Abstract
Fatigue may limit the ability of people with multiple sclerosis (MS) to participate in walking exercises, which could ultimately reduce their benefit from physical therapy. An exercise program that minimizes the fatigue experienced by people with MS during exercise may lead to an increase in the amount of exercise being performed. The purpose of this study was to determine whether subjective feelings of fatigue differ in people with MS depending on whether they exercised intermittently or continuously. Using a within-subjects, counterbalanced crossover design, a sample of 30 individuals with MS performed 6 minutes of continuous and 6 minutes of intermittent walking 1 week apart. Fatigue was measured on the Visual Analogue Scale of Fatigue (VASF) and recorded before and after the walking conditions. A 2 × 2 repeated-measures analysis of variance was used to assess the potential effects of intermittent versus continuous walking on self-reported fatigue. A significant interaction revealed that pre-post mean self-ratings of fatigue on the VASF increased less in the intermittent condition (from 37.93 mm to 44.83 mm; difference = 6.90 mm) compared with the continuous condition (from 34.33 mm to 54.43 mm: difference = 20.10 mm) (P < .001), suggesting that patients experienced less fatigue in the intermittent condition despite walking an equivalent total duration. The interaction effect was not influenced by age, gender, disease severity or duration, use of antispasticity medication, use of assistive devices, or mood. The results suggest that people with MS may be more tolerant of, and able to perform, greater amounts of exercise if they exercise intermittently.
Fatigue has been described as one of the most disabling symptoms of multiple sclerosis (MS)1 and has been reported to occur in 78% of patients.2 Not surprisingly, individuals with MS are limited in function owing, in part, to fatigue.3 Evidence is mounting that MS patients may benefit from exercise across a broad range of health outcomes, including fatigue.4–8 At the same time, fatigue likely adversely affects an MS patient's ability to participate in exercise,9 and little is known about how to maximize the benefits of exercise while managing fatigue in patients with MS.
Krupp et al.10 defined MS fatigue as having the following characteristics: a) comes on easily, b) prevents sustained physical functioning, c) is worsened by heat, d) prevents fulfillment of responsibilities, e) interferes with physical functioning, and f) causes frequent problems. The Multiple Sclerosis Council for Clinical Practice Guidelines defined fatigue as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities.”11 Paty and Ebers12 separated fatigue from MS into two subcategories: lassitude and motor fatigue. Lassitude has been described as a generalized feeling of exhaustion; motor fatigability refers to fatigue that occurs with repeated muscle contractions or motor activity.
Multiple factors have been associated with MS fatigue, including many symptoms commonly associated with MS such as pain,13 sleep disorders,14 depression,15 ataxia, spasticity, infections,16 and the use of multiple medications, which may have fatigue as a side effect. Theories about the pathophysiology of fatigue are varied but largely revolve around three perspectives: The first is the neuromodulation theory, which holds that fatigue may be related to proinflammatory cytokines activated from leukocytes participating in the autoimmune process.17 A second theory holds that fatigue in MS is due to demyelination and axonal loss in central pathways necessary for sustained motor activity. Consistent with this is the observation that fatigue is more common in patients with progressive MS, in which these pathologies are more predominant, than in patients with relapsing-remitting disease.18,19 Third, some fatigue in MS may also be due to secondary causes. Svensson et al.20 suggested that the motor fatigue seen in MS may be due in part to muscle disuse, rather than the central causes described above. If this is the case, some of the fatigue seen in MS may be alleviated through exercise.
