Abstract
Bladder dysfunction in multiple sclerosis (MS) can be socially disabling, have negative psychological and economic consequences, and impair patients' quality of life. Knowledge of the functional anatomy and physiology of the urinary tract is essential to understand the symptoms associated with central nervous system lesions and the pharmacotherapies used to treat them. Treatments for neurogenic detrusor overactivity (NDO) have consisted mainly of administration of anticholinergic drugs, which have been shown to provide suboptimal clinical benefits and be poorly tolerated. The US Food and Drug Administration (FDA) approval of intravesicular botulinum toxin therapy provides a second-line option for MS patients with NDO not responsive to anticholinergic drugs. We performed a review of key literature pertaining to the intravesicular application of botulinum toxin. In the management of NDO, administration of intravesicular botulinum toxin using clean intermittent catheterization decreases the incidence of urinary tract infections, promotes urinary continence, and improves quality of life for 9 months after a single injection; moreover, those benefits are maintained with repeated injections over time.
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system that affects over 400,000 people in North America and 2.1 million worldwide. Bladder dysfunction in MS can be socially disabling, have negative psychological and economic consequences, and impair patients' quality of life.1 Urinary incontinence is viewed by many people with MS as being their worst symptom.2 Urinary symptoms have been found to be severe enough to prevent 19% of MS patients from leaving their home and 15% from attending social functions.3 Urinary symptoms have been reported to occur in approximately 75% of people with MS4 and to appear on average 6 years after the onset of the disease.5 Bladder dysfunction also poses a threat to the upper urinary tract. Proper identification of bladder dysfunction and associated problems, combined with appropriate management to prevent upper-tract complications, results in improved quality of life and is therefore essential to the comprehensive care of MS patients.
Functional Anatomy of the Urinary Tract
The complete urinary tract includes the kidneys and the extrarenal excretory passages, consisting of the ureters, the urinary bladder, and the urethra. The transitional epithelial tissue lining the renal pelvis, ureters, bladder, and part of the urethra is the urothelium. The primary functions of the urinary tract are the production of urine, its storage, and its excretion from the body. To accomplish these functions, the urothelium must be compliant to accommodate increasing volumes of urine during storage, as well as contractile in order to expel the urine during micturition. The urothelium is therefore made up of a luminal transitional epithelium able to expand as needed during urine storage, and a deeper muscular layer able to contract during micturition. The transitional epithelium consists of five or six layers of polyploid cells. This arrangement provides the necessary compliance, as the initially cuboidal cells become squamous with stretching of the bladder wall during the filling phase. Deeper yet are the subepithelial connective tissue and the muscularis propria, which is composed of inner longitudinal and outer circular layers of smooth muscle. During peristalsis, the circular smooth muscle contracts to prevent retropulsion of urine; this is followed by contraction of the longitudinal smooth muscle to propel the urine unilaterally toward the bladder in the case of pyeloureteral peristalsis, and toward the urethral opening in the case of urethral peristalsis. The area between the two ureteral openings and the urethral opening is called the trigone. There are two sphincters surrounding the urethra. Proximally, at the junction between the bladder and the urethra is the internal sphincter, which consists of vertically oriented fibers of smooth muscle that provide the primary, and involuntary, prevention of urine leakage. Distally along the urethra, past the prostate gland in males, is the external sphincter, which consists of circular fibers of striated muscle encircling the urethra that provide secondary, and voluntary, prevention of urine leakage. Deep to the transitional epithelium in the bladder is the detrusor muscle, a smooth muscle that exerts the contraction necessary for bladder emptying during micturition.
Innervation of the Urinary Tract and Physiology of Micturition
The functions of storing and emptying are controlled by the sympathetic and parasympathetic nervous systems, respectively (Figure 1). Sympathetic input is mediated via the hypogastric nerve and originates from T10 to L2 in the spinal cord. Parasympathetic input is mediated via the pelvic splanchnic nerve and originates from S2 to S4 in the spinal cord. Somatic input to the distal urethral sphincter originates from the anterior horn of S2 to S4 in the Onuf nucleus and is mediated via the pudendal nerve. General visceral afferent (GVA) fibers are part of the autonomic nervous system (ANS), arise from the bladder, are especially dense in the trigone, monitor minute pressure and stretch changes in the urothelium, and carry sensory impulses to the sacral micturition center (SMC) and on to the periaqueductal gray, pontine micturition center, and cerebral cortex. The frequency of these impulses increases as the intravesicular volume and associated intravesicular pressure increase. At low volume, the reflex of micturition can be ignored and continence can be maintained voluntarily via constriction of the external sphincter by somatic input. Once the frequency of the afferent sensory impulses crosses a threshold, micturition becomes necessary.
