Abstract
Optimal health of people with multiple sclerosis (MS) can be promoted by patients' sharing of health information gained through periodic self-monitoring with their health-care providers. The purpose of this study was to develop a valid and reliable self-administered scale to obtain information about MS patients' health status and the impact of the disease on their daily lives. We named this scale “Monitoring My Multiple Sclerosis” (MMMS). A cross-sectional survey was conducted of 171 MS patients who completed the MMMS and Patient-Determined Disease Steps (PDDS) scales and provided information on their MS disease classification and demographic characteristics. Data analysis included several parametric procedures. Factor analysis of the 26-item MMMS resulted in four factors with satisfactory α reliability coefficients for the total scale (0.90) and factored subscales: Physical (0.85), Relationships (0.80), Energy (0.70), and Cognitive/Mental (0.67). Analysis of variance demonstrated that the total scale and the Physical subscale, but not the Relationships subscale, showed significantly worse functioning for patients with either moderate or severe disability as measured by the PDDS than for patients with mild disability (P < .001). The Cognitive/Mental subscale showed significantly worse functioning for patients with moderate disability than for patients with mild disability (P < .05). However, the Energy subscale showed significantly worse functioning among moderately disabled patients than among severely disabled patients (P < .01). Independent t tests demonstrated that patients classified as having secondary progressive multiple sclerosis had significantly worse scores on the total MMMS (P < .05) and the Physical subscale (P < .001) than those classified as having relapsing-remitting multiple sclerosis. The MMMS demonstrated satisfactory reliability and validity and is recommended for use by MS patients and their health-care providers as a mechanism to promote the sharing of health information, to the benefit of both patients and providers.
Multiple sclerosis (MS) is a chronic and progressive inflammatory autoimmune disease of the central nervous system (CNS), with onset usually occurring during young adulthood.1 The disease is initially characterized by relapsing-remitting symptoms in most patients and may later take a progressive course.2,3 The symptoms and signs of MS reflect the CNS area of neural impairment.1 Neural impairment in the cerebrum affects cognition; cerebellum impairment affects balance and produces tremors; brainstem impairment is associated with diplopia, vertigo, and impaired speech and swallowing; and spinal cord impairment causes weakness, stiffness, painful spasms, bladder dys-function, sexual dysfunction, and constipation. These symptoms are reflected in accumulating disabilities experienced by the person with MS that affect physical, social, and cognitive/mental functioning.
Currently there is no cure for MS, although there are many partially effective treatments and interventions that focus on symptom management. Because of the great variability in symptoms and disabling conditions experienced by MS patients, ongoing monitoring of physical, social, and cognitive/mental functioning is important to determine both appropriate counseling and treatment and the effectiveness of such interventions. Self-monitoring by MS patients who then share health-related information with their health-care providers holds promise for controlling or alleviating symptoms, resulting in improved health, functioning, and quality of life. It also promotes shared decision-making between the patient and health-care providers.4 Ideally, recorded information gained through self-monitoring could be sent to the health-care provider's clinic or office just before the patient's appointment or brought to the appointment for review and discussion by the patient, the nurse, and other appropriate health-care providers. Such information can help convey a holistic picture of the patient's health status as well as whether current treatments and interventions are effective or require adjustment. When self-monitored information is gathered at periodic intervals, current information can be compared with previous information to determine whether the patient's health has improved, remained the same, or worsened.
The value of self-monitoring of activity of daily living (ADL) functions and MS-related symptoms was shown in a longitudinal study spanning 27 months that involved three separate patient assessments; compared with the experimental group, the control group used significantly more professional health services.5 Feedback from the clinic nurse or patient-services coordinator given to the experimental group following each self-assessment and monitoring activity was thought to help participants more accurately estimate their health needs and evaluate the self-care actions they had taken.
A self-monitoring assessment scale must be representative of the physical, social, and cognitive/mental conditions common to MS as well as the patient's ability to manage everyday activities and relationships with others. Ross and associates6 noted that patient self-report questionnaires can increase communication between patients and health-care providers and may increase patient satisfaction with health care, improve disease management, and facilitate office and clinic visits by helping to set priorities among issues that must be addressed in a single visit. They also noted that such questionnaires can enable nurses to better recognize situations in which patients require referrals to other health-care or social-services providers; help differentiate MS diagnoses; and facilitate identification of new symptoms.
Current self-administered assessment measures used by MS patients to assess their functioning and the impact of the disease on their quality of life either fail to include important areas or are very long, burdening the patient and discouraging completion of the scale. Currently available measures generally include items pertaining to physical and psychological functioning; examples are the 36-item Short Form Health Status Survey (SF-36),7 the Multiple Sclerosis Quality of Life–54 (MSQOL-54),8 the Multiple Sclerosis Quality of Life Inventory (MSQLI),9 the Functional Assessment of Multiple Sclerosis (FAMS) quality of life instrument,10 and the Multiple Sclerosis Impact Scale (MSIS-29).11 All of these measures include assessment of physical and psychological conditions, common MS symptoms, energy/fatigue, and social relationships. Cognition and sexual functioning are also included in the MSQOL-54, MSQLI, and FAMS. Bowel and bladder functioning are included as separate items in the MSQLI but as a single combined item in the MSQOL-54. Sleep is represented as a single item only in the MSIS-29. Areas that are not included in the aforementioned measures include spirituality, coping, nutrition, financial situation, and, for the most part, sleep.