Although the pathophysiology of MS fatigue has yet to be fully elucidated, it is likely that fatigue limits patients' ability to engage in exercise and rehabilitation programs. An exercise protocol that minimizes fatigue may allow for more exercise. Intermittent exercise could result in less fatigue and a longer exercise period, thus enhancing the benefits of exercise and potentially of physical rehabilitation programs. Although some studies have examined intermittent exercise, it has not been examined in the context of MS. Clapp and associates21 reported good results with low-intensity, intermittent exercise in individuals with chronic fatigue syndrome. Murphy and Hardman22 reported similar improvements in fitness with long and short bouts of brisk walking in sedentary women. Schachter and associates23 examined the effects of one long exercise bout compared with two short bouts (the total amount of exercise in the two groups was the same) in sedentary women with fibromyalgia. However, no advantage for either group was found in terms of physical or psychological well-being, self-adherence, improvements in fibromyalgia symptoms, or physical function. Thus, evidence of the potential benefit of intermittent versus continuous exercise remains mixed, particularly as it pertains to the MS population. Moreover, due to the multifactorial and subjective nature of fatigue in MS, its measurement is challenging. Measurement of fatigue is usually accomplished by either self-report, where the individual rates or describes his or her fatigue, or a physical performance test in which a level of physical activity must be sustained for a period of time.
Although the issue is not yet resolved, literature and theory support the possibility that intermittent exercise, by potentially reducing MS fatigue, might be advantageous for MS patients. To this end, we assessed the effects of 6 minutes of intermittent walking versus 6 minutes of continuous walking on self-reported fatigue in patients with MS. We chose the 6-Minute Walk (6MW) test as a sample of exercise that can provoke fatigue in patients across a relatively broad range of disability.24 We hypothesized that the intermittent condition would be associated with less subjective fatigue than the continuous condition, despite the equivalence of the total duration of walking across conditions. Such a finding could guide improvements in exercise programs for patients with MS.
Methods
Research Design
We used a repeated-measures, crossover design, with each patient serving as his or her own control. To control for order effects, the design was counterbalanced such that half the patients did an intermittent 6-minute walking condition first, followed by a 6-minute continuous condition 1 week later, and the other half of the patients participated in the conditions in the reverse order. Fatigue was assessed for each patient before (pre) and after (post) each walking condition.
Participants
A convenience sample of 30 participants was recruited from an MS management practice in New York City where the first author worked as a physical therapist. After receipt of internal review board approval from St. Luke's Roosevelt Hospital, testing began with the first subjects who met the inclusion criteria and continued in an ongoing manner until recruitment was complete. Inclusion criteria included a) a clinical diagnosis of MS, b) ability to ambulate continuously for 6 minutes, with or without assistive devices, and c) ability to read and comprehend the informed consent form. Exclusion criteria included a) evidence of exacerbation as defined by the neurologist (patients were eligible to participate after 4 weeks of clinical stability), b) interferon injections within 48 hours of testing, c) recent treatment (within 4 weeks) with methylprednisolone, d) inability to ambulate for 6 minutes continuously, e) history of recent lower-extremity injury, cardiac problems, or respiratory complications interfering with the ability to perform a 6-minute continuous walk, f) use of antifatigue medications, and g) cognitive impairment that limited the ability to follow simple commands.
Walking Conditions
The walking conditions were as follows: 1) Continuous walking: Each subject walked for 6 minutes at his or her best comfortable pace. If the subject normally walked with an assistive device (cane, walker, brace), he or she used the device. The subject was guarded by a physical therapist for the entire walk. 2) Intermittent walking: The same subject walked for 6 minutes in three 2-minute intervals. At the end of each interval, the subject rested for 2 minutes in a seated position.
Although we did not use it as a dependent variable or endpoint, the 6MW is widely used in research with MS patients, with well-established reliability and validity.24 We selected the 6MW as a sample of walking exercise because it is easily reproducible, is long enough to detect fatigue effects,25 and reasonably represents an endurance activity, while still allowing for inclusion of a relatively broad range of MS disability. Because of the inclusion of rests after each 2-minute interval, the intermittent walking condition in the current study does not represent the 6MW in its standard form.
