Abstract
It has recently been suggested that the Lublin-Reingold clinical classification of multiple sclerosis (MS) be modified to include the use of magnetic resonance imaging (MRI). An international consensus conference sponsored by the Consortium of Multiple Sclerosis Centers (CMSC) was held from March 5 to 7, 2010, to review the available evidence on the need for such modification of the Lublin-Reingold criteria and whether the addition of MRI or other biomarkers might lead to a better understanding of MS pathophysiology and disease course over time. The conference participants concluded that evidence of new MRI gadolinium-enhancing (Gd+) T1-weighted lesions and unequivocally new or enlarging T2-weighted lesions (subclinical activity, subclinical relapses) should be added to the clinical classification of MS in distinguishing relapsing inflammatory from progressive forms of the disease. The consensus was that these changes to the classification system would provide more rigorous definitions and categorization of MS course, leading to better insights as to the evolution and treatment of MS.
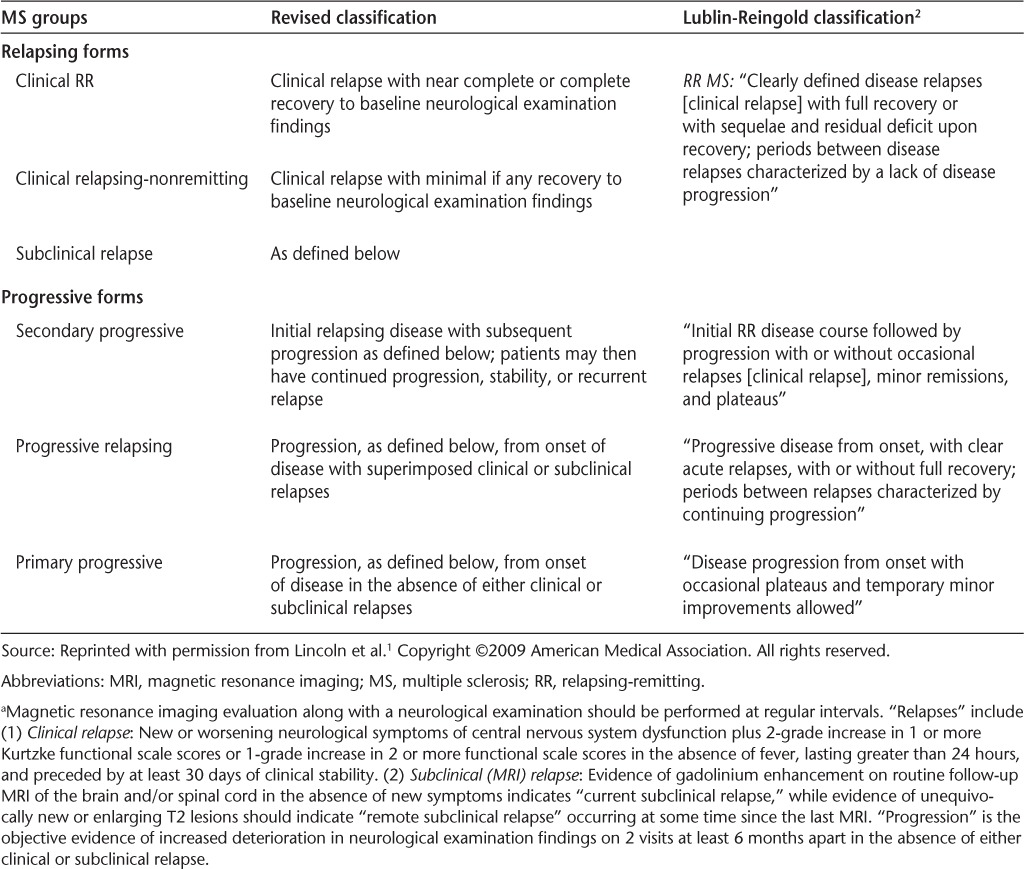
It was recently suggested by Lincoln et al.1 that the Lublin-Reingold clinical classification of multiple sclerosis (MS)2 for the assessment of MS phenotypes and patient evolution over time be modified to include magnetic resonance imaging (MRI). It was recommended that the classification incorporate the “conventional,” generally available MRI techniques of gadolinium (Gd) T1-weighted sequences and dual-echo and fluid-attenuated inversion recovery (T2-weighted and FLAIR) images (Table 1). It was recognized that other MRI modalities that are not currently widely available and other validated biomarkers might be added to the classification in the future.
Table 1.
Definitions of relapse by both clinical and MRI measures a
In response to the publication of Lincoln et al.,1 the Consortium of Multiple Sclerosis Centers (CMSC) sponsored an international consensus conference, which was held in Short Hills, New Jersey, from March 5 to 7, 2010. Participating in the conference were 28 invited MS experts from North and South America and Europe who were well versed in clinical trials, the management of MS, biostatistics, neuropathology, neuroimaging, and neuroimmunology. The goal of the meeting was to review the available evidence on the need for modification of the Lublin-Reingold criteria and whether addition of MRI and other biomarkers as proposed by Lincoln et al.1 would lead to a better understanding of MS pathophysiology and disease course over time and, in turn, more informed clinical trials and a better understanding of appropriate therapies.
Prior to the meeting, a survey of members of the CMSC was conducted on the need to modify the Lublin-Reingold classification. Over a 1-week period, 141 responses were received, representing 16% of the CMSC members polled. The results were as follows: A total of 70% of respondents indicated that the Lublin-Reingold classification did not sufficiently distinguish the different forms of MS; 87% felt that the Lublin-Reingold classification did not sufficiently distinguish MS disease activity even within a given category of the disease; and 84% indicated that it would be useful to include certain subclinical indices of disease activity in the clinical classification, such as MRI gadolinium-enhancing (Gd+) T1-weighted lesions or unequivocally new or enlarging T2-weighted lesions. Although this poll reflected the opinions of a relatively small proportion of the CMSC membership over a short period of time, the results supported the concept that changes to the Lublin-Reingold classification should be considered.
