocial components of the MFIS were noted in MS patients in the intervention group (P = .037, P = .032). No statistically significant changes were observed in the NIS for PN patients or in PDDS scores for MS patients. Meditation may be helpful in reducing pain and improving quality of life in patients with MS and PN. The lack of changes seen in mobility (MS) and sensorimotor deficits (PN) suggests that meditation may not affect the overall clinical course.
For patients with chronic neurologic diseases, subjective symptoms such as fatigue and pain are often disabling and refractory to conventional medical treatment. Many of these patients have joined the general population in the growing trend toward use of complementary and alternative medicine (CAM), with 67% of multiple sclerosis (MS) patients and 43% of peripheral neuropathy (PN) patients reporting the use of at least one form of CAM in the last 12 months.1,2 Mind-body therapies, a subset of CAM that includes meditation and yoga, have previously been shown to be of benefit in patients with chronic pelvic pain, sleep disturbances, mood disorders, Parkinson's disease, cancer-related fatigue, and stress, among other chronic conditions.3–8 A recent study found that yoga was helpful in reducing fatigue in MS patients as compared with controls placed on a waitlist.9 In this pilot study, we investigated the effects of meditation on pain and quality of life in MS and PN patients.
Methods
This was an 8-week prospective, nonrandomized controlled trial in which a Buddhist monk led study participants in a weekly 90-minute meditation class. A total of 61 eligible participants (30 with MS, 31 with PN) were recruited from the Cleveland Clinic Neurological Institute via flyers posted throughout the hospital and clinic, online advertising on the hospital website, and direct contact during an outpatient clinic visit or by phone. Patients who had previously practiced meditation in the last 6 months, had severe cognitive impairment, or were unable to ambulate without assistance (MS patients with Expanded Disability Status Scale [EDSS] score > 6.5) were excluded.
A total of 36 participants (19 with MS, 17 with PN) were assigned to the intervention group based on individual preference to participate in the meditation sessions. A cohort group of 25 participants was recruited as controls (11 with MS, 14 with PN). The diagnoses of definite MS and PN were confirmed by the investigators through chart reviews. All participants provided written informed consent, and the study was approved by the appropriate institutional review board.
During a 4-hour introductory session, intervention group study participants were instructed on how to perform three types of meditation: 1) samatha, a Buddhist form of sitting meditation that focuses on the breath, 2) moving meditation incorporating tai chi and qigong, and 3) a six-step focused walking meditation. Details regarding the methods of each type of meditation are in Appendix 1. Subsequent classes were held weekly and divided into three 30-minute sessions for each type of meditation practiced. The study subjects (both MS and PN) participated in each of the sessions together in a group setting with the Buddhist monk leading each session. Participants were encouraged to practice daily at home, but there was no documentation of individual practice time. Controls received usual care and were instructed to refrain from practicing meditation for the duration of the study. They were also offered an individual meditation teaching session to take place during a clinic visit after the end of the study.
Primary outcome assessments were based on the 36-item Short Form Health Status Survey (SF-36) for quality of life measurements and a visual analogue scale (VAS) for pain. Secondary outcome measures included (for PN patients) the Neuropathy Impairment Score (NIS), which was determined by the investigating neurologists (JT and MR) before and after completion of the study, and (for MS patients) the Patient-Determined Disease Steps (PDDS) questionnaire and the 5-item Modified Fatigue Impact Scale (MFIS-5). All study participants completed the questionnaires before and after the intervention, with control participants completing the surveys at baseline and 2 months after usual care.
Statistical Analysis
To increase statistical power, several analyses were performed on the MS and PN patients combined, although disease-specific analyses were also included. Pre- and postintervention measurements were analyzed using the paired t test. For each of the measured responses, pairing was done within each patient grouping. Results from the intervention groups (MS and PN separately and combined) were then compared with those from correlating controls. Any paired difference with a P value less than .05 was considered to be statistically significant. SAS 9.2 software (SAS, Cary, NC) was used for data analysis.
The Wilcoxon rank sum test was used to assess for demographic differences in age, years since disease onset, and EDSS scores (MS) between study intervention participants and controls who completed the study and those who did not.
Results
Of the 61 patients enrolled in the study, 16 withdrew (12 with MS, 4 with PN) because of transportation issues, lack of interest, and unknown reasons. Moreover, 5 patients were excluded from the final analysis because of hospitalizations for non-study-related reasons (n = 2), attendance at less than 50% of the intervention sessions (n = 2), and significant improvement in inflammatory neuropathy possibly due to immune-modulating treatment rather than study intervention (n = 1). The final analysis included 40 participants, with 22 (10 MS, 12 PN) in the intervention group and 18 (7 MS, 11 PN) controls. Participant characteristics are shown in Table 1.