Self-Rated Fatigue
We used the Visual Analogue Scale of Fatigue (VASF) as our primary outcome measure.26 Subjects rated fatigue by marking a 100-mm visual analogue scale. A mark on the extreme right of the scale indicated the worst fatigue, and that on the extreme left indicated the least fatigue. Each subject rated fatigue on the VASF 1 minute before and immediately after completion of the continuous or intermittent condition. The difference in millimeters between the pre- and post-walking scores was assessed. The VASF was reported by Krupp et al.27 to be more sensitive in detecting beneficial effects of fatigue treatment compared with two other well-known rating scales for fatigue in individuals with MS, the Fatigue Severity Scale (FSS)28 and the Fatigue Impact Scale (FIS).29 The VASF has been found to be well correlated with physical aspects of fatigue, but weakly correlated with psychosocial and cognitive aspects of weeks) fatigue.30 Reliability of the VASF has been established for other conditions but has not been examined in MS.31 Although the FIS and FSS scales are reported to have greater test-retest reliability than the VASF, they were designed for measuring the effects of fatigue over time, not at a particular point in time, as in the present study. We used the VASF as the primary measure because it is reflective of immediate changes in fatigue. We hypothesized that fatigue measured with the VASF would be less after intermittent compared with continuous walking. The FSS was used in this study to assess the background level of fatigue for each subject: A score of 4.0 or greater is considered to represent significant fatigue.32
Because of the well-known mood-enhancing effects of exercise in individuals with MS and evidence that depression may be a factor in MS-related fatigue,33 the Visual Analogue Mood Scale (VAMS) was administered immediately after administration of the VASF, prior to and after walking bouts. The VAMS was first used by Folstein and Luria34 and revised by Monk35 and consists of nine questions. It has been found to be a valid and reliable measure of mood in MS.36 The subject is instructed to draw a vertical line through a 100-mm horizontal VAS in accordance with how he or she is feeling at the time. Caruso and associates37 used the VAMS (and other measures) to examine the relationship between sleep disorders and MS fatigue. We hypothesized that there would be no significant change in mood over the testing period and that mood would not influence differential effects of condition on changes in fatigue.
Procedure
Participant characteristics, including age, gender, height, weight, type of MS (relapsing-remitting, primary progressive, secondary progressive), and years since diagnosis were recorded. Clinical information included the level of impairment (Expanded Disability Status Scale [EDSS]38 score, as measured by the subject's neurologist), overall level of fatigue (measured by the FSS), use of assistive devices (cane, walker, ankle-foot orthoses), and use of antispasticity medication.
Condition order was counterbalanced such that half the patients were randomly assigned to receive the conditions in one order, and the order was reversed for the other patients. The testing environment was thermostat controlled and maintained at a temperature of approximately 76°F. Patients remained in the testing environment for approximately 15 minutes before testing began to reduce the effects of outside weather variations. Testing began with patients completing a 5-minute seated rest, and the VASF and VAMS were administered 1 minute before the first walk. The walking area was a 200-foot, rectangular walkway with a linoleum surface requiring four 90° turns (one turn every 50 feet). Subjects were instructed not to talk during the walk, and others who might be in the hallway were previously instructed not to talk with the subjects during the walk or rest periods. The VASF and VAMS were administered within 1 minute of sitting down. One week later, subjects performed the other walking condition. After a 5-minute seated rest, the VASF and VAMS were again administered immediately before the walk and within 1 minute of sitting down after the walk. Subjects performed the different conditions during the same time of day, either before or after 12 p.m. They were asked to walk at their best comfortable pace and to wear the same footwear for both walks that they would normally wear if they were going to walk the same length of time. If they normally used an assistive device, they were instructed to use that device for both walks. The subject was guarded by a physical therapist for the entire walk in case of loss of balance. The therapist did not give any physical or verbal assistance unless loss of balance occurred. If loss of balance occurred or the subject needed to stop for any reason, the trial was halted and repeated at a later date. If the trial was halted a second time, the subject was removed from the study.