Presentations at the meeting included an overview of MRI in various categories of MS, from clinically isolated syndrome (CIS) to primary progressive MS (PPMS) (Li, Arnold); the pathology of MS and of MRI lesions from CIS to PPMS (Moore, Lucchinetti); a historical review of diagnostic and clinical classifications of MS as well as neuromyelitis optica (NMO), radiologically isolated syndrome (RIS), and CIS (Lublin, Kinkel); possible explanations for the so-called MRI-clinical paradox (Miller, Simon); recent evidence supporting a relationship between MRI and clinical endpoints (Sormani, Cutter); the concept of the disease-free state (Giovannoni, Naismith); the status of biomarkers for MS clinical activity (Dhib-Jalbut, Cadavid); and a proposal for modifying the Lublin-Reingold classification (Cook, Lisak). These presentations and reviews with updates are summarized below.
Background
Historical Evolution of MS Diagnostic Criteria
Criteria for establishing the diagnosis of MS have evolved considerably over almost 50 years. The Schumacher Committee recommendations issued in 1965 were purely clinical,3 while the Poser Committee revision of 1983 incorporated laboratory as well as clinical criteria.4 Most recently, the various iterations of the McDonald Committee criteria (2001, 2005, 2010) used MRI (Gd+ lesions, new T2 lesions) and other laboratory findings in addition to purely clinical criteria for disease diagnosis.5–7 The occurrence of at least two relapses and dissemination of lesions in space are fundamental to the diagnosis of relapsing-remitting MS (RRMS); however, conventional MRI (Gd+ lesions, new T2 lesions) has become a surrogate for the second relapse and dissemination of disease in space and time. New MRI lesions have expedited the diagnostic evolution of CIS to definite RRMS,5–7 resulting in earlier diagnosis and treatment of MS.8,9 Whether MRI can be used as a marker for the first subclinical attack of MS in RIS, as for the second attack in diagnosing MS, is under consideration.10,11
Historical Analysis and Critique of the Lublin-Reingold Criteria
The Lublin-Reingold MS classification has made important contributions to the definition of various MS phenotypes and classical stages on purely clinical grounds. The classification consists of four categories of MS: 1) relapsing-remitting MS (RRMS), characterized by clearly defined episodes of acute neurologic worsening with a variable degree of recovery and a stable course between attacks; 2) secondary progressive MS (SPMS), in which one or more relapses are followed by mainly progressive deterioration, possibly with some plateaus, new relapses, and even slight or transient improvement over time; 3) primary progressive MS (PPMS), consisting of continuous, often slow progression from the onset of symptoms, possibly with some plateauing without obvious relapses; and 4) progressive relapsing MS (PRMS), in which progression occurs from the onset of neurologic symptoms, with occasional relapses thereafter. Whether the latter is really a distinct category or represents a variant of relapsing or another progressive form of MS is unclear. Moreover, it may be the case that each of these categories is actually heterogeneous, with mild clinical or subclinical relapses not being adequately recognized.1,12–14 Despite these limitations, the Lublin-Reingold classification has facilitated patient selection for enrollment in clinical trials and determination of which patients may be most likely to respond to approved drugs.
A potential shortcoming of the Lublin-Reingold classification is that it is often difficult to determine the transition from a relapsing to a progressive form of the disease on purely clinical grounds.15 Using MRI metrics to assess subclinical disease activity, in addition to diagnosing disease, might identify patients with subclinical relapses rather than true progression. A purely clinical classification depends heavily on the patient history, which may not always be a sensitive or accurate measure, particularly over long periods of time. Progression of MS may also be difficult to assess by treating physicians because of inter- and intra-examiner variability, limitations in the accuracy and comprehensiveness of the Expanded Disability Status Scale (EDSS) as a measurement tool, the slow rate of deterioration in some 2-year clinical trials, and other possible factors such as medication side effects, lack of sleep, depression, and time of day.16–18 Furthermore, even sustained EDSS progression in research studies may reverse after study completion.19
Moreover, it is likely that an inflammatory component of disease is present either always or at some time in all stages of MS.20–24 Indeed, Gd+ lesions, which indicate both an abnormality in blood-brain barrier (BBB) and central nervous system (CNS) inflammation, can occur not only in RRMS but in all Lublin-Reingold forms of MS, including in 14.1% to 42% of patients with PPMS.12–14 Pathologically inflammatory CNS lesions have also been described in all forms of MS, with or without the presence of Gd+ lesions, although the distribution of inflammatory lesions can vary in location or intensity in different forms of the disease.20–24
When sensitive and frequent MRI studies are performed, Gd+ and unequivocally new or enlarging T2 brain lesions (subclinical relapses) can occur much more commonly than clinical symptoms (relapses or progression).25–28 This may be explained at least in part by several factors: lesions in “eloquent” brain areas such as the optic nerves are more likely to be clinically expressed than lesions in less eloquent pathways; the degree of matrix and axonal destruction may vary; and the potential exists for rapid symptom recovery.29–38 Even patients with progressive forms of MS, particularly those with Gd+ lesions indicating a subclinical relapse, may respond clinically to disease-modifying therapies (DMTs). For example, in a post hoc analysis of the Olympus trial of rituximab in PPMS, patients with Gd+ lesions had a significantly better response to treatment than those without Gd+ lesions.13
In summary, magnetic resonance imaging is the “gold standard” for evaluating drugs in phase 2 trials and is often used by clinicians, along with assessment of the presence of clinical relapses and progression, in making therapeutic decisions. Indeed, “freedom from disease activity” assessed both clinically and using MRI is increasingly regarded as an optimal outcome by which to judge the effectiveness of therapy.39,40
Relationship of MRI to CNS Pathology