Spirituality, coping, sleep, nutrition, and financial adequacy are important areas that need assessment, as they are related to physical and psychological conditions, relationships with family members and others, cognition, and energy/fatigue status. Woods and Ironson12 noted that spirituality can promote a sense of well-being and empowerment, facilitate coping, and aid in personal learning and growth in chronically ill patients. While maladaptive coping has been shown to precede depression, active coping can mediate the relationship between cognitive dysfunction and depression.13 Sleep disturbance at different times during the night can be due to various MS symptoms as well as environmental factors and is associated with feelings of fatigue.14 Adequate dietary intake is essential for optimal health.15 Conversely, malnutrition is associated with impairment of the immune system and adversely affects mental function.16 The economic costs of MS increase with disease severity; although direct medical costs (health-care providers, drugs, assistive devices, therapy) are high in the early stages of the disease, they are outweighed by indirect costs (work loss, impact on family and friends) at later disease stages.17 Direct and indirect costs of MS vary across different countries because of substantial differences in the availability of services and resource use patterns.17,18
Given that existing self-administered assessment measures either do not include a number of functional areas important to MS patients or are so lengthy as to create response burden on the MS patient (or both), this study was undertaken to develop a reliable and valid self-administered measure that both included such often-neglected areas and was user-friendly. The scale items were intended to generate discussion during the patient's visit with the nurse or other appropriate health-care provider, and the purpose of the scale was to allow MS patients to monitor their physical, social, and cognitive/mental functioning and related conditions, as well as the impact of the disease on their daily activities. The ultimate aim was to enable patients to share the information gathered through this self-monitoring process with their health-care providers in order to improve patient care.
Methods
Two separate studies were undertaken in order to develop the self-assessment scale.
Study 1: Initial Scale
Recognizing the benefits to MS patients and their health-care providers of information gained through patient self-monitoring, the International Organization of Multiple Sclerosis Nurses (IOMSN) convened a meeting in September 2007 to discuss the need for a self-assessment scale for MS patients that could help them monitor their condition and evaluate outcomes of care. At this initial meeting, participants discussed what kinds of health information would be most useful for assessing the patient's condition and determining the need for changes in treatment or interventions. Several existing patient assessment scales were reviewed for possible adoption. The Quality of Life in Alzheimer's Disease (QOL-AD) scale consists of 13 domains such as physical health, energy level, and mood, which are also important among MS patients. Satisfactory internal consistency, stability, and construct validity have been reported for the QOL-AD.19,20 With permission from Logsdon and associates,19 the authors of the QOL-AD, the IOMSN committee made some small changes in the wording of the 13 QOL-AD items and added 10 more items to reflect the health conditions of people with MS and the impact of the disease on daily activities and interpersonal relationships.
The 10 new items specific to MS pertained to mobility, bowel and bladder functioning, sexuality, personal care, nutrition, pain, communication, future goals, and motivation. Most items used a 4-point rating scale from 1 (poor) to 4 (excellent). Responses to items pertaining to future goals and motivation were rated from 1 (not at all) to 4 (a great deal). Administration of the 23-item revised QOL-MS scale to 91 MS patients resulted in an α coefficient of 0.89. However, one of the 23 QOL-MS items pertaining to nutrition failed to demonstrate a satisfactory item–total scale correlation (r = 0.135). The 23-item QOL-MS scale demonstrated a moderate correlation (r = 0.54, P < .01) with the MSQOL-54 scale8 and differentiated patients by disease classification of relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS) (t = 3.89, P < .001).21 Concerns about general health behaviors, especially nutrition, led the IOMSN committee to recommend revision of the nutrition item and further examination of the scale.
Study 2: Revised Scale
Examination of the initial QOL-MS scale led to several item changes. Many of the items were shortened to make the scale more user-friendly. The “nutrition” item was rewritten to be more explanatory, and the single item pertaining to “bowel and bladder” functions was separated into two items, one for each function. Two items pertaining to “life as a whole” were merged into one item to avoid duplication. Finally, three new items were added pertaining to “spiritual well-being,” “feeling rested after sleeping,” and “satisfaction with level of physical activity.” The 26-item scale was renamed “Monitoring My Multiple Sclerosis” to reflect IOMSN's objective of patient self-monitoring. After approval of the study by the institutional review boards for protection of human subjects of the first author's institution and the participating MS clinics, the scale was administered to 171 MS patients who were attending several different outpatient MS clinics. All items were rated on a 4-point scale. Most item ratings were 1 (poor), 2 (fair), 3 (good), and 4 (excellent). Three items were rated as 1 (not at all), 2 (somewhat), 3 (mostly), and 4 (a great deal). One item was rated as 1 (not at all), 2 (very little), 3 (somewhat), and 4 (completely). The total possible score ranged from 26 to 104, with higher scores indicating higher satisfaction with the patient's condition and functions. In reporting results in the current study, in order to facilitate comparisons, summed scores for the total scale and each subscale (described in the Results section) were converted to averages by dividing them by the respective number of items, resulting in a score range of 1 to 4 for each scale.