Figure 1.
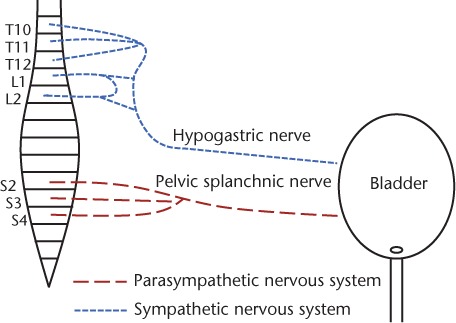
Autonomic bladder innervation
While the internal sphincter remains tonic under sympathetic input during the filling phase, the maintenance of this tone is reversed and the internal sphincter is allowed to relax under parasympathetic input when micturition is needed. The detrusor muscle is relaxed under sympathetic input during the filling phase and contracts under parasympathetic input during micturition. The explanation of why the detrusor and the internal sphincter react in opposite fashion to input from the sympathetic and parasympathetic systems is simple. The detrusor, in addition to several subtypes of muscarinic cholinergic receptors, also contains beta-adrenergic catecholamine receptors. The internal sphincter also contains catecholamine receptors but of the alpha-adrenergic subtype. In the presence of catecholamines (epinephrine or norepinephrine), beta-adrenergic receptors trigger relaxation while alpha-adrenergic receptors trigger contraction, thus explaining the opposing responses found in the detrusor and the internal sphincter during sympathetic input. The external urethral sphincter is under somatic control and contracts voluntarily to occlude the urethra and provide continence until micturition can be conveniently initiated.
Nervous System Lesions and Associated Bladder Dysfunctions
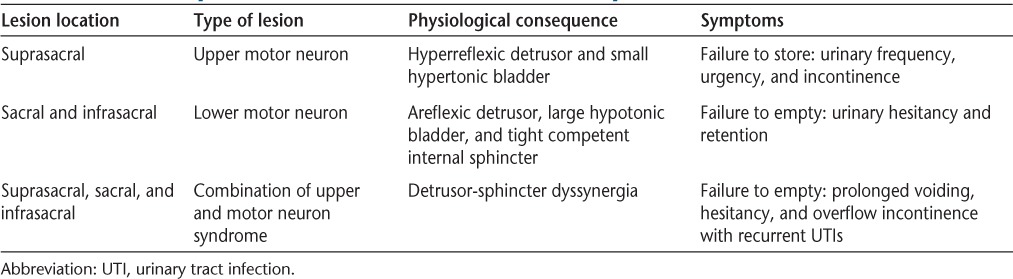
Any central nervous system lesions above the SMC (S2-S4 cord level) will result in upper motor neuron bladder and lead to small hyperreflexic, overactive bladder and failure to store (Table 1). Lower motor neuron bladder will result from complete destruction of the SMC or lesions of the conus medullaris, cauda equina, or peripheral nerves to the bladder and will lead to an areflexic detrusor, a large hypotonic bladder, a tight and competent internal sphincter, and failure to empty. In MS, cord lesions above S2 are quite frequent, while cord lesions below S2 are more rare. These relative incidences are due to locational opportunity, as the majority of the cord lies above S2 and is therefore more likely to be affected. In fact, the majority of people with MS have cervical cord lesions6 while less than 20% have sacral lesions on autopsy.7 Urinary urgency, frequency, and incontinence are therefore more common than urinary retention in people with MS.8 Another type of dysfunction is a combination of both upper and lower motor lesions called detrusor-sphincter dyssynergia (DSD), which leads to mixed symptoms.1
Table 1.