A limited number of biopsy and necropsy studies have been carried out involving Gd+ T1-weighted lesions, T2-weighted lesions, and T1 hypointense lesions or “black holes” in an attempt to correlate CNS pathology with identified MRI abnormalities.41–48 In considering the histologic characteristics of classic MS MRI lesions, it is important to recognize that when frequent, sensitive longitudinal (weekly or monthly)37,49 MRI studies are performed in RRMS patients, almost all new T2 lesions (95–100%) as well as acute, transient, and chronic black holes (those lasting for more than 12 months) occur coincidentally with or evolve from Gd+ T1-weighted lesions.37,49 However, Gd+ lesions evolve into chronic black holes less commonly than they do into T2 lesions or transient black holes.37 This indicates that unequivocally (not related to technique, positioning, quality of scan, or questionable reader interpetation) new T2 lesions or black holes in RRMS patients had a prior inflammatory phase with alteration of the BBB of a degree sufficient to be detected, even though this may not always be documentable with less frequent or sensitive MRI.37
It has been shown that T2 lesions have heterogeneous pathology, with varying degrees of abnormality ranging from edema and inflammation with phagocytosis of myelin, to demyelination, gliosis, and axonal loss, which can be mild to severe.41–48 However, it is generally recognized that the underlying pathology responsible for enhancement is inflammation with accompanying BBB breakdown. In contrast to T2 lesions, it has been reported that T1 hypointensities or black holes, particularly chronic black holes, correlate better with physical disability, tissue injury including severe demyelination and axonal injury, and brain atrophy.42,45,50–52 Recent evidence suggests that the size and duration of enhancing lesions, increased radial diffusion on diffusion tensor imaging (DTI), and lower magnetic transfer ratio (MTR) predispose to chronic black holes, matrix destruction, and brain atrophy.34,45,46,53–60 Some controversy remains as to the degree of inflammation found in MS cortical lesions as compared with white matter lesions on histologic examination, but these differences are probably related to stage of MS (early vs. chronic disease).61–63
Newer MRI techniques, currently experimental in nature, are giving us a glimpse of what these modalities may tell us in the future about MRI brain pathology and clinical relevance. These include magnetic transfer imaging (MTI), DTI, proton MRI spectroscopy, functional MRI (fMRI), double inversion recovery (DIR) MRI, subtraction techniques, and ultra-high-field MRI scanners.64–66 For example, the degree of increase in fMRI activity has been reported to be proportional to the sensitivity of T2 lesion load up to a critical limit, at which time these important compensatory mechanisms (ie, plasticity) fail.34 Gray matter lesion conspicuity is increased with DIR techniques as well as with ultra-high-field MRI scanners.67,68 The latter may even allow one to identify remyelination and distinguish perivenous lesions caused by demyelinating diseases from those caused by other neurologic disorders.69,70 There is also recent evidence that focal MRI white matter lesions in the optic radiations may lead to retrograde gray matter atrophy in the lateral geniculate nucleus.71 Even in normal-appearing white matter, abnormalities have been detected with DTI, MTI, and pathologic analysis that may be associated with clinical and cognitive worsening.72–74
In summary, a limited number of studies of classic MRI abnormalities show CNS pathology, with virtually all MRI lesions being associated with CNS injury of varying degrees. It is hoped that other easily obtained biomarkers will also emerge over time,75 particularly as the etiology and pathophysiology of the disease become better understood.
Relationship Between MRI Disease Activity and MS Clinical Course: The MRI-Clinical Paradox
Although currently Gd+ and new or enlarging T2 lesions are the standard conventional MRI features used in screening for therapeutic efficacy and are important secondary outcome measures in phase 3 trials, their relationship to clinical prognosis has been less robust than anticipated.76–83 This has led to the concept of a mismatch between MRI findings and clinical outcome, referred to as the “MRI-clinical paradox.” In fact, some researchers have suggested that, given the absence of a clear correlation between presence of Gd+ or T2 lesions and clinical findings, MRI evidence of inflammation may not be of great value as a surrogate for relapses, progression in disability, or the need to reconsider choice of therapeutic drugs.19,77–83 However, many plausible reasons can be put forward to explain why the MRI-clinical paradox may no longer be the enigma it once was, and indeed the paradigm is now shifting.31 For example, mild clinical relapses may not be documentable if they do not meet established EDSS or Functional Systems Scale (FSS) criteria, patients do not bring them to medical attention, or they occur in less eloquent brain areas. Subclinical MRI exacerbations are quite common, being detected up to 10 times more frequently than clinical symptoms or findings in RRMS patients.25–28,49
Further, as previously indicated, progression can be difficult to assess.16–18 The rate of deterioration in patients with established disability may be slow, in both relapsing and progressive forms of MS18,19,31; the EDSS is a relatively insensitive barometer of progression; and pseudoprogression may occur, making it difficult to accurately assess stability, worsening, or even mild improvement over the short term.16,17,19 Progression of cognitive dysfunction is more problematic to clearly document, by either routine clinical or routine imaging metrics. Mild-to-moderate cognitive impairment may not be readily detected even by competent physicians or on EDSS testing, which is insensitive to such changes,16–18 nor can cortical gray matter lesions be easily detected with conventional MRI techniques.67,68 However, cortical lesions are much more commonly detected pathologically and on MRI with DIR and high-field scans and documented much more easily with neuropsychological testing, even early in the patient's course.18,74,84–86
Furthermore, in both clinical practice and study trials, neither frequent brain MRI scans nor even infrequent spinal cord MRI scans are regularly obtained, so that many overt MRI lesions in the neuraxis can be missed, and previously hypointense or hyperintense lesions can become isointense and be difficult to detect unless prior MRI studies have been done.18,87,88 In such instances, structural damage may still be identified with DTI or MTI in former T2 lesions or black holes that have subsequently become isointense.87,88
Lastly, although most if not all focal Gd+, T2, and T1 hypointense lesions may cause damage to the brain parenchyma, there may be sufficient reserves in neural pathways—with redundancies in critical thresholds of axonal function, or even compensatory mechanisms including plasticity and regeneration (particularly early in the course of MS)—so that new neurologic deficits or progression in disability may not be readily seen.19,31,89 Ultimately, however, sufficient axonal loss may accumulate over time (albeit at a unique rate in each patient) so that deterioration occurs, even when overt new MRI lesions are not identified.89
All of the above could decrease MRI lesion conspicuity on conventional scanning and affect the correlation of MRI lesions with clinical phenotypes, relapses, and progression.