Other Measures
Another measure used in the study was the Patient-Determined Disease Steps (PDDS),22 a 9-item patient-administered measure of MS-related disability. Its content validity is indicated by consistency of the items with the Expanded Disability Status Scale (EDSS).23 Construct validity is indicated by a correlation between the PDDS and EDDS scales of 0.96.24 The PDDS was used to categorize participants into three groups according to level of disability: a score of 0 to 2 indicated mild disability, represented by sensory symptoms but no limitations in walking; a score of 3 to 5 indicated moderate disability, represented by symptoms that interfered with daily activities, especially walking, and need for a cane; and a score of 6 to 8 indicated severe disability, represented by the need for bilateral support, wheelchair use, or being bedridden.
Demographic information collected from study participants included their current age, age at onset of MS symptoms, age at diagnosis with MS, educational level, gender, family status, ethnicity, and MS disease classification (RRMS or SPMS).
Procedure
An MS clinic nurse described the study to MS patients attending the clinic and invited them to participate. Patients who agreed to participate in the study were given a packet containing an informed consent form, the MMMS, the PDDS, and a demographic form for completion either at home or during their clinic visit. Completed study materials were forwarded by the clinic nurse to the first author for data analysis. Of the sample, 23 respondents completed the MMMS a second time to determine stability of the scale over a 2-week period (15 respondents) and a 3-month period (8 respondents).
Data Analysis
Data analysis was performed with PASW Statistics 18 software (SPSS, Chicago, IL). Descriptive statistics were used to describe the study participants' demographic characteristics and scale scores and to confirm that statistical assumptions were met for the statistical procedures. Principal components analysis and varimax rotation factor analysis were used to estimate the structure of the MMMS scale. The known-groups validity procedure was used to examine differences in MMMS scores by PDDS group, with one-way analysis of variance and the post hoc Bonferroni correction procedure used to adjust the αlevel of significance for number of group pairs being tested. The independent t test was used to estimate differences in MMMS scores by MS disease classification and gender. The Pearson correlation procedure was used to determine associations between the factored MMMS subscales. The α level of statistical significance was set at .05.
Results: Study 2
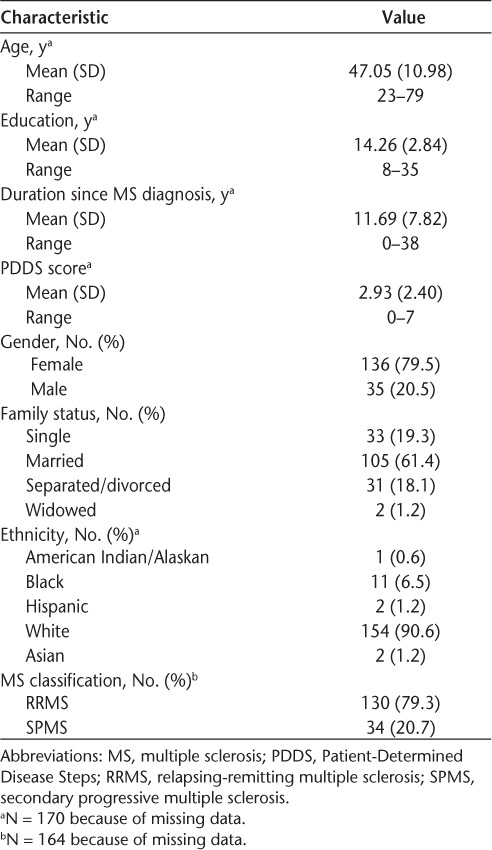
The 171 participants were recruited from four out-patient MS centers located in Minnesota, Ohio, Texas, and Virginia. The mean age was 47 years, mean amount of education was 14 years, and mean duration since diagnosis of MS was 12 years (Table 1). Most of the participants were white, 80% were female, almost two-thirds were married, and 79% reported having RRMS.
Table 1.
Demographic characteristics
Content validity for the MMMS was indicated by the 100% agreement among the IOMSN committee members regarding the scale items' representativeness of MS-related conditions and their impact on MS patients' daily activities.
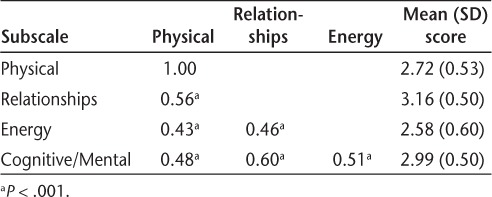
Construct validity was estimated by performing principal components analysis and varimax rotation factor analysis of the 26-item MMMS, and by determining differences in MMMS scores by PDDS group and MS classification. Factor analysis resulted in four factors: 1) Physical (10 items), 2) Relationships (8 items), 3) Energy (3 items), and 4) Cognitive/Mental (5 items). Physical items included mobility, bladder and bowel functioning, sexual and intimate relationships, personal care, work, and overall satisfaction with physical activity. Relationship items included communication with others; living situation; relationships with family, spouse/significant other, and friends; and financial situation. Energy items included energy level, mood, and feeling rested after sleeping. Cognitive/Mental items included thinking and memory, coping ability, spiritual well-being, life as a whole, and dietary adequacy. Eigen values for the factors ranged from 4.09 to 2.43. Fifty-one percent of the variance in the MMMS scale was explained (Table 2). Moderate correlations were found between the factored subscales, indicating substantial interaction among Physical, Relationships, Energy, and Cognitive/Mental conditions (Table 3). Female participants had a significantly lower mean (SD) score on the subscale measuring physical conditions than male participants (2.76 [0.53] vs. 2.56 [0.51]; t = 1.99, P = .048).