Statistical Analysis
All analyses were performed with SPSS, version 12.0 (SPSS, Chicago, IL). Descriptive statistics were calculated for all variables and participant characteristics. Means and standard errors were calculated for VASF scores before (pre) and after (post) each walking condition (continuous vs. intermittent). Since each participant provided his or her own control in this crossover design, there were no baseline group characteristics to compare. No impact was found of condition order on pretest-posttest fatigue score differences. In order to assess the effects of intermittent versus continuous walking conditions on pretest-posttest differences in self-reported fatigue, we conducted a two-way, repeated-measures analysis of variance (ANOVA). Prior to performing the ANOVA, the assumptions of normality and sphericity of the fatigue scores were tested and met (P < .05). Bonferroni corrections were applied to all pairwise comparisons to control for family-wise error. All tests were two-tailed, with the significance level set at α ≤ .05. The potential impacts of participant characteristics on the repeated-measures ANOVA were assessed by running the main ANOVA multiple times, once for each characteristic entered as a covariate. Potential bivariate associations between the VASF and participant characteristics were assessed with Pearson correlations for continuous interval variables and Spearman correlations for dichotomous or noninterval variables.
Results
Demographic and background sample characteristics are summarized in Table 1 and are consistent with those of the urban MS management practice from which subjects were recruited. All participants completed the study. As expected, the two-way repeated-measures ANOVA of the effects of Time (pretest vs. posttest) and Condition (continuous vs. intermittent walking) on VASF self-ratings of fatigue revealed two significant effects. First, the main effect of Time was significant (F = 36.65, P < .001), indicating that patients were more fatigued after walking than before walking, regardless of condition. This finding supports the use of 6 minutes of walking as a sample of exercise that can elicit fatigue. Most importantly, the interaction effect was significant (F = 16.54, P < .001), reflecting a greater increase in fatigue in the continuous-walking condition relative to that in the intermittent condition (Figure 1). Further supporting the main hypothesis, pairwise comparisons revealed a significant difference between conditions for postwalking VASF scores (P < .05) and not for prewalking VASF scores (P = .28). The latter finding reflects that patient fatigue was equivalent across days at the beginning of each session prior to walking. Pre-post mean VASF ratings increased from 34.33 mm to 54.43 mm (difference = 20.10 mm; 59%) in the continuous-walking condition, whereas they increased from 37.93 mm to 44.83 mm (difference = 6.90 mm; 18%) in the intermittent-walking condition. Clearly, fatigue increased less with rest breaks.
Table 1.
Demographic and background characteristics of study participants (N = 30)
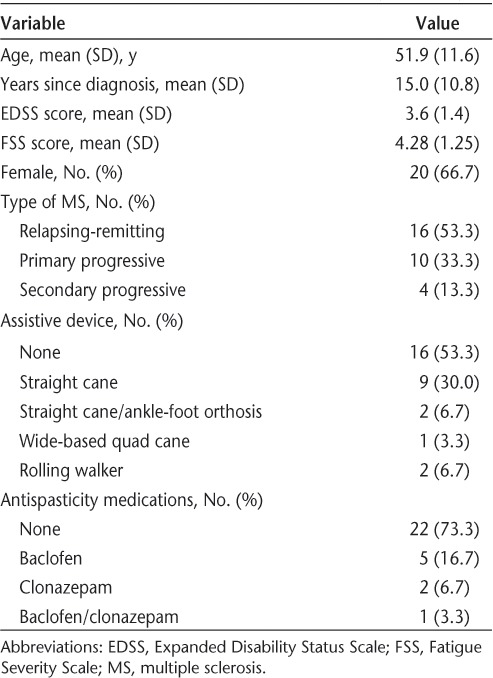
Figure 1.