Nervous system lesions and associated bladder dysfunctions
Neurogenic Detrusor Overactivity
Neurogenic detrusor overactivity (NDO) refers to a hyperreflexic, overactive detrusor muscle from a neurogenic etiology. Detrusor hyperreflexia is an upper motor neuron syndrome that leads to brisk detrusor responses to low-frequency sensory input from the GVA fibers and occurs at low intravesicular volumes. Overactivity results in unregulated sporadic contractions of the detrusor muscle that lead to increases in intravesicular pressure, causing urgency, frequency, and incontinence.
Primary management of NDO should incorporate behavioral approaches such as increasing the frequency of micturition, limiting fluid intake in the evening, limiting intake of diuretics1 including caffeine and alcohol, and using absorbent undergarments and bed pads. Next in the management of this condition are pharmacologic approaches. Anticholinergic medications can be tried, as they theoretically decrease the detrusor muscle activation by antagonizing the cholinergic muscarinic receptors. These agents are available both orally and transdermally, are nonspecific, and can lead to systemic anticholinergic effects including xeropthalmia, keratoconjunctivitis sicca, and ciliary muscle paralysis leading to mydriasis and cycloplegia. Reduced motility and secretion in the gastrointestinal system may also be seen and can lead to halitosis, dental caries, and constipation. An important adverse effect of anticholinergic therapy to consider in MS patients is decreased sweating, which prevents cooling and exacerbates overheating in patients already suffering from heat intolerance. Moreover, in a thorough review of 33 randomized controlled studies, Nicholas and associates9 concluded that the use of anticholinergics in MS could not be advocated from the evidence. Another study showed that 10% to 15% of patients have no response or cease anticholinergic treatment deliberately because of various side effects or inadequate efficacy.10 Two comprehensive Cochrane reviews showed that up to 30% of patients treated with anticholinergic medications cease to utilize them because of either poor efficacy or intolerable side effects.11
Botulinum Toxin: Background and Mechanism of Action
When anticholinergic medications fail to prove efficacious, the next option is the neutralization of the detrusor muscle with transient paresis from intradetrusor injections of botulinum toxin. Botulinum neurotoxin is secreted by Clostridium botulinum, a gram-negative, rod-shaped, obligate anaerobe,12 spore-forming bacterium that secretes seven different serotypes of botulinum toxin labeled A, B, C, D, E, F, and G.12,13 These different serotypes are antigenically dissimilar, utilize comparable but distinct modes of action, and are not interchangeable.12 Only serotypes A and B are being used in the clinical setting. Of the several forms of botulinum toxin available commercially, only onabotulinumtoxinA, marketed under the brand name Botox (Allergan, Irvine, CA), and abobotulinumtoxinA, marketed under the brand name Dysport (Ipsen Biotech, Paris, France), have been evaluated for use in the detrusor muscle for the management of NDO.
Botulinum toxin is a polypeptide consisting of a heavy chain (100 kDa) and a light chain (50 kDa) held together by a disulfide bond. Once introduced near the synaptic cleft, the toxin is endocytosed in a vesicle within the terminal bouton.12,13 Once inside the vesicle, the two chains separate. The heavy chain remains inside the bouton while the light chain, a protease,12,14 travels through the vesicular membrane, where, depending on its serotype, it proteolytically cleaves different soluble N-ethylmaleimide attachment receptor proteins essential for exocytosis (SNARE proteins).12–14 SNARE proteins are docking proteins essential for the fusion of the vesicular membrane with the plasmalemma. There are three different SNARE proteins in the synaptic cleft. Synaptobrevin, also known as vesicle-associated membrane protein (VAMP), is attached to the vesicular membrane, and syntaxin and synaptosomal-associated protein of 25 kDa (SNAP25)15 are both attached to the plasmalemma. Upon arrival of an action potential in the terminal bouton, calcium enters the presynaptic terminal and triggers the three SNARE proteins to form a stable multimeric SNARE complex that results in the fusion of the vesicular membrane with the plasmalemma, thus allowing for the acetylcholine contained in the vesicles to be exocytosed in the synaptic cleft,12 where it binds with muscarinic receptors and causes, in the case of the bladder, contraction of the detrusor muscle. Serotypes B, D, F, and G proteolytically cleave VAMP. Serotypes A, C, and E proteolytically cleave SNAP25, and serotype C also cleaves syntaxin.13,14 The cleavage of any of the SNARE proteins will effectively prevent formation of the SNARE complex and subsequent exocytosis of acetylcholine into the synaptic cleft.13