Table 2.
Factor analysis: Monitoring My Multiple Sclerosis scale (N = 171)
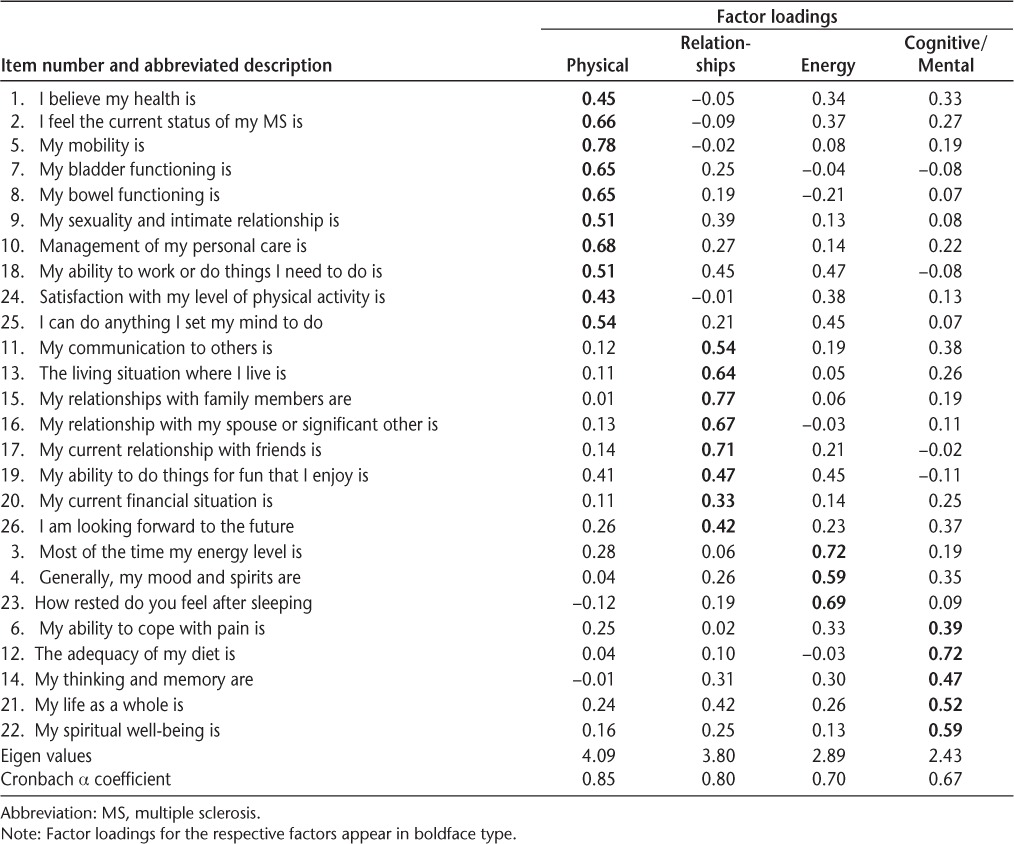
Table 3.
Pearson correlations of factored subscales and mean scores
The differences in mean scores on the MMMS total scale and four subscales between the PDDS disability groups were determined (Table 4). Participants with mild disability (PDDS 0–2) reported significantly fewer negative effects from MS (higher functioning) for the total MMMS scale and for the Physical subscale than did those with severe disability (PDDS 6–8). Those with moderate disability (PDDS 3–5) reported significantly more negative effects (worse functioning) for the Energy subscale than did those with severe disability (PDDS 6–8). Those with mild disability (PDDS 0–2) reported significantly fewer negative effects for the Cognitive/Mental subscale (higher functioning) than did those with moderate disability (PDDS 3–5). No significant differences among the PDDS groups were observed for the Relationships subscale. Additionally, no differences were found between the mild disability group (PDDS 0–2) and the other two PDDS groups for the Energy subscale, and no differences were found between the severe disability group (PDDS 6–8) and the other two PDDS groups for the Cognitive/Mental subscale. Participants with RRMS had significantly higher scores on the total MMMS scale and the Physical subscale than did those with SPMS (Table 5). However, no significant differences were found between the RRMS and SPMS groups for the Relationships, Energy, and Cognitive/Mental subscales.
Table 4.
