Abstract
Research has found that people with multiple sclerosis (MS) who engage in exercise programs experience improvements in physical and psychological health, resulting in enhanced quality of life. These studies have involved structured exercise protocols, but few have examined the effects of an individualized exercise program allowing for peer socialization. The purpose of this study was to investigate the effects of a 10-week individualized exercise program offering opportunities to socialize with peers on fatigue and physical functioning in people with MS. Thirteen individuals with a physician diagnosis of MS were enrolled in a 10-week exercise program at Nazareth College in Rochester, New York. Eleven participants (9 female, 2 male; mean ± SD age, 55.0 ± 7.06 years) completed the study. The following qualitative and quantitative measures were used for evaluation before and after the exercise program: Multiple Sclerosis Quality of Life–54 (MSQOL-54), Activities-specific Balance Confidence (ABC) scale, Modified Fatigue Impact Scale (MFIS), Timed Up and Go (TUG) test, Timed 10-Meter Walk (T10MW) test, functional reach test, and single-leg stance (SLS) test. Statistically significant differences were found for the TUG (P = .005), T10MW (P = .014), and MFIS physical functioning subscore (P = .039). The results showed significant increases in gait speed and mobility as well as decreased impact of fatigue on physical functioning after the 10-week exercise program.
Multiple sclerosis (MS) is a chronic neurologic disease that affects the myelin sheaths in the central nervous system, resulting in various types of functional impairment. The exact cause is currently unknown, as is the precise number of people affected by the disease. The National Multiple Sclerosis Society estimates that approximately 400,000 individuals in the United States have the disease, with 200 new cases diagnosed per week,1 while the National Institutes of Health places this number at 250,000 to 350,000 individuals.2 One reason for the discrepancy is the lack of a comprehensive national registry for collecting prevalence data. Individuals with MS require assistance in relieving the symptoms of the disease and improving their functional status. In the past, individuals with MS were advised to avoid participating in exercise in order to reduce the risk of disease exacerbation or symptoms of fatigue.3 However, recent research indicates that people with MS who engage in exercise programs show benefits in both physical4 and psychological5 health, resulting in improved quality of life (QOL).6–9 Specifically, studies have found improvement in cardiorespiratory fitness10–12 and muscle strength.13,14 In several of these studies,4,5,10,12,14 the exercise protocol was performed by the participant in a testing environment that included supervision by an exercise specialist but no opportunity for social interaction with other study participants. In addition, the exercise programs consisted of identical exercises for every participant, with no consideration given to differences in their strength.4,5,14 Dodd et al.5 assessed not only the physical outcomes of a structured exercise program but also the psychological and social aspects of the program by means of interviews with study participants. The results showed that the participants enjoyed interacting with other people with MS during the program, felt increased motivation to exercise, and noticed a reduction in fatigue.
In a meta-analysis using the Cochrane database of systematic reviews, Rietberg et al.15 aimed to assess the effects of exercise therapy on patients with MS in terms of activities of daily living (ADLs) and QOL. Nine randomized controlled trials (RCTs) met the selection criteria; these studies reported on exercise therapy for adults with MS who were not presently experiencing an exacerbation, and reported outcomes including measures of QOL and/or activity limitation. The exercise programs varied in intensity, duration, and frequency as well as setting. However, none examined the effects of interaction with peers during the exercise program. The assessment of the RCTs found improvements in muscle strength, exercise tolerance functions, and mobility-related activities, as well as anxiety and depression. No evidence was found supporting positive effects of exercise therapy on fatigue or perception of handicap, nor was there any evidence that specific exercise programs were more successful than others in improving activities and functions.15 Other studies, however, have found exercise to positively affect both fatigue and QOL.8,16,17 McCullagh and colleagues17 investigated the effects of a 3-month exercise program on QOL and fatigue. Outcome measures included the Functional Assessment of Multiple Sclerosis (FAMS) and the Multiple Sclerosis Impact Scale–29 (MSIS-29) to measure QOL and the Modified Fatigue Impact Scale (MFIS) to measure fatigue. At the completion of the 3-month program, significant differences from baseline were found for the FAMS and MFIS, which persisted at 6 months after completion.