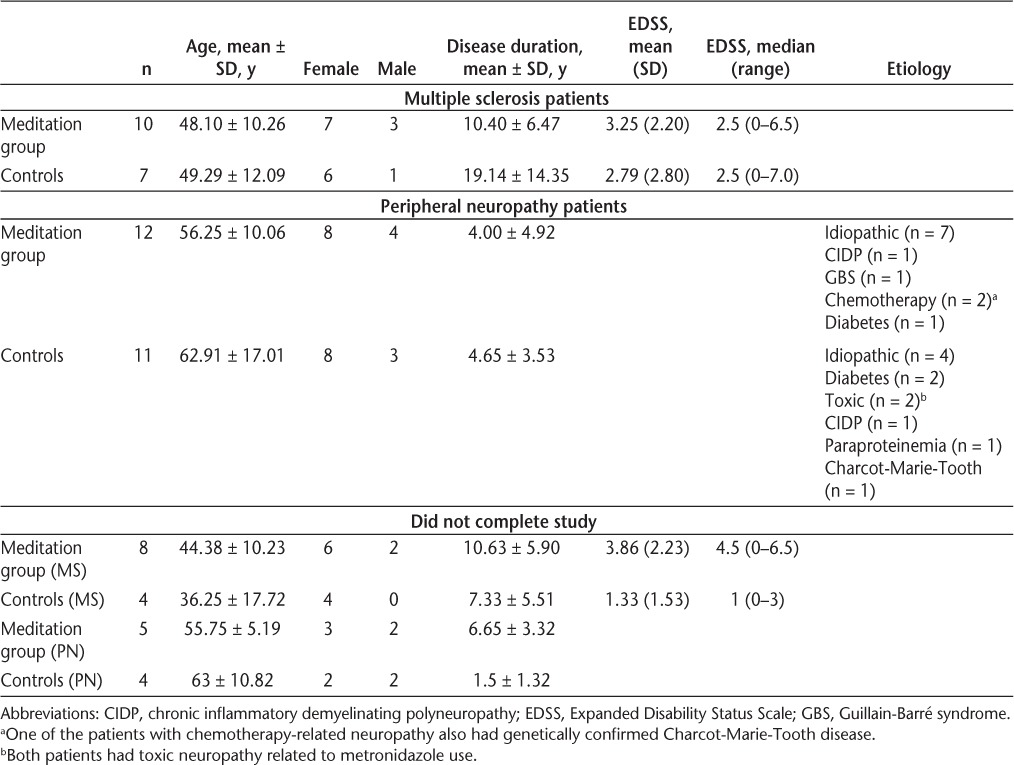
Table 1.
Baseline demographics
After 8 weeks, study participants in the intervention group showed a significant improvement in pain scores on the VAS (P = .035 combined group, P = .044 PN) and SF-36 scores for overall summed physical health (P = .011 MS, P = .014 PN), mental health (P = .02 combined group), vitality (P = .005 combined group), and physical role (P = .003 combined group), while no change was observed in controls (Figure 1). MS patients in the intervention arm also demonstrated a significant improvement in scores for bodily pain (P = .031).
Figure 1.
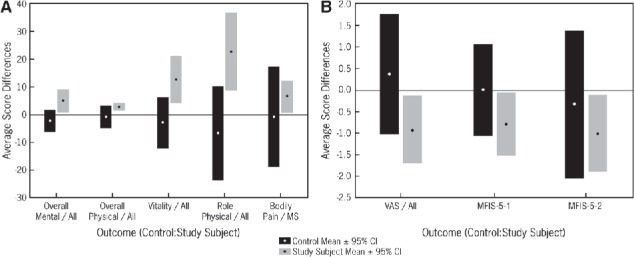
Changes in quality of life, pain, and fatigue before and after the intervention
A, Changes in quality of life (SF-36). B, Changes in pain and fatigue (VAS and MFIS-5). CI, confidence interval; MFIS-5, 5-item Modified Fatigue Impact Scale; MFIS-5-1 and MFIS-5-2, cognitive and psychosocial components of MFIS-5; MS, multiple sclerosis; SF-36, 36-item Short Form Health Status Survey; VAS, visual analogue scale.
For the secondary outcome measure of fatigue in MS patients, improved scores were seen in the intervention group with regard to the cognitive and psychosocial components of the MFIS (P = .037, P = .032) (Figure 1). For PN patients, a slight trend toward improvement of the motor and sensory portions of the NIS was seen (mean reduction of −0.29 and −1.5 on the NIS-M and NIS-S, respectively), but the difference did not reach statistical significance (P = .66, P = .20). No statistically significant changes were seen in PDDS scores for MS patients in the intervention group.