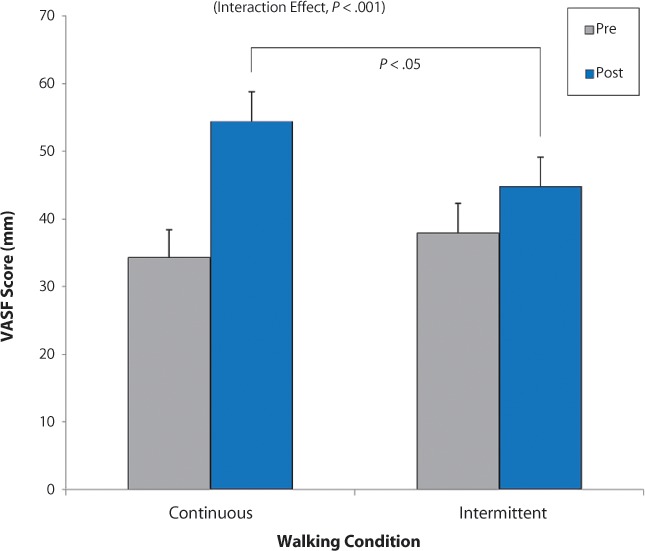
Change in mean self-rated fatigue from before walking (pre) to after walking (post) by experimental condition (continuous vs. intermittent; N = 30) with standard error bars
The overall interaction was significant (P < .001) owing to a greater increase in VASF scores in the continuous condition relative to the intermittent condition. The prewalking scores were statistically equivalent across conditions, whereas there was a significant difference between the postwalking scores of the two conditions (P < .05). VASF, Visual Analogue Scale of Fatigue. Note: Possible VASF scores range from 0 mm to 100 mm, but the vertical axis was truncated to save space.
Type of MS, EDSS score, FSS score, years since diagnosis, gender, age, use of assistive devices, medication, and mood (VAMS) did not significantly affect fatigue differences across conditions. VASF scores were not significantly correlated with EDSS scores or other measures.
Discussion
The present results showed that patients with MS found intermittent walking less fatiguing than continuous walking, even though the total amount of time walked was the same. This suggests that greater amounts of activity could be performed with intermittent versus continuous exercise. Given that MS-related fatigue often limits participation in exercise programs, a strategy that reduces fatigue (intermittent exercise) while maintaining a substantial duration of activity might allow for greater exercise benefits. Baseline patient characteristics, such as gender, age, baseline disability, and disease duration, did not diminish the fatigue-reducing effect of intermittent exercise relative to continuous exercise. To our knowledge, this is the first study to examine the potential benefits of intermittent versus continuous exercise for patients with MS, and the results may be of value to health-care providers, therapists, and patients living with MS. Additionally, the utility of the VASF demonstrated in the present study suggests that it may be of benefit to rehabilitation therapists for assisting in rapid, real-time assessments of fatigue that could be used to better individualize therapy programs. Disease severity did not alter the subjective effect of intermittent versus continuous walking on fatigue, despite the range of EDSS scores in the present study (1.5–6.0) reflecting a substantial portion of the MS continuum. Although subjects with an EDSS score greater than 6.0 would not have been able to complete the study, it seems reasonable that ambulatory subjects at the higher end of MS disability (EDSS score 6.5–7.0) might find intermittent walking less fatiguing than continuous walking. Despite inclusion of a substantial range of EDSS scores in the present study, the mean EDSS of 3.6 may suggest that our sample was most representative of a mildly disabled population, thus potentially limiting the generalizability of the findings. Future research should examine the effects of intermittent versus continuous exercise in a more disabled population.
The decision to exclude subjects taking antifatigue medication is potentially problematic because it may have resulted in the omission of subjects who were most easily fatigued. However, ethical issues arise in asking subjects to forgo medications they normally require. In addition, evidence of the efficacy of antifatigue medications in patients with MS is mixed, suggesting that exclusion of subjects on antifatigue medication may not have biased the sample much.39,40 The subjects in this study all noted fatigue as a significant symptom despite a wide range of disability, yet it is not known whether subjects on antifatigue medication would have reacted in a similar manner. Future studies could examine the effect of intermittent exercise on individuals with MS who are taking antifatigue medication. Also regarding medications, although we found no differential effect based on whether a patient was taking antispasticity medications, we did not capture the onset or duration of use of this medication with regard to the timing of the study. It would seem prudent in future research to record such information to increase confidence that antispasticity medications did not represent a confounding factor.