Differences in MMMS total and subscale scores by PDDS group
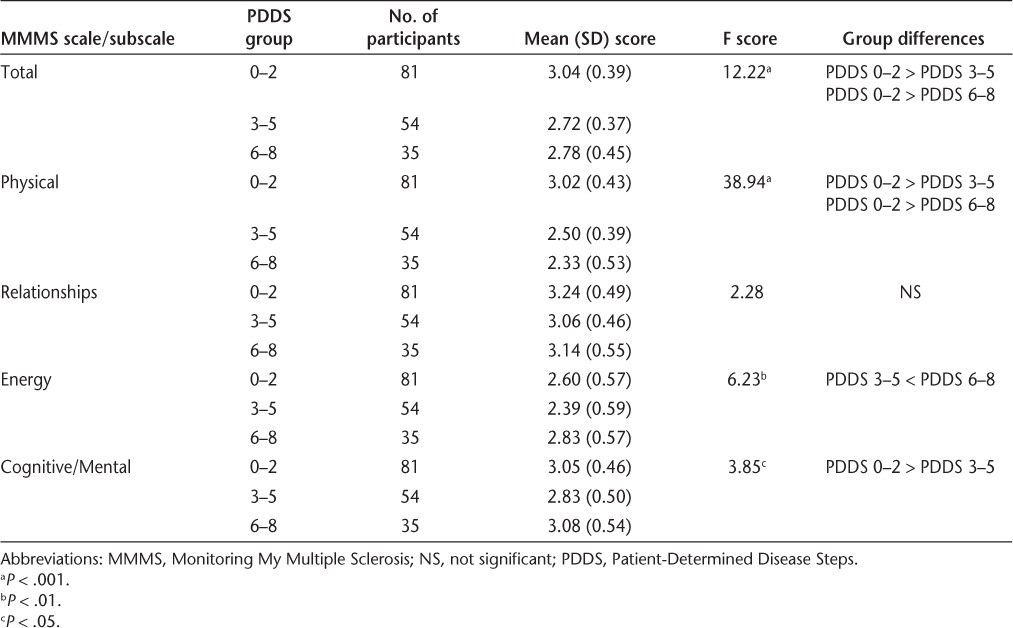
Table 5.
Differences in MMMS total and subscale scores by MS disease classification
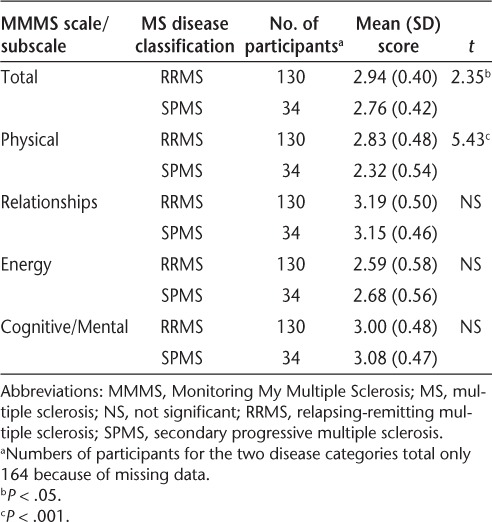
The Cronbach α coefficient for internal consistency is an indicator of reliability, or the extent to which the items on a scale or subscale measure the same underlying dimension. The reliability coefficient for the total MMMS scale was 0.90, and those for the subscales were 0.85 (Physical), 0.80 (Relationships), 0.70 (Energy), and 0.67 (Cognitive/Mental), indicating satisfactory reliability. Stability of the MMMS scale over a period of 2 weeks to 3 months was measured by the intraclass correlation coefficient; the coefficient for the total scale was 0.94, and those for the subscales were 0.96 (Physical), 0.91 (Relationships), 0.92 (Energy), and 0.87 (Cognitive/Mental), based on responses from 23 participants.
Discussion
Principal components analysis and varimax rotation factor analysis of the 26-item MMMS resulted in four subscales with satisfactory internal consistency and stability. Moderate correlations found between the MMMS subscales indicate that participants experienced interactions among Physical, Relationships, Energy, and Cognitive/Mental functions.
The factored subscale Physical explained 17.4% of the variance in the MMMS. Many physical conditions influence MS patients' level of health and functioning. Effects on mobility of leg weakness, spasms, tremors, knee locking, balance problems, and falling have been shown to increase significantly over a 10-year period.25 Integrity of one's motor functions is required for participating in one's work, exercise, leisure activities, and personal care.26 Dysfunctional bowel (constipation, fecal incontinence, or both) and bladder (hesitancy, frequency, urgency, urge incontinence, retention) conditions have an estimated prevalence of between 44% and 73%27–30 and correlate positively with disability level.31 Sexual dysfunction, particularly erectile problems in men and difficulty having an orgasm in women, worsen with increased disability.31
The factored subscale Relationships explained 14.9% of the variance in the MMMS. Relationships with family members and others are an important source of social support that includes availability of aid32 and emotional well-being.33 Communication within relationships that demonstrate support, caring, sincerity, and concern enhances the patient's emotional well-being.34
The factored subscale Energy explained 10.4% of the variance in the MMMS. Interestingly, the Energy subscale score for the moderately disabled (PDDS 3–5) group was significantly lower than that for the severely disabled (PDDS 6–8) group. Similar findings regarding higher perceived negative effects pertaining to energy, mood, and cognition in moderately compared with severely disabled patients were reported by Johansson and associates.35 The loss of energy and strength and feelings of a strained body with diminished power characterize fatigue among women with MS.36 Vucic and associates37 noted that fatigue among MS patients may be manifested as exhaustion, lack of energy, increased somnolence, or worsening of symptoms. Conditions associated with fatigue among those with MS include mood and sleep disorders. Sleep disturbance, with a prevalence of 51.5%,38 may be due to leg spasms, pain, immobility, nocturia, or medication39 and can consist of difficulty falling asleep, frequent awakenings, snoring, and nocturia.39
The factored subscale Cognitive/Mental explained 8.3% of the variance in the MMMS. Cognitive dysfunction has a prevalence of up to 65% of individuals with MS.40 Cognitive areas most often affected are memory, attention, information processing, abstract/conceptual reasoning, and visuospatial skills.41,42 Memory, assessed by the Buschke Selective Reminding Test,43 and depression, assessed by the Beck Depression Inventory,44 are reportedly related to each other.45 Arnett and associates46 suggested that reduced working memory capacity and generalized psychomotor slowing may explain the association between depression and impaired performance on speeded attentional capacity tasks. A composite index of coping obtained by computing the difference between the MS patient's active coping score (eg, planning) and avoidance coping score (eg, mental disengagement, denial) was shown to be a significant moderator and mediator of the relationship between cognitive function and depression.13 Thus, interventions that promote active coping behavior may be successful in preventing or alleviating depression in some people with MS. Effective coping and a sense of well-being and empowerment can accrue from spirituality.47 Spirituality is considered an individual's search for meaning and purpose in life47 based on belief in and a feeling of interconnectedness with a power greater than self.48
Adequate dietary intake is essential for optimal health in all individuals.15 Unfortunately, overweight is prevalent among people with MS,49 as is malnutrition.50 Malnutrition is associated with impairment of the immune system, and it affects mental function and respiratory muscle strength as well as increasing the risk of specific nutrient deficiencies.16
In summary, many Physical, Relationships, Energy, and Cognitive/Mental conditions can individually or interactively affect the health and functioning of people with MS. Thus, it is important for health-care providers to obtain a full picture of the MS patient's health status and functional level by partnering with the patient to optimize his or her health. Patient self-monitoring of health status can help achieve this goal.