Evidence That the MS MRI-Clinical Paradox Is Disappearing
Individual MRI-Clinical Studies
The relevance of MRI-clinical relationships has changed remarkably in recent years.31 A large and growing number of reports from observational, retrospective, randomized, and prospective studies show a much stronger correlation than was found previously between MS MRI and clinical disease activity.18,59,90–100 For example, RRMS and CIS patients with elevated Gd+ and T2 lesion activity, in terms of number or volume of lesions before or after therapy, have a greater likelihood of subsequent clinical relapses, disability progression, cognitive impairment, decreased quality of life, and even death as compared with patients with lower or absent MRI activity.18,59,90–100 Although most of these studies have been relatively short term, some have followed study cohorts for 20 or more years.100–102
Patterns of MRI Activity and Clinical Course
In the BECOME study, a sensitive monthly (for up to 24 months) MRI technique (3-T scanner, up to a 40-minute delay in postcontrast imaging, and triple-dose Gd administration, all of which increase lesion conspicuity)102–105 was used along with periodic clinical and cognitive assessments to study patients with early MS and CIS randomized to interferon beta-1b (IFNβ-1b) or glatiramer acetate (GA).18,26,37 Scans were obtained at baseline before patients were started on IFNβ-1b or GA and regularly thereafter. On post hoc analysis, three categories of MRI patterns were apparent.18 One patient group never had Gd+ or new T2 lesions (23.2%); another group had continuous Gd+ or new T2 lesions with the exception of at most 1 inactive month between active MRI months (30.4%); and a third group had an intermediate pattern of MRI monthly activity (46.4%). Overall, no clinical relationship was found in this relatively small number of study patients between MRI pattern and drug treatment. However, subset analysis showed that patients with active MRI patterns had a higher relapse rate, greater sustained worsening in the Timed 25-Foot Walk, and more cognitive impairment—the latter both at baseline and subsequently during the study—than those without active MRIs. This is the first time that such clinical outcomes have been so clearly related to MRI patterns; moreover, it is remarkable that this could be seen with so few patients, all of whom were on therapy. In addition, 75% to 80% of patients had MRI activity at some point during the BECOME study.26 This reinforces that what we see in clinical practice or during clinical trials using infrequent, less sensitive MRIs represents just a fraction of what is actually occurring in the neuraxis.
Meta-analysis of Large Randomized MS Trials
Until recently, attempts to show that conventional MRI is a surrogate for clinical relapses and progression were limited and not very robust.76–82 This situation started to change when it was shown that MRI reflected not only pathology but also clinical prognosis and response to therapy. Subsequently, a meta-analysis of all large randomized placebo-controlled studies of patients with RRMS was carried out by Sormani et al.106–109 In the initial study, 23 trials involving 6591 patients were analyzed. It was demonstrated that the therapeutic response effect on new MRI T2 lesions correlated strongly with the effect of drugs on relapses (adjusted R2 = 0.81). In a second study, these investigators carried out a similar analysis of 11 trials involving 10,009 patients with complete data on MRI and disability progression. This meta-analysis also showed a significant correlation of MRI response to therapy with effect on disability progression (adjusted R2 = 0.57) over the 2 to 3 years of the study. These and two subsequent studies based on individual patient analysis showed that MRI markers satisfy the rigorous Prentice criteria for consideration as a valid surrogate marker in group studies of RRMS.108–110 In considering the impact of these studies, it is important to remember that while one may not always see a robust relationship between MRI and clinical course in individual study patients, neither does one always see a robust effect of treatment with a drug that has been proven clinically effective in a cohort of patients in all individual members of the cohort.
At present, sophisticated research MRI studies such as fMRI, MTI, DTI, spectroscopy, and the use of stronger magnets or other MRI outcomes are not being considered for incorporation into the current Lublin-Reingold clinical classification because they are not generally available for use in clinical practice and do not show clear differences in MRI subclinical relapses, including alteration of BBB and inflammation, between Lublin-Reingold patient categories. For the same reasons, we did not take into consideration data on brain atrophy for this discussion on clinical classification.
Discussion
After the presentations further group discussions were held (Durelli, Traboulsee), as well as a final discussion to review the evidence available and assess the desirability of incorporating standard MRI metrics into the current Lublin-Reingold criteria for MS disease classification. There was general agreement that conventional MRI activity including Gd+ lesions or unequivocally new or enlarging T2 lesions (activity not due to marginally enlarging lesions or related to technique, positioning, quality of scan, or questionable reader interpretation) indicates contemporary or prior inflammation, respectively, associated with CNS tissue damage; that the effect of therapy on new lesions correlates with the effect on relapses in RRMS; and that MRI is a surrogate for relapses in these studies. It was agreed that conventional MRI is readily available and should be performed according to consensus guidelines.111 Lastly, it was also agreed that the clinical classification for distinguishing between relapsing and progressive forms of MS should be supplemented with MRI findings.
Conclusion
At the end of the meeting, it was unanimously recommended that evidence of new MRI Gd+ lesions, and probably unequivocally new or enlarging T2 lesions (subclinical activity), should be added to the clinical classification of MS disease types, as suggested by Lincoln et al.1 There was also a strong consensus that Gd+ lesions and possibly unequivocally new or enlarging T2 lesions should be recognized as contemporary or prior subclinical relapses, respectively. It was felt that these changes would lead to a better understanding of the evolution of MS, with more rigorous definitions and categorization of MS course.
Further, it was felt that at the present time no available biomarker was superior to MRI in combination with clinical criteria for use in disease classification or distinguishing between relapsing and progressive forms of MS, although it was hoped that this situation would change in the future. Moreover, there was a consensus that, as with MS diagnostic criteria, the clinical classification likely would undergo periodic modification, particularly as metrics based on currently experimental quantitative measures become generally available and relevant.
PracticePoints.
The widely used Lublin-Reingold classification of MS disease course is based purely on clinical measures of disease activity.
An increasing body of literature shows a much stronger correlation between magnetic resonance imaging and MS clinical disease activity than was found in previous studies.
Thus magnetic resonance imaging should be used in the clinical classification of MS, particularly to distinguish inflammatory relapsing forms, which might be amenable to treatment, from progressive forms.
Footnotes
Consensus Conference Participants: Stuart D. Cook, MD (Chair), Douglas Arnold, MD, Diego Cadavid, MD, Gary Cutter, PhD, Suhayl Dhib-Jalbut, MD, FAAN, Peter Dowling, MD, Luca Durelli, MD, Corey Ford, MD, PhD, Gavin Giovannoni, MD, June Halper, MSN, APN-C, MSCN, FAAN, Colleen Harris, MN, NP, MSCN, Joseph Herbert, MD, R. Philip Kinkel, MD, David Li, MD, John A. Lincoln, MD, PhD, Robert Lisak, MD, Fred D. Lublin, MD, FAAN, Claudia F. Lucchinetti, MD, David Miller, MD, Robert T. Naismith, MD, Carlos Oehninger, MD, Jim Quinless, NP, Maria Sepulveda, MD, Jack Simon, MD, Maria Pia Sormani, PhD, Lael Stone, MD, Deborah Thurston, RN, Anthony Traboulsee, MD.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: The consensus conference and this resulting publication were supported through Foundation of the Consortium of Multiple Sclerosis Centers grants from Bayer HealthCare Pharmaceuticals, Biogen Idec, and Genzyme Corporation. Grant support for the conference only was provided by Teva Neuroscience.