Participants in the MS and PN control groups showed no significant improvement at the end of the trial in any of the parameters measured except for a minor improvement in mobility seen in MS patients (P = .03). Furthermore, PN controls reported worsened scores for both the VAS (mean increase in pain of 1.21 ± 2.32) and all SF-36 measures except for bodily pain and mental health. Similarly, the MS control group showed increased pain on the VAS at the end of the trial (0.50 ± 2.74) and worsened SF-36 scores for all parameters except for vitality and social functioning (Figure 1).
Discussion
As demonstrated in this pilot study, 2 months of meditation may be helpful in reducing pain and improving scores on quality of life measures in MS and PN patients as compared with controls who have these disorders. In conjunction with a recently published study evaluating the effects of mindfulness in MS patients, these findings provide additional evidence that meditation may be beneficial in patients with chronic neurologic diseases.10 Notably, this is the first pilot study that showed a positive effect on pain and measures of well-being in PN patients.
The effectiveness of mind-body therapies may lie in their ability to facilitate stress reduction, relaxation, and improvement of mood, which in turn may affect the degree to which psychosocial factors can negatively affect quality of life in MS and PN patients.8,11 In addition, recent studies incorporating functional magnetic resonance imaging, electroencephalography, and evoked potentials have shown that meditation may result in reduced activation of the prefrontal cortex, amygdala, and hippocampus during exposure to pain stimuli, which may result in a more neutralized perception of pain.12,13
The lack of statistically significant changes observed for the objective measures of mobility (MS) or NIS scores (PN) may suggest that meditation does not change the underlying disease course. However, as there was a trend toward improvement in the NIS scores, a larger sample size and more extended length of study (>8 weeks) may result in a finding of statistical significance.
The major limitations of this study are the small sample size and the nonrandomized allocation of participants. The study was initially designed to be a randomized controlled trial. However, because of difficulty in recruiting participants for the study, volunteers who were approached in the clinic or called in after seeing the flyers were simply asked if they would like to participate in the intervention group. After enrollment of the intervention group was complete, controls were recruited from the population of clinic patients already under the care of the investigators (JT, MR, LS) and were asked either in the clinic or by phone to participate in the nonintervention group. All were offered a separate meditation class or individual teaching session that would take place after the study was completed. This lack of randomization could certainly have led to a self-efficacy effect, in which an individual's more active role in his or her treatment plan leads to an increased perception of the ability to cope; this is a limitation of many clinical trials involving mind-body therapies.14,15 Another major limitation was the lack of a sham intervention for controls, which would have served to control for the nonspecific benefits of the intervention, such as more frequent contact with the medical care providers.
Although the study may have also been somewhat limited by the combination of two groups of patients with different neurologic diagnoses (MS and PN), the research project was an intentional joint effort between the Neuromuscular Center and Multiple Sclerosis Center. The study was designed accordingly, with separate secondary measures that were specific to each pathology, but the intervention was the same for both groups.
Also of note, the retention rate in the MS intervention group was only 53%, compared with 79% for the corresponding PN group. However, a similarly poor retention rate of 64% was noted in MS controls, who were only required to fill out paperwork to participate. There was no demographic variable that appeared to be associated with a higher attrition rate in either intervention group. Although the MS control participants who withdrew from the study tended to have a shorter duration of disease, lower EDSS scores, and younger age, there were no significant differences (P < .05) between those who completed the study and those in the corresponding disease group who did not complete the study with respect to age, years since disease onset, and EDSS scores (MS). In most of these cases, the reasons for withdrawal were unknown, but the comparable dropout rates of the intervention group and controls suggest that the intervention itself was unlikely to be the primary reason. Similar studies evaluating the use of CAM in neurology patients have revealed attrition rates of 9% to 21% in control groups versus 3% to 16% in intervention groups.8–10
Despite the small sample size, our pilot study demonstrated a positive effect of meditation in patients with MS and PN. Future studies are needed to further support the growing evidence that meditation may be helpful in these patients. Ideally, the studies would feature a larger sample size, randomized allocation of participants with a sham intervention group, and documentation of daily practice at home for the duration of the study in order to assess the presence of a dose-response effect. In an era of rising health-care expenditures in which patients are searching for holistic therapies that are both effective and affordable, the practice of meditation should be considered a therapeutic option for symptomatic management of chronic neurologic diseases.
Appendix 1. Meditation Techniques
During each session, the patients practiced three forms of meditation in a group setting that was divided into three parts, each lasting 30 minutes. The first part of the session consisted of walking meditation, the second part consisted of moving meditation, and the third part consisted of sitting meditation. Details regarding each technique are provided below.
Part I: Walking Meditation
With the six-part walking meditation technique, patients were instructed to focus their attention on each movement of the foot as they took a step forward. They were told to walk for 10 to 20 paces total, then turn around and walk back the same way. This was repeated for the 30-minute duration of the walking session. The six movements of the foot that they were instructed to concentrate on are as follows:
Lift heel
Lift toe
Move forward
Lower the foot
Heel down
Toe down
Participants were told to think of the movement first, and then concentrate as they physically took each step.