Previous studies examining physical4,10–14 and QOL6–9 outcomes have shown that individuals with MS benefit from participation in an exercise program. However, these studies used varying exercise protocols; in addition, the programs were conducted in a structured and controlled environment, with no opportunities to tailor the program to a participant's specific deficits. Finally, the programs did not provide opportunities for social interaction among the participants. Thus, the purpose of the present study was to investigate the effects of a 10-week individualized exercise program allowing for peer socialization on physical functioning and fatigue in people with a clinical diagnosis of MS.
Methods
Participants
Thirteen individuals with a physician diagnosis of MS were enrolled in a 10-week exercise program at Nazareth College in Rochester, New York. Two of the participants did not complete the final questionnaires and thus were not included in the data analysis. Eleven participants (9 female, 2 male; mean ± SD age, 55.0 ± 7.06 years) completed the study. Participants were a sample of convenience and were referred for participation in the study by their neurologists. Inclusion criteria consisted of a diagnosis of MS as determined by a physician, medical clearance from a primary-care physician to engage in an exercise program, and magnetic resonance imaging findings of sclerotic plaques in the brain. Participants were excluded from the study if they had solitary spinal cord lesions.
Instruments
Multiple Sclerosis Quality of Life–54 (MSQOL-54)
The MSQOL-54 is a questionnaire used to determine health-related QOL (HRQOL). The questionnaire consists of 54 items divided into 12 multiple-item scales and 2 single-item scales. The higher the score obtained, the better the HRQOL.18 Both reliability and validity of the MSQOL-54 have been established for individuals with MS.18,19 Internal consistency of the instrument is considered good, with a value of 0.84 found overall19 and values of 0.75 to 0.96 found for the 12 individual items in the instrument.18
Modified Fatigue Impact Scale (MFIS)
Few instruments with established psychometric properties have been developed to measure fatigue in patients with MS. One such instrument, the Fatigue Impact Scale (FIS), developed by Fisk et al.,20 was found to have high internal consistency (>0.93). The extensive 40-item FIS was later shortened to the MFIS.21 The MFIS is a 21-item scale used to evaluate the impact of fatigue with regard to psychosocial, cognitive, and physical functioning. The self-reported ratings of each item range from 0 (never) to 4 (almost always), for a total possible score ranging from 0 to 84 points. Higher scores indicate a stronger impact of fatigue on QOL. In a comparison of different rating scales for fatigue in MS including the MS-specific Fatigue Severity Scale (FSS) and the visual analogue scale (VAS), the MFIS was identified as a discriminative scale to measure fatigue in individuals with MS.22
Timed Up and Go (TUG) Test
The TUG test involves determining the time it takes the participant to rise from a chair, walk 3 m, turn around, walk back to the chair, and resume sitting.23 In addition to measuring gait speed for a shorter distance, the TUG also provides information regarding physical mobility in everyday skills including getting up and sitting down and turning.24 In testing of individuals with MS, the TUG had an intraclass correlation coefficient (ICC) of 0.9824 but was less discriminatory in predicting risk of falls compared with the Activities-specific Balance Confidence (ABC) scale and the Dizziness Handicap Inventory (DHI).25
Activities-specific Balance Confidence (ABC) Scale
The ABC scale is a self-report measure that assesses the confidence level of an individual while completing 16 ADLs.25 This scale has been shown to distinguish between various levels of functional mobility.26,27
Timed 10-Meter Walk (T10MW)
The T10MW is administered by measuring a 10-m course and marking its end with tape on the floor. The individual starts approximately 3 m behind the start tape and is instructed to walk as fast as possible to the end tape. An assistive device may be used if required for safe ambulation. Time is recorded when the person completely crosses over the end tape.28 This test has been found to be valid and reliable in measuring mobility and gait speed of patients with neurologic impairment29 and reliable (ICC = 0.97) when testing individuals with MS.24
Single-Leg Stance (SLS) Test