References
- 1.Lincoln J, Cadavid D, Pollard J. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66:412–414. doi: 10.1001/archneurol.2009.26. et al. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher GA, Beebe G, Kebler RF. Problems of experimental trials of therapy in multiple sclerosis: report by the Panel on the Evaluation of Experimental Trials of Therapy in Multiple Sclerosis. Ann NY Acad Sci. 1965;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. et al. [DOI] [PubMed] [Google Scholar]
- 4.Poser CM, Paty DW, Scheinberg L. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. et al. [DOI] [PubMed] [Google Scholar]
- 5.McDonald WI, Compston DA, Edan G. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. et al. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Edan G. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. et al. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappos L, Polman CH, Freedman MS. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. et al. [DOI] [PubMed] [Google Scholar]
- 9.Kinkel RP, Kollman C, O'Connor P. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006;66:678–684. doi: 10.1212/01.wnl.0000200778.65597.ae. et al.; CHAMPIONS Study Group. [DOI] [PubMed] [Google Scholar]
- 10.Okuda DT, Mowry EM, Cree BAC. Asymptomatic spinal cord lesions precede disease progression in radiologically isolated syndrome. Neurology. 2011;76:686–692. doi: 10.1212/WNL.0b013e31820d8b1d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourdette D, Simon J. The radiologically isolated syndrome: it is very early multiple sclerosis. Neurology. 2009;72:780–781. doi: 10.1212/01.wnl.0000337255.89622.ce. [DOI] [PubMed] [Google Scholar]
- 12.Wolinsky JS, Narayana PA, O'Connor P. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61:14–24. doi: 10.1002/ana.21079. et al. [DOI] [PubMed] [Google Scholar]
- 13.Hawker K, O'Connor P, Freedman MS. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo controlled multicenter trial. Ann Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. et al.; for the OLYMPUS Trial Group. [DOI] [PubMed] [Google Scholar]
- 14.Ingle GT, Sastre-Garriga J, Miller DH, Thompson AJ. Is inflammation important in early PPMS? a longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2005;76:1255–1258. doi: 10.1136/jnnp.2004.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palace J. Clinical and laboratory characteristics of secondary progressive MS. J Neurol Sci. 2003;206:131–134. doi: 10.1016/s0022-510x(02)00419-7. [DOI] [PubMed] [Google Scholar]
- 16.Willoughby EW, Pary DW. Scales for rating impairment in multiple sclerosis: a critique. Neurology. 1988;38:1793–1798. doi: 10.1212/wnl.38.11.1793. [DOI] [PubMed] [Google Scholar]
- 17.Hobart J, Kalkers N, Barkhof F, Uitdehaag B, Polman C, Thompson A. Outcome measures for multiple sclerosis clinical trials: relative measurement precision of the Expanded Disability Status Scale and Multiple Sclerosis Functional Composite. Mult Scler. 2004;10:41–46. doi: 10.1191/1352458504ms983oa. [DOI] [PubMed] [Google Scholar]
- 18.Cadavid D, Kim S, Peng B. Clinical consequences of MRI activity in treated multiple sclerosis. Mult Scler. 2011;17:1113–1121. doi: 10.1177/1352458511405375. et al. [DOI] [PubMed] [Google Scholar]
- 19.Ebers GC, Heigenhauser L, Daumer M, Lederere C, Noseworthy JH. Disability as an outcome in MS clinical trials. Neurology. 2008;71:624–631. doi: 10.1212/01.wnl.0000313034.46883.16. [DOI] [PubMed] [Google Scholar]
- 20.Howell OW, Reeves CA, Nicholas R. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(pt 9):2755–2771. doi: 10.1093/brain/awr182. et al. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann H. Pathophysiology of inflammation and tissue injury in multiple sclerosis: what are the targets for therapy. J Neurol Sci. 2011;306:167–169. doi: 10.1016/j.jns.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Bramow S, Frischer JM, Laursen H, Koch-Henriksen N, Lassmann H, Sorensen PS. Progressive multiple sclerosis: inflammatory demyelination correlates with the rate of clinical progression prior to death. Paper presented at: 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); September 10, 2009; Dusseldorf, Germany.