Application of Botulinum Toxin Type A in the Management of NDO
In 2000, Schurch and colleagues16,17 introduced and demonstrated the safety and efficacy of intradetrusor onabotulinumtoxinA injections in the management of NDO and incontinence refractory to high-dose anticholinergic therapy in 19 patients with spinal cord injuries and severe detrusor hyperreflexia. All 19 patients used clean intermittent catheterization (CIC) as a means of bladder emptying.16 The benefits of onabotulinumtoxinA included a significant improvement in continence, patient satisfaction, and urodynamic parameters at up to 9-month follow-up. It was noted that the only two patients who did not achieve full continence received 200 units, and it was concluded that 300 units of onabotulinumtoxinA seemed necessary to counteract an overactive detrusor.16 In 2004, a much larger study by Reitz and colleagues18 including 231 patients with NDO treated with 300 units of onabotulinumtoxinA into the detrusor showed similar benefits over the same period of time. The cost-effectiveness of this therapy was evaluated by Kalsi and colleagues19 in a study including 101 patients with urodynamically proven detrusor overactivity. It was determined that 82% of patients showed at least 25% improvement in at least two parameters among urinary frequency, urgency, urgency incontinence episodes, maximum cystometric capacity, and maximum detrusor pressure at the 4-week follow-up and 65% of patients continued to show at least 25% improvement after 16 weeks. The largest cost components were the operating room time and pre-procedure urodynamics. It was concluded that intradetrusor onabotulinumtoxinA is a cost-effective treatment for overactive bladder. In 2008, Giannantoni and colleagues20 echoed the fact that the remarkable quality of life improvement justified the cost of the procedure. In 2007, Ehren and colleagues21 demonstrated improved quality of life after treatment with 500 units of intradetrusor abobotulinumtoxinA in 31 patients with NDO and incontinence in a double-blind placebo-controlled study. The improvements in quality of life and continence were significant at 26 weeks after treatment.
In 2008, Gamé and colleagues22 studied the incidence of symptomatic urinary tract infections (UTIs) 6 months before and 6 months after intradetrusor injection of 300 units of onabotulinumtoxinA in 30 patients with NDO refractory to anticholinergic therapy and using CIC. A significant reduction in the incidence of symptomatic UTIs after onabotulinumtoxinA treatment was observed in patients using CIC as a means of emptying their bladder.
While the benefits of botulinum toxin type A were found to last up to 9 months, repeat treatment is necessary for the benefits to be prolonged. Grosse and colleagues23 studied the efficacy of repeat injections for the management of recurrent symptoms in 66 patients, between 9 and 11 months from an initial injection. The study showed that repeat injections of botulinum toxin type A were as effective as the first one. Continued efficacy of onabotulinumtoxinA was also seen in 17 patients with refractory NDO after 6 years of repeated treatment.20 That study showed a sustained decrease in the frequency of incontinence, and 88% of patients were still fully continent at 6 years. Quality of life remained improved throughout the duration of the study, and the 11 cases of renal pelvis dilatation and the 3 cases of vesicoureteral reflux that had been noted before treatment were no longer seen after the 6-year period. It was concluded that onabotulinumtoxinA intravesicular treatment controls detrusor overactivity and urinary incontinence and preserves upper urinary tract function over a 6-year follow-up.20 Another study also demonstrated persistence of urodynamic and clinical benefits after 5 repetitive intradetrusor botulinum toxin A treatments in patients with NDO.18
For unclear reasons, the trigone has been excluded as an injection site for botulinum toxin since the implementation of this therapy. The trigone, as previously described, is rich in GVA fibers, and its muscle fibers are thought to be the place where the initial detrusor contraction occurs before spreading to the rest of the detrusor around the bladder.24 Suburothelium cells have been shown to be electrically active,25 and while they do not play an obvious role as a pacemaker,26 their extensive linkage by gap junctions27 may enable them to act in concert as an “electrical syncytium” to amplify excitatory effect.28 Kumar and colleagues28 further explained that the urothelium/suburothelium played a key role in mechanosensory transduction in that there may be a release of neurotransmitters in these compartments in response to stretch, to modulate the reflex afferent nerves.