The single-leg stance (SLS) test is a measure of static balance. The individual is instructed to stand on one leg, with the arms across the chest and the hands touching the shoulders and with the nontested leg bent at the knee. The test is timed for the duration the person can remain standing without the nontested leg touching the floor or the tested leg, the tested leg moving from its static position on the floor, or the arms moving from the starting position. This test can be administered with eyes open and eyes closed.30,31 Individuals with MS have significantly less ability to maintain balance in SLS compared with those without MS.32
Functional Reach Test
The functional reach test can be used as a measure of balance. The test measures the distance an individual can reach forward beyond arm's length while remaining standing in a stationary position. The individual stands erect with the arm forward at shoulder height and is instructed to reach forward as far as possible without losing the base of support. Measurement, using a tape measure, is recorded in inches as distance from the starting point of the forward arm at the tip of the middle finger to the ending point reached by the tip of the middle finger. The reliability and validity of this test have been established.33
Procedures
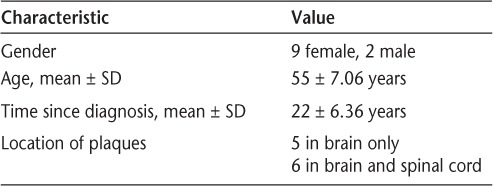
The Human Subjects Review Committee at Nazareth College granted approval for this study. The participants completed a written consent form and provided information on their medical history including year of MS diagnosis and area of plaques, as well as demographic information (Table 1). The participants were asked to fill out three questionnaires: the MSQOL-54, MFIS, and ABC (pretest, time 1). Baseline measures (pretest, time 1) of the TUG, T10MW, functional reach test, and SLS test were completed by physical therapy (PT) students under supervision by licensed PTs. The PT students had been previously instructed on procedures for proper administration of the testing instruments. They were aware that a study was being conducted, but because this exercise program did not consist only of participants in the study, they did not know whether the person they worked with was a participant. The PT students developed individualized intervention programs that took into account the participants' specific deficits based on the outcomes of a PT assessment; thus there was not a standardized exercise protocol for all participants. The participants' impairments and functional limitations included gait deviations, decreased static and dynamic balance, decreased endurance with daily activities, and decreased strength, especially in the lower extremities. Interventions including gait and functional mobility training and resistive, aerobic, and balance exercises were provided weekly for 1 hour for a total of 10 weeks. Exercise principles were observed, including overload and specificity. The students adjusted the programs as tolerated by the participants, taking into consideration fatigue level during the sessions. Although each participant had an individualized program and worked one-onone with a PT student, there were opportunities to interact with other participants before and after the sessions and during rest periods. These interactions were not structured and were initiated by the participants. At the completion of the 10-week program, participants completed the qualitative measures MSQOL-54, MFIS, and ABC and were reassessed on the quantitative measures TUG, T10MW, functional reach, and SLS (posttest 1, time 2). Ten weeks after completion of the program, the participants were asked to complete the qualitative measures again (posttest 2, time 3).
Table 1.
Demographic characteristics of study participants (N = 11)
Statistical Analysis
Data were analyzed using SPSS version 16.0 (SPSS, Chicago, IL). Quantitative measures were analyzed using a paired t test. Qualitative measures were analyzed using one-way analysis of variance (ANOVA) and the Friedman test. The P value considered to indicate statistical significance was set at P < .05.
Results
One participant was unable to walk or stand without assistance and was therefore excluded from data analysis for the TUG, T10MW, SLS, and functional reach tests. Seven participants were unable to perform SLS with eyes closed for at least 1 second and thus were excluded from data analysis for this test. However, only five participants were able to perform SLS with eyes open for more than 10 seconds, and only two participants could perform it for 30 seconds. Interestingly, the participants' left leg did not perform as well. One participant had an exacerbation during the 10-week exercise program; however, this was a higher-level individual, and the posttest 1 (time 2) quantitative scores improved.