We did not measure distance walked, as the purpose of the present study was to measure the effects of intermittent walking on subjective perceptions of fatigue. Therefore, although it seems unlikely, we cannot rule out the possibility that shorter distances walked, rather than rest periods, led to the observed reductions in fatigue associated with the intermittent condition. Moreover, if the reductions in fatigue could be shown to translate into farther distances walked, it would further strengthen the case for the potential beneficial effects of intermittent walking in MS. Preliminary findings in our laboratory suggest that this may, in fact, be the case. Measuring distance could also address the potential bias introduced by allowing patients to determine their own pace in a design where blinding is not feasible. Thus, future studies should assess distance walked and potential associations of distance with condition (intermittent vs. continuous) and fatigue.
Moreover, future examinations should compare outcomes on a variety of exercise tasks in individuals with MS using intermittent versus continuous protocols. Such tasks could be selected to allow participation by MS patients that were outside the range set by the 6MW in the present study. In the present study, we instructed patients to walk at their best comfortable pace, hoping that this would elicit a balance between intensity and safety. However, our procedure likely did not represent aggressive or vigorous exercise, and an important direction for future research will be to replicate these findings using more vigorous types of exercise. We used the VAMS to control for the effects of mood on perceptions of fatigue and found that changes in mood did not have a significant effect. However, this could have been reflective of a single bout of exercise. If a lengthier intervention were used, the effects of mood might be more prominent.
Clinical Implications
Rehabilitation programs have been used to aid in recovery in major neurologic conditions. Multiple sclerosis stands apart in that a predominant feature of the condition itself, fatigue, limits the ability to undergo rehabilitation programs of sufficient intensity to realize maximal benefits. For this reason, individuals with MS may be unable to obtain rehabilitation gains similar to those of patients with other neurologic diseases. Although we did not examine the effects of a rehabilitation program per se, the present findings suggest that incorporating rests into an exercise program may reduce fatigue and ultimately allow patients to exercise for greater amounts of time and potentially at higher intensities. Such gains could reasonably be expected to translate to greater potential participation in rehabilitation programs.
Rehabilitation programs comprise much more than 6-minute bouts of walking exercise. Thus, if the intermittent strategy of including rests increases an MS patient's capacity for exercise, then multiple types of exercise at various durations and intensities should be explored. For example, diminished strength is a common finding in MS; however, building strength via continuous repetitions of resistance exercise is difficult, in part owing to fatigue. If an individual with MS performed intermittent repetitions (eg, resting at the onset of fatigue and resuming when recovered, as opposed to continuing until fatigue prevents further work), a greater number of repetitions could be achieved, potentially resulting in the realization of greater strength gains.
A typical aim of an exercise program for MS patients is for gains to generalize to multiple areas of the patient's life. Although this has been found to be the case in other populations, it remains unclear whether performing more work intermittently will result in better functional outcomes compared with performing work continuously in MS patients: the complexities of MS may yield different results. Nevertheless, the exciting possibility remains that an intermittent exercise strategy may lead to improvements in quality of life for MS patients that would not otherwise be possible. Thus, future studies should examine whether intermittent exercise leads to gains that translate to other areas of functioning by incorporating it into full programs of longer duration and including prospective measures of quality of life.
Patients in the present study indicated that they experienced less fatigue in the intermittent walking condition, suggesting that patients with MS may better tolerate intermittent exercise, potentially leading to more beneficial participation in exercise programs. It will be the work of future research first to explore whether reduced fatigue associated with intermittent walking is associated with greater distance, to examine the intermittent strategy with a variety of exercises of varying durations and intensities, and ultimately to investigate its potential utility for MS patients engaging in exercise and rehabilitation programs.
PracticePoints.
People with MS are limited in their ability to participate in walking-related exercise activities, likely in part due to the presence of neurogenic fatigue.
The results of this study show that intermittent walking is less fatiguing than continuous walking in people with MS.