Part II: Moving Meditation
Qigong and tai chi are Chinese mind-body exercises that are considered moving meditation techniques in which awareness and concentration are placed on breathing and specific movements of the body. Study participants performed basic tai chi maneuvers, including neck rolls in which the head was moved slowly from side to side, ankle rolls, shoulder rolls, hip rotations, knee bends, and alternating pulling and pushing movements with the arms. This was followed by more well-known forms such as “cloud hands,” in which they slowly rotated their body from left to right with sweeping motions of the arms in front of them.
Qigong is a more physically rigorous form of moving meditation with shortened and very quick but much simpler movements coupled with deep inhalations and forced exhalations. Focus is placed internally with this type of moving meditation. In contrast, the forms in tai chi are more complex and require outward focus.16 Study participants performed the 12-step Yi Jin Jing form of qigong.
Participants unable to completely perform all movements while standing because of fatigue, instability, or weakness were allowed to sit in a chair and practice with their arms.
Part III: Sitting Meditation
Study participants performed samatha sitting meditation, a form of Buddhist concentration meditation in which the mind is focused on one point. Patients sat in a chair or on a cushion on the floor and were told to close their eyes and focus their attention solely on their breathing. They were instructed to breathe normally and observe the movements of the abdomen with each inhalation and exhalation.
Footnotes
Financial Disclosures: Dr. Stone has received speaking fees from Biogen Idec and is the Editor in Chief of the International Journal of MS Care.
Funding/Support: This study was supported in part by a grant from the Bakken Heart and Brain Institute through the Cleveland Clinic Foundation.
PracticePoints
People with MS often experience pain and reduced quality of life. Efforts to improve these parameters can enhance care of the patient.
MS patients have increasingly turned to complementary and alternative medicine (CAM) for symptomatic management of their disease. Treating physicians should be aware of potentially helpful CAM therapies.
Meditation in the form of sitting, walking, or moving may improve scores on pain and quality of life measures in MS patients.
References
- 1.Brunelli B, Gorson KC. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci. 2004;218:59–66. doi: 10.1016/j.jns.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Marrie RA, Hadjimichael O, Vollmer T. Predictors of alternative medicine use by multiple sclerosis patients. Mult Scler. 2003;9:461–466. doi: 10.1191/1352458503ms953oa. [DOI] [PubMed] [Google Scholar]
- 3.Fox SD, Flynn E, Allen RH. Mindfulness meditation for women with chronic pelvic pain: a pilot study. J Reprod Med. 2011;56:158–162. [PubMed] [Google Scholar]
- 4.Carlson L, Speca M, Faris P, Patel K. One year pre-post intervention followup of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Blank S, Kittel J, Haberman MR. Active practice of Iyengar Yoga as an intervention for breast cancer survivors. Int J Yoga Ther. 2005;15:51–59. [Google Scholar]
- 6.Yook K, Lee SH, Ryu M. Usefulness of mindfulness-based cognitive therapy for treating insomnia in patients with anxiety disorders: a pilot study. J Nerv Ment Dis. 2008;196:501–503. doi: 10.1097/NMD.0b013e31817762ac. et al. [DOI] [PubMed] [Google Scholar]
- 7.Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psycho-Oncology. 2009;18:571–579. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- 8.Oken BS, Kishiyama S, Zajdel D. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. doi: 10.1212/01.wnl.0000129534.88602.5c. et al. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Hübsch T, Pyfer D, Kielwein K, Fimmers R, Klockgether T, Wüllner U. Qigong exercise for the symptoms of Parkinson's disease: a randomized controlled pilot study. Mov Disord. 2006;21:543–548. doi: 10.1002/mds.20705. [DOI] [PubMed] [Google Scholar]
- 10.Grossman P, Kappos L, Gensicke H. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75:1141–1149. doi: 10.1212/WNL.0b013e3181f4d80d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger B, Owen D. Stress reduction and mood enhancement in four exercise modes: swimming, body conditioning, Hatha yoga, and fencing. Res Q Exerc Sport. 1988;59:148–159. [Google Scholar]
- 12.Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–156. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Brown CA, Jones AKP. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150:428–438. doi: 10.1016/j.pain.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Wahbeh H, Elsas SM, Oken BS. Mind-body interventions. Neurology. 2008;70:2321–2328. doi: 10.1212/01.wnl.0000314667.16386.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandura A. Self Efficacy: The Exercise of Control. New York, NY: WH Freeman and Company; 1997. [Google Scholar]
- 16.Ospina MB, Bond K, Karkhaneh M. Meditation practices of health: state of the research. Evid Rep Technol Assess (Full Rep) 2007;155:1–263. et al. [PMC free article] [PubMed] [Google Scholar]