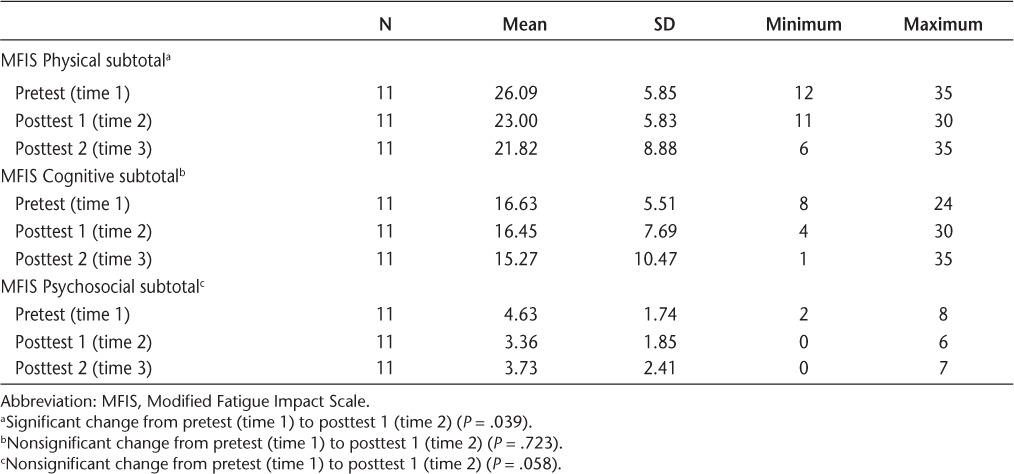
Statistically significant decreases in mean time to complete the TUG (P = .005) and the T10MW (P = .014) were found between the pretest (time 1) and posttest 1 (time 2) (Table 2). No statistically significant differences were found between the pretest (time 1) and posttest 1 (time 2) in functional reach (P = .257), right SLS with eyes open (P = .277), right SLS with eyes closed (P = .084), left SLS with eyes open (P = .326), or left SLS with eyes closed (P = .734) (Table 2). A significant decrease (P = .039) was found in the impact of fatigue on physical function after the 10-week program (Table 3). The impact of fatigue on cognitive function (P = .723) and psychosocial function (P = .058) decreased after the 10-week program, but not significantly (Table 3). None of the 16 question scores on the ABC scale changed significantly (P > .05) across the data-collection period. Positive trends were seen in the outcome measures of the MSQOL-54 across the study in various item categories, although none were significant.
Table 2.
Change in quantitative measures from pretest (time 1) to posttest 1 (time 2)
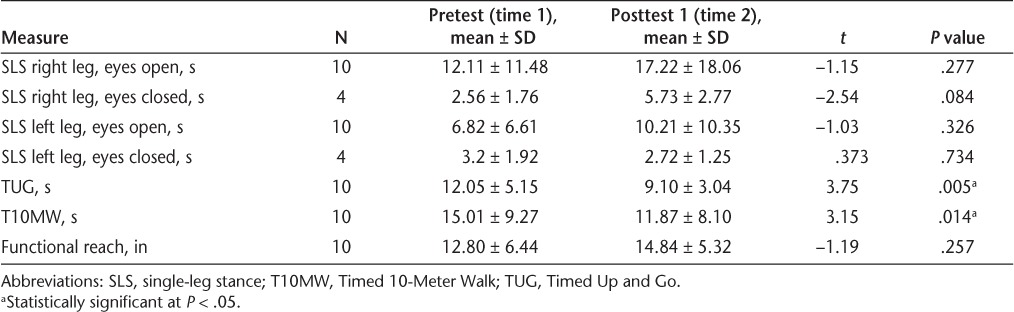
Table 3.