- 23.Brick W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(suppl 5):v3–9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 24.Bradl M, Lassmann H. Progressive multiple sclerosis. Semin Immunopathol. 2009;31:455–465. doi: 10.1007/s00281-009-0182-3. [DOI] [PubMed] [Google Scholar]
- 25.Willoughby EW, Grochowski E, Li DKB, Oger J, Kastrukoff LF, Paty DW. Serial magnetic resonance scanning in multiple sclerosis: a second prospective study in relapsing patients. Ann Neurol. 1989;25:43–49. doi: 10.1002/ana.410250107. [DOI] [PubMed] [Google Scholar]
- 26.Cadavid D, Wolansky LJ, Skurnick J. Efficacy of treatment of MS with 1FNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72:1976–1983. doi: 10.1212/01.wnl.0000345970.73354.17. et al. [DOI] [PubMed] [Google Scholar]
- 27.Miller DH. Guidelines for MRI monitoring of the treatment of multiple sclerosis: recommendations of the US Multiple Sclerosis Society's task force. Mult Scler. 1996;1:335–338. doi: 10.1177/135245859600100610. [DOI] [PubMed] [Google Scholar]
- 28.McFarland HF, Frank JA, Albert PS. Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann Neurol. 1992;32:758–766. doi: 10.1002/ana.410320609. et al. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe JW, Kidd D, Moseley IF. Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing-remitting multiple sclerosis. Neurology. 1996;46:373–378. doi: 10.1212/wnl.46.2.373. et al. [DOI] [PubMed] [Google Scholar]
- 30.Bodini B, Battaglini M, De Stefano N. T2 lesions location really matters: a 10 year follow-up study in primary progressive multiple sclerosis. Neurol Neurosurg Psychiatry. 2011;82:72–77. doi: 10.1136/jnnp.2009.201574. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkhof F, Filippi M. MRI—the perfect surrogate marker for multiple sclerosis? Nat Rev Neurol. 2009;5:182–183. doi: 10.1038/nrneurol.2009.31. [DOI] [PubMed] [Google Scholar]
- 32.Youl BD, Turano G, Miller DH. The pathophysiology of acute optic neuritis: an association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114(pt 6):2437–2450. doi: 10.1093/brain/114.6.2437. et al. [DOI] [PubMed] [Google Scholar]
- 33.Vellinga MM, Geurts JJ, Rostrup E. Clinical correlations of brain lesion distribution in multiple sclerosis. J Magn Reson Imaging. 2009;29:768–773. doi: 10.1002/jmri.21679. et al. [DOI] [PubMed] [Google Scholar]
- 34.Traboulsee A. MRI relapses have significant pathologic and clinical implications in multiple sclerosis. J Neurol Sci. 2007;256(suppl 1):519–522. doi: 10.1016/j.jns.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 35.Chiaravalloti N, Wylie G, Leavitt V, DeLuca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol. 2012;259:1337–1346. doi: 10.1007/s00415-011-6353-x. [DOI] [PubMed] [Google Scholar]
- 36.Filippi M, Riccitelli G, Mattioli F. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures—an explorative study. Radiology. 2012;262:932–940. doi: 10.1148/radiol.11111299. et al. [DOI] [PubMed] [Google Scholar]
- 37.Cadavid D, Cheriyan J, Skurnick J, Lincoln JA, Wolansky LJ, Cook SD. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta-1b or glatiramer acetate. J Neurol Neurosurg Psychiatry. 2009;80:1337–1343. doi: 10.1136/jnnp.2008.171090. [DOI] [PubMed] [Google Scholar]
- 38.Bermel RA, Fox RJ. Picturing injury and recovery with diffusion sensor imaging: the eyes have it. Neurology. 2009;72:584–585. doi: 10.1212/01.wnl.0000336559.52816.16. [DOI] [PubMed] [Google Scholar]
- 39.Havrdova E, Galetta S, Hutchinson M. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. et al. [DOI] [PubMed] [Google Scholar]
- 40.Giovannoni G, Cook SD, Rammohan K. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10:329–337. doi: 10.1016/S1474-4422(11)70023-0. et al.; CLARITY study group. [DOI] [PubMed] [Google Scholar]
- 41.Nesbit GM, Forbes GS, Scheithauer BW, Okazaki H, Rodriguez M. Multiple sclerosis histopathologic and MR and/or CT correlation in 37 cases at biopsy and three cases at autopsy. Radiology. 1991;1880:467–474. doi: 10.1148/radiology.180.2.2068314. [DOI] [PubMed] [Google Scholar]
- 42.Maarouf M, Kuchta J, Miletic H. Acute demyelination: diagnostic difficulties and the need for brain biopsy. Acta Neurochir (Wien) 2003;145:961–969. doi: 10.1007/s00701-003-0113-3. et al. [DOI] [PubMed] [Google Scholar]
- 43.Bitsch A, Kuhlmann T, Stadelmann C, Lassmann H, Lucchinetti C, Bruck W. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol. 2001;49:793–796. doi: 10.1002/ana.1053. [DOI] [PubMed] [Google Scholar]
- 44.van Walderveen MA, Kamphorst W, Scheltens P. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. 1998;50:1282–1288. doi: 10.1212/wnl.50.5.1282. et al. [DOI] [PubMed] [Google Scholar]
- 45.De Groot CJ, Bergers E, Kamphorst W. Post-mortem MRI-guided sampling of multiple sclerosis brain lesions increased yield of active demyelinating and (p) reactive lesions. Brain. 2001;124:1635–1645. doi: 10.1093/brain/124.8.1635. et al. [DOI] [PubMed] [Google Scholar]
- 46.Van Waesberghe JH, Kamphorst W, De Groot CJ. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. et al. [DOI] [PubMed] [Google Scholar]
- 47.Nijeholt GJ, Bergers E, Kamphorst W. Post-mortem high-resolution MRI of the spinal cord in multiple sclerosis: a correlative study with conventional MRI, histopathology and clinical phenotype. Brain. 2001;124:154–166. doi: 10.1093/brain/124.1.154. et al. [DOI] [PubMed] [Google Scholar]
- 48.Moore GR, Leung E, MacKay AL. A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology. 2000;55:1506–1510. doi: 10.1212/wnl.55.10.1506. et al. [DOI] [PubMed] [Google Scholar]
- 49.Tortorella C, Codella M, Rocca MA. Disease activity in multiple sclerosis studied by weekly triple-dose magnetic resonance imaging. J Neurol. 1999;246:689–692. doi: 10.1007/s004150050433. et al. [DOI] [PubMed] [Google Scholar]
- 50.Truyen L, van Waesberghe JH, van Walderveen MA. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996;47:1469–1476. doi: 10.1212/wnl.47.6.1469. et al. [DOI] [PubMed] [Google Scholar]
- 51.Barkhof F, Karas GB, van Walderveen MA. T1 hypointensities and axonal loss. Neuroimaging Clin N Am. 2000;10:739–752. [PubMed] [Google Scholar]
- 52.Barkhof F, McGowan JC, van Waesberghe JH, Grossman RI. Hypointense multiple sclerosis lesions on T1-weighted spin echo magnetic resonance images: their contribution in understanding multiple sclerosis evolution. J Neurol Neurosurg Psychiatry. 1998;64(suppl 1):577–579. [PubMed] [Google Scholar]
- 53.Fox RJ, Cronin T, Lin J. Measuring myelin repair and axonal loss with diffusion tensor imaging. AJNR Am J Neuroradiol. 2011;32:85–91. doi: 10.3174/ajnr.A2238. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filippi M. Magnetization transfer imaging to monitor the evolution of multiple sclerosis. Ital J Neurol Sci. 1999;20(5 suppl):S232–S240. doi: 10.1007/s100729970003. [DOI] [PubMed] [Google Scholar]
- 55.Simon JH, Lull J, Jacobs LD. A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon beta-1a. Neurology. 2000;55:185–192. doi: 10.1212/wnl.55.2.185. et al.; Multiple Sclerosis Collaborative Research Group. [DOI] [PubMed] [Google Scholar]