Additional support for the construct validity of the MMMS total scale and the Physical and Cognitive/Mental subscales was shown by the finding that lower MMMS scores were related to higher PDDS scores, indicating that increased disability is associated with worsening of both Physical and Cognitive/Mental functions and conditions. The finding of no difference in scores on the Relationships subscale between PDDS groups suggests that relationships with spouse, signifi-cant other, family, and friends were relatively unaffected by disease severity; in fact, the mean scores on the Relationships subscale for all three PDDS groups were higher than 3.0, exceeding the mean scores on the Physical, Energy, and Cognition/Mental subscales. A factor that may have contributed to the lower Energy and Cognition/Mental subscale scores for the moderately disabled group compared with the severely disabled group was the composition of the PDDS 6–8 group with respect to MS classification: of the 35 group members, 17 (48.6%) had RRMS and 18 (51.4%) had SPMS. Additional research is needed to confirm or refute these findings and interpretations.
The finding of significantly lower mean scores on the MMMS total scale and the Physical subscale for SPMS participants compared with RRMS participants further supports the construct validity of the MMMS scale. As expected, those with RRMS reported better health status than did those with SPMS. Again, the scores on the Relationships subscale did not differ significantly by MS classification, nor did scores on the Energy and Cognitive/Mental subscales. The fact that mean scores on the Energy and Cognitive/Mental subscales differed significantly by PDDS group but not by MS disease classification may be due to the purposes of the respective categorization methods. The PDDS largely measures mobility function,11 rather than disease course. Although there is not 100% agreement among MS clinicians regarding the definitions of RRMS and SPMS, RRMS generally indicates disease relapses with full recovery or with sequelae and residual deficit upon recovery, while SPMS indicates progression with or without occasional relapses, minor remissions, and plateaus.2 Some of the study participants may have been undergoing a transition in disease classification from RRMS to SPMS. Also, as noted previously, the PDDS 6–8 group had similar percentages of RRMS and SPMS participants (48.6% and 51.4%, respectively).
Limitations of the present study must be considered. The data were obtained through MS patient self-report, and were not validated by clinical assessments performed by MS professionals. However, the measures either were previously validated (PDDS) or demonstrated satisfactory reliability and validity in the current study (MMMS). Although the sample of participants with SPMS was considerably smaller (n = 34) than the sample with RRMS (n = 130), the sample proportions of RRMS (79%) and SPMS (21%) reflect those found in the total MS population. Given the fairly high educational level of the study participants and the fact that the vast majority were white, the generalizability of the study findings may be limited to MS patients with these characteristics. Further research should be performed involving MS patients with a broader range of education levels and ethnicities. Moreover, the MMMS should undergo ongoing psychometric testing by comparison with other scales designed to measure the functioning of MS patients, as well as testing for scale stability over a 2-to 3-week period.
Conclusion
The MMMS demonstrates satisfactory reliability and validity for use among relatively well educated and white MS patients. Patient self-monitoring using this scale can promote partnership between MS patients and their health-care providers to achieve optimal patient health and activity. Information collected using the scale may facilitate health-care providers' assessment of the patient's condition and guide the selection of treatments and interventions. Further psychometric testing using samples with more varied ethnicities and education levels is recommended.
PracticePoints.
Self-monitoring of health status enables patients to determine which treatments and interventions are successful and whether their health is improving, remaining the same, or worsening. In MS, such self-monitoring can help motivate the patient to take an active role in managing the disease.
Sharing patient health status information from self-monitoring with health-care providers can enhance the providers' understanding of the patients' needs in terms of diagnostic tests and treatments.
The Monitoring My Multiple Sclerosis patient-administered health-assessment scale can provide important health status information to both the MS patient and his or her health-care providers, helping to optimize patient care.