MFIS scores for physical, cognitive, and psychosocial functioning
Discussion
The results of this pilot study show that participation in a 10-week individualized exercise program focusing on the participants' functional deficits significantly increased mobility and gait speed in individuals with MS as assessed by the TUG and T10MW. In addition, results for the MFIS physical functioning items showed a significant decrease in the impact of fatigue on physical function. Although the MFIS scores for cognitive and psychosocial functioning items did not show significant improvements, a positive trend was found toward decreased impact of fatigue on these items. Our results also show that the exercise program did not have a significant effect on balance as measured by SLS, functional reach, and ABC scale, nor on HRQOL as measured by the MSQOL-54.
The improvements seen in the TUG and T10MW tests may be attributed to exercise program specificity. In contrast to previously reported exercise program designs that focused on strengthening specific muscles, the current exercise program focused on functional improvements such as gait, as this was a personal goal of many of the participants. The increase in gait function may have contributed to a perceived improvement in impact of fatigue on physical functioning, as shown by the MFIS scores. The lack of significant changes in the SLS, functional reach, and ABC scores may also be related to exercise program specificity. An increased emphasis on balance exercises in the exercise program would have been beneficial.
Exercising in the presence of peers promotes socialization through observation and potential interaction. It has been suggested that the receipt of encouragement, confidence, and improved mood5 may contribute to an exercise program's success. In our study the participants could observe other people with MS exercising and had the opportunity for social interaction during rest periods. The positive trend toward decreased impact of fatigue on psychosocial and cognitive functioning as measured by the MFIS may be attributed partly to peer socialization.
A limitation of this study is that the quantitative measures were not retested 10 weeks after completion of the exercise program. Such retesting would have provided additional information on the participants' retention of the benefits of the exercise program over time. Other limitations include the lack of a control group, possible bias of the PT students providing interventions and measuring outcomes, and small sample size. Future studies should explore the benefits of an exercise program over time and examine any improvements in disability as measured by the Expanded Disability Status Scale.
Conclusion
This study shows that individuals with MS who participate in an individualized exercise program that emphasizes function can experience marked improvements in gait speed and mobility. Participation in this 10-week program also reduced the perceived impact of fatigue on physical function. This study is unique in that while participants exercised and progressed at their own pace, they exercised alongside peers. Peer socialization may have contributed to the beneficial effects of the program.
PracticePoints.
Few previous studies of the effects of participation in exercise programs among people with MS have examined the effects of an individualized program allowing for peer socialization.
In this study, people with MS who participated in a 10-week individualized exercise program allowing for peer socialization showed improvements in gait speed, mobility, and impact of fatigue on physical functioning.
Peer socialization may have contributed to the beneficial effects of the exercise program observed in this study.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.National Multiple Sclerosis Society. Who gets MS. National Multiple Sclerosis Society Web site. http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx. Accessed October 2010.
- 2.National Institutes of Health. How many people have MS. National Institute of Neurologic Disorders and Stroke Web site. http://www.ninds.nih.gov/disorders/multiple_sclerosis/detail_multiple_sclerosis.htm#158953215. Updated 2010. Accessed October 2010.