- 56.Ciccarelli O, Giugni E, Paolillo A. Magnetic resonance outcome of new enhancing lesions in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 1999;6:455–459. doi: 10.1046/j.1468-1331.1999.640455.x. et al. [DOI] [PubMed] [Google Scholar]
- 57.Naismith RT, Xu J, Tutlam NT. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology. 2010;74:1694–1701. doi: 10.1212/WNL.0b013e3181e042c4. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rovira A, Alonso J, Cucurella G. Evolution of multiple sclerosis lesions on serial contrast-enhanced T1-weighted and magnetization-transfer MR images. AJNR Am J Neuroradiol. 1999;20:1939–1945. et al. [PMC free article] [PubMed] [Google Scholar]
- 59.Morgen K, Crawford AL, Stone RD. Contrast-enhanced MRI lesions during treatment with interferon beta-1b predict increase in T1 black hole volume in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2005;11:146–148. doi: 10.1191/1352458505ms1147oa. et al. [DOI] [PubMed] [Google Scholar]
- 60.Bagnato F, Jeffries N, Richert ND. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782–1789. doi: 10.1093/brain/awg182. et al. [DOI] [PubMed] [Google Scholar]
- 61.Luccinetti CF, Popescu BF, Bunyan RF. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2189. doi: 10.1056/NEJMoa1100648. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geurts JJ, Bö L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol. 2005;2:572–577. [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neuritis, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 64.Bakshi R, Thompson AJ, Rocca MA. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–626. doi: 10.1016/S1474-4422(08)70137-6. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filippi M, Rocca MA, De Stefano N. Magnetic resonance techniques in multiple sclerosis: the present and the future. Arch Neurol. 2011;68:1514–1520. doi: 10.1001/archneurol.2011.914. et al. [DOI] [PubMed] [Google Scholar]
- 66.Liguori M, Meier DS, Hildenbrand P. One year activity on subtraction MRI predicts subsequent 4 year activity and progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:1125–1131. doi: 10.1136/jnnp.2011.242115. et al. [DOI] [PubMed] [Google Scholar]
- 67.Geurts JJ, Pouwels PJ, Uitdehaag BM. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. et al. [DOI] [PubMed] [Google Scholar]
- 68.Mainero C, Benner T, Radding A. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;3:941–948. doi: 10.1212/WNL.0b013e3181b64bf7. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tallantyre EC, Dixon JE, Donaldson I. Ultra-high field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534–539. doi: 10.1212/WNL.0b013e31820b7630. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmierer K, Parkes HG, So PW. Direct visualization of remyelination in multiple sclerosis using T2-weighted high-field MRI. Neurology. 2009;72:472. doi: 10.1212/01.wnl.0000341878.80395.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sepulcre J, Goni J, Masdeu JC. Contribution of white matter lesions to gray matter atrophy in multiple sclerosis: evidence from voxel-based analysis of T1 lesions in the visual pathway. Arch Neurol. 2009;66:173–179. doi: 10.1001/archneurol.2008.562. et al. [DOI] [PubMed] [Google Scholar]
- 72.Parry A, Clare S, Jenkinson M, Smith S, Palace J, Matthews PM. White matter and lesion T1 relaxation times increase in parallel and correlate with disability in multiple sclerosis. J Neurol. 2002;249:1279–1286. doi: 10.1007/s00415-002-0837-7. [DOI] [PubMed] [Google Scholar]
- 73.Mistry N, Tallantyre EC, Dixon JE. Focal multiple sclerosis lesions abound in “normal appearing white matter.”. Mult Scler. 2011;17:1313–1323. doi: 10.1177/1352458511415305. et al. [DOI] [PubMed] [Google Scholar]
- 74.Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology. 2011;76:1161–1167. doi: 10.1212/WNL.0b013e318212a8be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graber JJ, Dhib-Jalbut S. Biomarkers of disease activity in multiple sclerosis. J Neurol Sci. 2011;305:1–10. doi: 10.1016/j.jns.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Rashid W, Davies GR, Chard DT. Relationship of triple dose contrast enhanced lesions with clinical measures and brain atrophy in early relapsing-remitting multiple sclerosis: a two-year longitudinal study. Mult Scler. 2007;13:178–185. doi: 10.1177/1352458506070758. et al. [DOI] [PubMed] [Google Scholar]
- 77.Daumer M, Neuhaus A, Morrissey S, Hintzen R, Ebers GC. MRI as an outcome in multiple sclerosis clinical trials. Neurology. 2009;72:705–711. doi: 10.1212/01.wnl.0000336916.38629.43. [DOI] [PubMed] [Google Scholar]
- 78.Tourbah A, Berry I. Magnetic resonance imaging in multiple sclerosis. Pathol Biol (Paris) 2000;48:151–161. [PubMed] [Google Scholar]
- 79.Petkau J, Reingold S, Held U. Magnetic resonance imaging as a surrogate outcome for multiple sclerosis relapses. Mult Scler. 2008;14:770–778. doi: 10.1177/1352458507088104. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miki Y, Grossman RI, Udupa JK. Relapsing-remitting multiple sclerosis: longitudinal analysis of MR images—lack of correlation between changes in T2 lesion volume and clinical findings. Radiology. 1999;213:395–399. doi: 10.1148/radiology.213.2.r99oc01395. et al. [DOI] [PubMed] [Google Scholar]
- 81.Filippi M, Paty DW, Kappos L. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology. 1995;45:255–260. doi: 10.1212/wnl.45.2.255. et al. [DOI] [PubMed] [Google Scholar]
- 82.Goodin DS. Magnetic resonance imaging as a surrogate outcome measure of disability in multiple sclerosis: have we been overly harsh in our assessment? Ann Neurol. 2006;59:597–605. doi: 10.1002/ana.20832. [DOI] [PubMed] [Google Scholar]
- 83.Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. Ernst Schering Res Found Workshop. 2005;53:23–38. doi: 10.1007/3-540-27626-2_3. [DOI] [PubMed] [Google Scholar]
- 84.Khalil M, Enzinger C, Langkammer C. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult Scler. 2011;17:173–180. doi: 10.1177/1352458510384009. et al. [DOI] [PubMed] [Google Scholar]
- 85.Reuter F, Zaaraoui W, Crespy L. Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:1157–1159. doi: 10.1136/jnnp.2010.213744. et al. [DOI] [PubMed] [Google Scholar]
- 86.Rocca MA, Colombo B, Falini A. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. et al. [DOI] [PubMed] [Google Scholar]
- 87.Vavasour IM, Li DK, Laule C, Traboulsee AL, Moore GR, Mackay AL. Multi-parametric MR assessment of T(1) black holes in multiple sclerosis: evidence that myelin loss is not greater in hypointense versus isointense T(1) lesions. J Neurol. 2007;254:1653–1659. doi: 10.1007/s00415-007-0604-x. [DOI] [PubMed] [Google Scholar]
- 88.Qian P, Lancia S, Xu J. Multiple sclerosis T2 lesions which disappear may still show persistent tissue injury. et al. Poster presented at: 2011 Annual Meeting of the American Academy of Neurology; April 13, 2011; Honolulu, HI.