Thus people with MS may be able to increase their walking exercise participation by walking intermittently rather than continuously.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose. Although Dr. Rzetelny is employed by Acorda Therapeutics, Ardsley, NY, that company provided no financial support and was not associated with this research.
References
- 1.Murray TJ. Amantadine therapy for fatigue in multiple sclerosis. Can J Neurol Sci. 1985;12:251–254. doi: 10.1017/s0317167100047107. [DOI] [PubMed] [Google Scholar]
- 2.Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1984;65:135–138. [PubMed] [Google Scholar]
- 3.Olgiati R, Burgunder JM, Mumenthaler M. Increased energy cost of walking in multiple sclerosis: effect of spasticity, ataxia, and weakness. Arch Phys Med Rehabil. 1988;69:846–849. [PubMed] [Google Scholar]
- 4.Learmonth YC, Paul L, Miller L, Mattison P, McFadyen AK. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. 2012;26:579–593. doi: 10.1177/0269215511423946. [DOI] [PubMed] [Google Scholar]
- 5.Andreasen AK, Stenager E, Dalgas U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult Scler. 2011;17:1041–1054. doi: 10.1177/1352458511401120. [DOI] [PubMed] [Google Scholar]
- 6.Dalgas U, Stenager E, Jakobsen J. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16:480–490. doi: 10.1177/1352458509360040. et al. [DOI] [PubMed] [Google Scholar]
- 7.Filipi ML, Kucera DL, Filipi EO, Ridpath AC, Leuschen MP. Improvement in strength following resistance training in MS patients despite varied disability levels. NeuroRehabilitation. 2011;28:373–382. doi: 10.3233/NRE-2011-0666. [DOI] [PubMed] [Google Scholar]
- 8.Huisinga JM, Filipi ML, Stergiou N. Elliptical exercise improves fatigue ratings and quality of life in patients with multiple sclerosis. J Rehabil Res Dev. 2011;48:881–890. doi: 10.1682/jrrd.2010.08.0152. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Olson K, Hale LA, Baxter D, Schneiders AG. How does fatigue influence community-based exercise participation in people with multiple sclerosis? Disabil Rehabil. 2011;33:2362–2371. doi: 10.3109/09638288.2011.573054. [DOI] [PubMed] [Google Scholar]
- 10.Krupp LB, Alvarez LA, LaRocca NG. Fatigue in multiple sclerosis. Arch Neurol. 1988;45:435–437. doi: 10.1001/archneur.1988.00520280085020. et al. [DOI] [PubMed] [Google Scholar]
- 11.Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans Association; 1998. [Google Scholar]
- 12.Paty DW, Ebers GC. Clinical features. In: Paty DW, Ebers GC, editors. Multiple Sclerosis. Philadelphia, PA: FA Davis; 1998. pp. 135–182. [Google Scholar]
- 13.Kerns RD, Kassirer M, Otis J. Pain in multiple sclerosis: a biopsychosocial perspective. J Rehabil Res Dev. 2002;39:225–232. [PubMed] [Google Scholar]
- 14.Clarke CM, Fleming JA, Li D. Sleep disturbance, depression and lesion site in patients with multiple sclerosis. Arch Neurol. 1992;49:641–664. doi: 10.1001/archneur.1992.00530300077013. et al. [DOI] [PubMed] [Google Scholar]
- 15.Minden SL, Schiffer RB. Affective disorders in multiple sclerosis: review and recommendations for clinical research. Arch Neurol. 1990;47:98–104. doi: 10.1001/archneur.1990.00530010124031. [DOI] [PubMed] [Google Scholar]
- 16.Buljevac D, Flach HZ, Hop WC. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. et al. [DOI] [PubMed] [Google Scholar]
- 17.Hemmer B, Cepok S, Nessler S. Pathogenesis of multiple sclerosis: an update on immunology. Curr Opin Neurol. 2002;15:227–231. doi: 10.1097/00019052-200206000-00001. et al. [DOI] [PubMed] [Google Scholar]
- 18.Colosimo C, Millefiorini E, Grasso MG. Fatigue in MS is associated with specific clinical features. Acta Neurol Scand. 1995;92:353–355. doi: 10.1111/j.1600-0404.1995.tb00145.x. et al. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77:165–170. doi: 10.1016/s0003-9993(96)90162-8. [DOI] [PubMed] [Google Scholar]