Additional mechanisms and therapeutic applications of botulinum toxin A have been recently proposed.29 Apostolidis and colleagues30 found that botulinum toxin leads to central desensitization through a decrease in central uptake of neurotrophic factors and leads to inhibition of afferent mechanisms. Therefore, botulinum toxin injections in the trigone not only could lead to inhibition of the GVA fibers affecting the overactive reflex of micturition with low intravesicular volumes but also could modulate the onset and propagation of the detrusor contraction in NDO.
Abdel-Meguid24 studied the effect of including rather than excluding the trigone as an injection site for onabotulinumtoxinA in 36 patients with refractory NDO divided into two groups. The first group received 300 units of onabotulinumtoxinA divided into 30 injection sites in the detrusor, sparing the trigone. The second group, labeled the combined group, underwent injection of 200 units of onabotulinumtoxinA in the detrusor and 100 units of onabotulinumtoxinA in the trigone. The combined group showed a statistically significant improvement over the detrusor-only group with respect to reduction of episodes of incontinence, complete dryness, and urodynamic parameters at 4 weeks. The benefits of trigone inclusion lasted up to 18 weeks, at which point the use of anticholinergic medication was reduced by more than half in the combined group compared with the detrusor-only group.24
Optimal dosage of intravesicular onabotulinumtoxinA for the management of NDO was recently examined in a study that included 275 patients with refractory NDO.31 Patients were divided into three treatment groups: the first group underwent treatment with 300 units of onabotulinumtoxinA into the detrusor sparing the trigone, the second group underwent treatment with 200 units of onabotulinumtoxinA, and the third group received placebo. Endpoints included the rates of full continence, the number of episodes of urinary incontinence, quality of life, and urodynamic parameters. The rates of full continence and the number of episodes of incontinence were improved in both onabotulinumtoxinA groups over the placebo group. Quality of life as well as urodynamic parameters were also improved in both onabotulinumtoxinA groups to a similar degree over placebo. There was no distinct gain in benefits between the 200-unit and 300-unit onabotulinumtoxinA groups. Evaluation of adverse events showed an increase in the likelihood of having to use CIC and an increase in the incidence of UTIs in both treatment groups, but to a greater degree in the 300-unit group. Overall, it was concluded that 200 units of onabotulinumtoxinA provided the best benefit–to–adverse events ratio.31
Intravesicular Electromotive Drug Treatments for NDO
The application of botulinum toxin into the detrusor requires the insertion of a cystoscope in the urethra and the bladder. Depending on whether or not the patient has preserved sensation, some level of anesthesia is usually needed. This can be achieved by a variety of techniques ranging from simple intravesicular lidocaine injections to general anesthesia.
Another mode of anesthetic drug administration, electromotive drug administration (EMDA) of lidocaine, epinephrine, and dexamethasone, was examined in 84 patients with urge syndrome and urodynamically proven idiopathic detrusor overactivity.32 This treatment improved urodynamic parameters, quality of life, and pad usage.32 Comparison of the effect of lidocaine application via injections versus electromotive administration on pain scores during subsequent botulinum toxin injections indicated that electromotive lidocaine administration was associated with a significant decrease in the pain score and ensured painless application of botulinum toxin in patients with preserved sensation.33 This concept of electromotive drug administration was later applied to botulinum toxin to evaluate whether electromotively driven botulinum toxin application was effective in rabbits.34 Compared with injections, electromotive lidocaine administration resulted in more uniform and homogeneous botulinum spread throughout the urothelium on immunohistologic staining.34 This application was then tested by the same authors in 15 children with refractory NDO and preserved bladder sensation.35 The electromotive administration of botulinum toxin led to significant improvements in terms of urodynamic parameters, severity of incontinence, and grade of vesicoureteral reflux.35 It was concluded that electromotive botulinum toxin type A administration is a feasible, efficacious, and safe method with no need for anesthesia.