- 89.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 90.Rio J, Castillo J, Rovira A. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler. 2009;15:848–853. doi: 10.1177/1352458509104591. et al. [DOI] [PubMed] [Google Scholar]
- 91.Tomassini V, Paolillo A, Russo P. Predictors of long-term clinical response to interferon beta therapy in relapsing multiple sclerosis. J Neurol. 2006;253:287–293. doi: 10.1007/s00415-005-0979-5. et al. [DOI] [PubMed] [Google Scholar]
- 92.Swanton JK, Fernando KT, Dalton CM. Early MRI in optic neuritis: the risk for disability. Neurology. 2009;72:542–550. doi: 10.1212/01.wnl.0000341935.41852.82. et al. [DOI] [PubMed] [Google Scholar]
- 93.Rudick RA, Lee JC, Simon J, Ransohoff RM, Fisher E. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol. 2004;56:548–555. doi: 10.1002/ana.20224. [DOI] [PubMed] [Google Scholar]
- 94.Durelli L, Barbero P, Bergui M. MRI activity and neutralizing antibody as predictors of response to interferon beta treatment in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:646–651. doi: 10.1136/jnnp.2007.130229. et al. [DOI] [PubMed] [Google Scholar]
- 95.Prosperini L, Gailo V, Petsas N, Borriello G, Pozzilli C. One-year scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol. 2009;16:1202–1209. doi: 10.1111/j.1468-1331.2009.02708.x. [DOI] [PubMed] [Google Scholar]
- 96.Pozzilli C, Prosperini L, Sbardella E, de Giglio L, Onesti E, Tomassini V. Post-marketing survey on clinical response to interferon beta in relapsing multiple sclerosis: the Roman experience. Neurol Sci. 2005;4:S174–S178. doi: 10.1007/s10072-005-0510-x. [DOI] [PubMed] [Google Scholar]
- 97.Tintore M, Rovira A, Rio J. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006;67:968–972. doi: 10.1212/01.wnl.0000237354.10144.ec. et al. [DOI] [PubMed] [Google Scholar]
- 98.Mowry EM, Beheshtian A, Waubant E. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisniku LK, Brex PA, Altmann DR. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131:808–817. doi: 10.1093/brain/awm329. et al. [DOI] [PubMed] [Google Scholar]
- 100.Kappos L, Moeri D, Radu EW. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Lancet. 1999;353:964–969. doi: 10.1016/s0140-6736(98)03053-0. et al.; Gadolinium MRI Meta-analysis Group. [DOI] [PubMed] [Google Scholar]
- 101.Goodin DS, Reder AT, Ebers GC. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78:1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolansky LJ, Finden SG, Chang R. Gadoteridol in multiple sclerosis patients: a comparison of single and triple dose with immediate vs delayed imaging. Clin Imaging. 1998;22:385–392. doi: 10.1016/s0899-7071(98)00072-2. et al. [DOI] [PubMed] [Google Scholar]
- 103.Wolansky LJ, Bardini JA, Cood SD, Zimmer AF, Sheffet A, Lee HJ. Triple-dose vs. single-dose gadoteridol in multiple sclerosis patients. J Neuroimaging. 1994;4:141–145. doi: 10.1111/jon199443141. [DOI] [PubMed] [Google Scholar]
- 104.Filippi M, Campi A, Martinelli V. Comparison of triple dose versus standard dose gadolinium-DTPA for detection of MRI enhancing lesions in patients with primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995;59:540–544. doi: 10.1136/jnnp.59.5.540. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filippi M, Yousry T, Camp A. Comparison of triple dose versus standard dose gadolinium-DTPA for detection of MRI enhancing lesions in patients with MS. Neurology. 1996;46:379–384. doi: 10.1212/wnl.46.2.379. et al. [DOI] [PubMed] [Google Scholar]
- 106.Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65:268–275. doi: 10.1002/ana.21606. [DOI] [PubMed] [Google Scholar]
- 107.Sormani MP, Bonzano L, Roccatagliata L, Mancardi GL, Uccelli A, Bruzzi P. Surrogate endpoints for EDSS worsening in multiple sclerosis: a meta-analytic approach. Neurology. 2010;75:302–309. doi: 10.1212/WNL.0b013e3181ea15aa. [DOI] [PubMed] [Google Scholar]
- 108.Sormani MP, Li DK, Bruzzi P. Combined MRI lesions and relapses as a perfect surrogate for disability in multiple sclerosis. Neurology. 2011;77:1684–1690. doi: 10.1212/WNL.0b013e31823648b9. et al. [DOI] [PubMed] [Google Scholar]
- 109.Sormani MP, Stubinski B, Cornelisse P. Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult Scler. 2011;17:541–549. doi: 10.1177/1352458510391837. et al. [DOI] [PubMed] [Google Scholar]
- 110.Prentice RL. Surrogate endpoints in clinical trials: definition and operation criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 111.Simon JH, Li D, Traboulsee A. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27:455–461. et al. [PMC free article] [PubMed] [Google Scholar]