Acknowledgments
The authors would like to acknowledge the following MS nurses for their recruitment of MS patients into the study: Patricia Blake, RN, BSN, CMSN, MSCN, Oak Clinic for Multiple Sclerosis (Uniontown, OH, USA); Maria Buccilli, RN, Mellen Center for Multiple Sclerosis Treatment and Research (Cleveland, OH, USA); Jane Iverson, RN, MSCN, Multiple Sclerosis Treatment and Research Center at the Minneapolis Clinic of Neurology (Minneapolis, MN, USA); Lori Mayer, RN, MSCN, CCRP, MS Clinic of Central Texas (Round Rock, TX, USA); Marie Namey, RN, MSN, MSCN, Mellen Center for Multiple Sclerosis Treatment and Research (Cleveland, OH, USA); Carol Saunders, RN, BSN, MSCN, Neurology Center of Fairfax (Fairfax, VA, USA).
Footnotes
Financial Disclosures: Dr. Gulick has no conflicts of interest to disclose. Ms. Namey has served as a paid consultant and speaker for Biogen Idec, Teva Neuroscience, EMD Serono, Acorda Therapeutics, Pfizer, Questcor Pharmaceuticals, and Novartis Pharmaceuticals and is on the advisory board of Genzyme. Ms. Halper has served as a paid consultant to Questcor Pharmaceuticals, Biogen Idec, Acorda Therapeutics, and Novartis Pharmaceuticals and is on the speakers' bureau of Acorda Therapeutics.
Funding/Support: This study was funded by a grant from Genentech to the International Organization of Multiple Sclerosis Nurses (IOMSN).
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 3.Weinshenker BG, Bass B, Rice GPA. The natural history of multiple sclerosis: a geographically based study. Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. et al. [DOI] [PubMed] [Google Scholar]
- 4.Sandman L, Munthe C. Shared decision-making and patient autonomy. Theor Med Bioeth. 2009;30:289–310. doi: 10.1007/s11017-009-9114-4. [DOI] [PubMed] [Google Scholar]
- 5.Gulick EE. Self-assessed health and use of health services. West J Nurs Res. 991;13:195–219. doi: 10.1177/019394599101300204. [DOI] [PubMed] [Google Scholar]
- 6.Ross AP, Hackbarth N, Rohl C, Whitmyre K. Effective multiple sclerosis management through improved patient assessment. J Neurosci Nurs. 2008;40:150–157. doi: 10.1097/01376517-200806000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36), part I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 8.Vickrey BG, Hays RD, Harooni R. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. et al. [DOI] [PubMed] [Google Scholar]
- 9.Fischer JS, LaRocca NG, Miller DM. Recent developments in the assessment of quality of life in multiple sclerosis (MS) Mult Scler. 1999;5:251–259. doi: 10.1177/135245859900500410. et al. [DOI] [PubMed] [Google Scholar]
- 10.Cella DF, Dineen K, Arnason B. Validation of the Functional Assessment of Multiple Sclerosis quality of life instrument. Neurology. 1996;47:129–139. doi: 10.1212/wnl.47.1.129. et al. [DOI] [PubMed] [Google Scholar]
- 11.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124:962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 12.Woods TE, Ironson GH. Religion and spirituality in the face of illness. J Health Psychol. 1999;4:393–412. doi: 10.1177/135910539900400308. [DOI] [PubMed] [Google Scholar]
- 13.Rabinowitz AR, Arnett PA. A longitudinal analysis of cognitive dys-function, coping, and depression in multiple sclerosis. Neuropsychol. 2009;23:581–591. doi: 10.1037/a0016064. [DOI] [PubMed] [Google Scholar]
- 14.Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler. 2006;12:481–486. doi: 10.1191/135248506ms1320oa. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans 2010. www.dietaryguidelines.gov. Accessed February 16, 2011.
- 16.Habek M, Hojsak I, Brinaar VV. Nutrition in multiple sclerosis. Clin Neurol Neurosurg. 2010;112:616–620. doi: 10.1016/j.clineuro.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28:363–379. doi: 10.2165/11532230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Trisolini M, Honeycutt A, Wiener J, Lesesne S. Global economic impact of multiple sclerosis. www.Global-economic-impact-of-MS-FINAL[1].pdf. Accessed February 16, 2011.
- 19.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Patterson MB, Whitehouse PJ, Edland SD. ADCS prevention instrument project: Quality of Life assessment (QOL) Alzheimer Dis Assoc Disord. 2006;20(suppl 3):S179–S190. doi: 10.1097/01.wad.0000213874.25053.e5. et al. [DOI] [PubMed] [Google Scholar]
- 21.Gulick EE, Halper J, Harris C. Quality-of-life multiple sclerosis scale: reliability and validity [CMSC abstract S26] Int J MS Care. 2008;10(suppl 1):18. et al. [Google Scholar]
- 22.Vollmer TI, Ni W, Stanton S, Hadjimichael O. The NARCOMS patient registry: a resource for investigators. Int J MS Care. 1999;1:12–15. [Google Scholar]
- 23.Kurtzke JF. Neurologic impairment in multiple sclerosis and the Disability Status Scale. Acta Neurol Scand. 1970;46:493–512. doi: 10.1111/j.1600-0404.1970.tb05808.x. [DOI] [PubMed] [Google Scholar]
- 24.Hadjimichael O. The NARCOMS registry data. Paper presented at: 18th Annual Meeting of the Consortium of Multiple Sclerosis Centers; June 2-6, 2004; Toronto, Ontario, Canada.