- 3.Rusk HA, editor. Rehabilitation Medicine. 3rd ed. St. Louis, MO: CV Mosby; 1971. [Google Scholar]
- 4.Kraft GH, Alquist AD, deLateur BJ. Effect of resistive exercise on physical function in multiple sclerosis. J Rehabil Res Dev. 1996;33:328–329. [Google Scholar]
- 5.Dodd KJ, Taylor NF, Denisenko S, Prasad D. A qualitative analysis of a progressive resistance exercise programme for people with multiple sclerosis. Disabil Rehabil. 2006;28:1127–1134. doi: 10.1080/09638280500531842. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland G, Andersen MB. Exercise and multiple sclerosis: physiological, psychological, and quality of life issues. J Sports Med Phys Fitness. 2001;41:421–432. [PubMed] [Google Scholar]
- 7.Motl RW, Snook EM. Physical activity, self-efficacy, and quality of life in multiple sclerosis. Ann Behav Med. 2008;35:111–115. doi: 10.1007/s12160-007-9006-7. [DOI] [PubMed] [Google Scholar]
- 8.Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008;14:129–135. doi: 10.1177/1352458507080464. [DOI] [PubMed] [Google Scholar]
- 9.Motl RW, McAuley E, Snook EM. Physical activity and quality of life in multiple sclerosis: possible roles of social support, self-efficacy, and functional limitations. Rehabil Psychol. 2007;52:143–151. [Google Scholar]
- 10.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 11.Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler. 2002;8:161–168. doi: 10.1191/1352458502ms779oa. [DOI] [PubMed] [Google Scholar]
- 12.Rampello A, Franceschini M, Piepoli M. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007;87:545–555. doi: 10.2522/ptj.20060085. et al. [DOI] [PubMed] [Google Scholar]
- 13.White LJ, McCoy SC, Castellano V. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler. 2004;10:668–674. doi: 10.1191/1352458504ms1088oa. et al. [DOI] [PubMed] [Google Scholar]
- 14.Kraft GH, Alquist AD, deLateur BJ. Effect of resistive exercise on strength in patients with multiple sclerosis. J Rehabil Res Dev. 1996;33:329–340. [Google Scholar]
- 15.Rietberg MB, Brooks D, Uitdehaag BM, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005;(1):CD003980. doi: 10.1002/14651858.CD003980.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroud NM, Minahan CL. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health Qual Life Outcomes. 2009;7:68. doi: 10.1186/1477-7525-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullagh R, Fitzgerald AP, Murphy RP, Cooke G. Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: a pilot study. Clin Rehabil. 2008;22:206–214. doi: 10.1177/0269215507082283. [DOI] [PubMed] [Google Scholar]
- 18.Vickrey B, Hays R, Harooni R, Myers L, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. [DOI] [PubMed] [Google Scholar]
- 19.Heiskanen S, Merilainen P, Pietila AM. Health-related quality of life-testing the reliability of the MSQOL-54 instrument among MS patients. Scand J Caring Sci. 2007;21:199–206. doi: 10.1111/j.1471-6712.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 20.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 21.Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS) Mult Scler. 1999;5:251–259. doi: 10.1177/135245859900500410. [DOI] [PubMed] [Google Scholar]
- 22.Flachenecker P, Kumpfel T, Kallmann B. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–526. doi: 10.1191/1352458502ms839oa. et al. [DOI] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 24.Nilsagard Y, Lundholm C, Gunnarsson LG, Dcnison E. Clinical relevance using timed walk tests and ‘timed up and go' testing in persons with multiple sclerosis. Physiother Res Int. 2007;12:105–114. doi: 10.1002/pri.358. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006;28:789–795. doi: 10.1080/09638280500404289. [DOI] [PubMed] [Google Scholar]
- 26.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1998;53:M287–294. doi: 10.1093/gerona/53a.4.m287. [DOI] [PubMed] [Google Scholar]
- 27.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AB, Carnel CT, Ditunno JF. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med. 2008;31:487–499. doi: 10.1080/10790268.2008.11753644. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW, Larkin PA, Cook AC, Gear J, Singer J. Decrease in timed balance test scores with aging. Phys Ther. 1984;64:1067–1070. doi: 10.1093/ptj/64.7.1067. [DOI] [PubMed] [Google Scholar]
- 31.Newton R. Review of tests of standing balance abilities. Brain Inj. 1989;3:335–343. doi: 10.3109/02699058909004558. [DOI] [PubMed] [Google Scholar]
- 32.Frzovic D, Morris ME, Vowels L. Clinical tests of standing balance: performance of persons with multiple sclerosis. Arch Phys Med Rehabil. 2000;81:215–221. doi: 10.1016/s0003-9993(00)90144-8. [DOI] [PubMed] [Google Scholar]
- 33.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]