- 20.Svensson B, Gerdle B, Elert J. Endurance training in patients with multiple sclerosis: five case studies. Phys Ther. 1994;74:1017–1026. doi: 10.1093/ptj/74.11.1017. [DOI] [PubMed] [Google Scholar]
- 21.Clapp LL, Richardson MT, Smith JF. Acute effects of thirty minutes of light-intensity, intermittent exercise on patients with chronic fatigue syndrome. Phys Ther. 1999;79:749–756. et al. [PubMed] [Google Scholar]
- 22.Murphy MH, Hardman AE. Training effects of short and long bouts of brisk walking in sedentary women. Med Sci Sports Exerc. 1998;30:152–157. doi: 10.1097/00005768-199801000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Schachter CL, Busch AJ, Peloso PM. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys Ther. 2003;83:340–358. et al. [PubMed] [Google Scholar]
- 24.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 25.Kesselring J. Disease Progression in Multiple Sclerosis II. Methods for the determination of walking impairment and its impact on activities and social participation. Eur Neurol Rev. 2010;5:61–68. [Google Scholar]
- 26.Weinshenker BG, Penman M, Bass B. A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology. 1992;42:1468–1471. doi: 10.1212/wnl.42.8.1468. et al. [DOI] [PubMed] [Google Scholar]
- 27.Krupp LB, Soefer MH, Pollina DA. Fatigue measures for clinical trials in multiple sclerosis. Neurology. 1998;50:A126. et al. [Google Scholar]
- 28.Krupp LB, LaRocca NC, Muir-Nash J, Steinberg AD. The fatigue severity scale applied to multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 29.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 30.Kos D, Nagels G, D'Hooghe MB, Duportail M, Kerckhofs E. A rapid screening tool for fatigue impact in multiple sclerosis. BMC Neurol. 2006;6:27. doi: 10.1186/1471-2377-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng BY, Gajewski BJ, Kluding PM. Reliability, responsiveness, and validity of the visual analog fatigue scale to measure exertion fatigue in people with chronic stroke: a preliminary study. Stroke Res Treat. 2010 doi: 10.4061/2010/412964. 2010. Article ID 412964. Epub 2010 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egner A, Phillips VL, Vora R, Wiggers E. Depression, fatigue, and health-related quality of life among people with advanced multiple sclerosis: results from an exploratory telerehabilitation study. NeuroRehabilitation. 2003;18:125–133. [PubMed] [Google Scholar]
- 33.Petajan JH, Gappmaier E, White AT. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. et al. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Luria R. Reliability, validity, and clinical application of a Visual Analog Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 35.Monk TH. A Visual Analog Scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 36.Groom MJ, Lincoln NB, Francis VM, Stephan TF. Assessing mood in patients with multiple sclerosis. Clin Rehabil. 2003;17:847–857. doi: 10.1191/0269215503cr688oa. [DOI] [PubMed] [Google Scholar]
- 37.Caruso LS, LaRocca NG, Spielman AJ. Sleep disruption in fatigued versus nonfatigued persons with multiple sclerosis. et al. Paper presented at: Annual Meeting of the Consortium of Multiple Sclerosis Centers; 1998; Cleveland, OH.
- 38.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 39.Brown JN, Howard CA, Kemp DW. Modafinil for the treatment of multiple sclerosis-related fatigue. Ann Pharmacother. 2010;44:1098–1103. doi: 10.1345/aph.1M705. [DOI] [PubMed] [Google Scholar]
- 40.Pucci E, Branãs P, D'Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2007 Jan;24(1):CD002818. doi: 10.1002/14651858.CD002818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