- 25.Gulick EE. Model for predicting work performance among persons with multiple sclerosis. Nurs Res. 1992;41:266–272. [PubMed] [Google Scholar]
- 26.Gulick EE. Symptom and activities of daily living trajectory in multiple sclerosis: a 10-year study. Nurs Res. 1998;47:137–146. doi: 10.1097/00006199-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hennessey A, Robertson NP, Swingler R, Compston DAS. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. J Neurol. 1999;246:1027–1032. doi: 10.1007/s004150050508. [DOI] [PubMed] [Google Scholar]
- 28.Nortvedt MW, Riise T, Frugard J. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–112. doi: 10.1177/1352458506071210. et al. [DOI] [PubMed] [Google Scholar]
- 29.Nordenbo AM, Andersen JR, Andersen JT. Disturbances of anorectal function in multiple sclerosis. J Neurol. 1996;243:445–451. doi: 10.1007/BF00900497. [DOI] [PubMed] [Google Scholar]
- 30.Gulick EE. Comparison of prevalence, related medical history, symptoms, and interventions regarding bowel dysfunction in persons with multiple sclerosis. J Neurosci Nurs. 2010;42:E12–E23. [Google Scholar]
- 31.Bakke A, Myhr K, Gronning M, Nyland H. Bladder, bowel, and sexual dysfunction in patients with multiple sclerosis—a cohort study. Scand J Urol Nephrol. 1996;179(suppl):61–66. [PubMed] [Google Scholar]
- 32.Gulick EE. Social support among persons with multiple sclerosis. Res Nurs Health. 1994;17:195–206. doi: 10.1002/nur.4770170307. [DOI] [PubMed] [Google Scholar]
- 33.Krokavcova M, Dijk JP, Nagyova I. Social support as a predictor of perceived health status in patients with multiple sclerosis. Patient Educ Counsel. 2008;73:159–165. doi: 10.1016/j.pec.2008.03.019. et al. [DOI] [PubMed] [Google Scholar]
- 34.Kleiboer AM, Kuijer RG, Hox JJ. Daily negative interactions and mood among patients and partners dealing with multiple sclerosis (MS): the moderating effects of emotional support. Soc Sci Med. 2007;64:389–400. doi: 10.1016/j.socscimed.2006.07.016. et al. [DOI] [PubMed] [Google Scholar]
- 35.Johansson S, Ytterberg C, Claesson IM. High concurrent presence of disability in multiple sclerosis: associations with perceived health. J Neurol. 2007;254:767–773. doi: 10.1007/s00415-006-0431-5. et al. [DOI] [PubMed] [Google Scholar]
- 36.Olsson M, Lexell J, Soderberg S. The meaning of fatigue for women with multiple sclerosis. J Adv Nurs. 2005;49:7–15. doi: 10.1111/j.1365-2648.2004.03258.x. [DOI] [PubMed] [Google Scholar]
- 37.Vucic S, Burke D, Kiernan MC. Fatigue in multiple sclerosis: mechanisms and management. Clin Neurophys. 2010;121:809–817. doi: 10.1016/j.clinph.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008;14:1127–1130. doi: 10.1177/1352458508092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tachibana N, Howard RS, Hirsch NP, Miller DH, Moseley IF, Fish D. Sleep problems in multiple sclerosis. Eur Neurol. 1994;34:320–323. doi: 10.1159/000117070. [DOI] [PubMed] [Google Scholar]
- 40.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, part I: frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 41.Rao SM. Cognitive function in patients with multiple sclerosis: impairment and treatment. Int J MS Care. 2004;6:9–22. [Google Scholar]
- 42.Winkelmann A, Engel C, Apel A, Zettl UK. Cognitive impairment in multiple sclerosis. J Neurol. 2007;254(suppl 2):11/35–42. doi: 10.1007/s00415-007-2010-9. [DOI] [PubMed] [Google Scholar]
- 43.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RG, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 45.Figved N, Benedict R, Klevan G. Relationship of cognitive impairment to psychiatric symptoms in multiple sclerosis. Mult Scler. 2008;14:1084–1090. doi: 10.1177/1352458508092262. et al. [DOI] [PubMed] [Google Scholar]
- 46.Arnett PA, Higginson CI, Wurst JM. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychol. 1999;13:434–446. doi: 10.1037//0894-4105.13.3.434. et al. [DOI] [PubMed] [Google Scholar]
- 47.McBrien B. A concept analysis of spirituality. Br J Nurs. 2006;15:42–45. doi: 10.12968/bjon.2006.15.1.20309. [DOI] [PubMed] [Google Scholar]
- 48.Haase JE, Britt T, Coward DD. Simultaneous concept analysis of spiritual perspective, hope, acceptance, and self-transcendence. Image: J Nurs Scholar. 1992;24:141–147. doi: 10.1111/j.1547-5069.1992.tb00239.x. et al. [DOI] [PubMed] [Google Scholar]
- 49.Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47–57. [Google Scholar]
- 50.Goldberg JP, Belury MA, Elam P. The obesity crisis: don't blame it on the Pyramid. J Am Diet Assoc. 2004;104:1141–1147. doi: 10.1016/j.jada.2004.04.026. et al. [DOI] [PubMed] [Google Scholar]