Possible Complications of Botulinum Toxin
Application of botulinum toxin into the detrusor will weaken the detrusor and lead to bladder paresis, which is the goal of the procedure. It may therefore lead to urinary retention and increased postvoid residual (PVR) volume. In turn, PVRs over 50 mL will increase the risk of UTIs. In patients not using CIC as a means of bladder emptying after botulinum toxin treatment, the incidence of UTIs was found to be increased. Conversely, those using CIC had a lower incidence of UTIs.22
Another possible complication is systemic spread of the toxin. It is assumed that a small fraction of the toxin will travel distally from the area of therapeutic administration. In the great majority of cases, this systemic spread will not be symptomatic. This spread will be noticeable, however, in the few patients in need of all of their strength to be able to independently carry out their activities of daily living. Weakness after botulinum toxin injection in the detrusor has been reported in five spinal cord–injured patients. Two of them, a woman with paraplegia and a woman with tetraplegia, suffered from transient generalized weakness after receiving 1500 units of abobotulinumtoxinA, which corresponds to approximately 500 units of onabotulinumtoxinA.36,37 The three other cases included two men with tetraplegia and one woman with paraplegia who suffered from transient weakness that negatively interfered with their ability to carry out transfers after receiving 300 units of onabotulinumtoxinA.31,37 It is important to note that these cases involved large doses of botulinum toxin A and that no report of weakness was noted at doses of 200 units of onabotulinumtoxinA or 750 units of abobotulinumtoxinA. As far as we are aware, no event of generalized weakness after intradetrusor botulinum toxin treatment has been reported in the MS population.
Why Does the Benefit of Botulinum Toxin Go Away with Time?
Botulinum toxin permanently disables the SNARE protein it cleaves, but its clinical effects have limited duration. In the detrusor, the duration of the benefits extends to 9 months. The reason for the symptom relapse is resprouting, in which new nerve terminals are created to compensate for the loss of old ones. Detrusor biopsies following botulinum toxin injections showed no resprouting in four of four cases after 3 months but significant resprouting in three of seven biopsies at a mean follow-up of 8.8 months.38 This correlates with the commonly seen persistence of benefits at 9 months with intradetrusor injections.16,18
Conclusion
Neurogenic detrusor overactivity is a common diagnosis encountered in people with MS. Its social impact is greater than that of any other complication associated with MS.2 The use of anticholinergic therapies is a good first-line treatment approach but unfortunately is either inefficacious or poorly tolerated in up to 30% of patients.11 A second-line therapy now exists for those suffering from NDO who are refractory to anticholinergic therapy. Botulinum toxin application into the detrusor has been shown to decrease the occurrence of incontinence, ameliorate urodynamic parameters, and improve quality of life for 9 months after a single injection,16,18,20,21 and these benefits are being maintained with repeated injections at 6 years.20,37 Botulinum toxin also leads to a decreased incidence of UTIs when associated with CIC22 and protects against vesicoureteral reflux and associated upper urinary tract pathologies.20 Botulinum toxin intradetrusor injections are a cost-effective way to control the symptoms of NDO.19 Currently, one of the largest cost components of the procedure is the need for anesthesia.19 Fortunately, a new method of administration using electromotive forces may eliminate the need for anesthesia in patients with preserved bladder sensation.34,35
PracticePoints.
Bladder dysfunction in MS can be socially disabling, have negative psychological and economic consequences, and impair patients' quality of life.
Treatments for neurogenic detrusor overactivity (NDO) have consisted mainly of administration of anticholinergic drugs, which have been shown to provide suboptimal clinical benefits and be poorly tolerated.
The introduction of botulinum neurotoxin for the management of NDO offers a cost-effective second-line treatment method that controls NDO symptoms and improves quality of life in MS patients.
Acknowledgments
The authors would like to thank the following people for their valuable contributions to the manuscript editing process: James Burns, MD, John Greenlee, MD, and Dana Dewitt, MD, of the Department of Neurology at the University of Utah Hospital.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Kim JH. Management of urinary and bowel dysfunction in multiple sclerosis. In: Giesser BS., editor. Primer on Multiple Sclerosis. New York, NY: Oxford University Press; 2011. pp. 197–206. [Google Scholar]
- 2.Hemmet L, Holmes J, Barnes M et al. What drives quality of life in multiple sclerosis? QJM. 2004;97:671–676. doi: 10.1093/qjmed/hch105. [DOI] [PubMed] [Google Scholar]
- 3.Hennessey A, Robertson NP, Swingler R et al. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. J Neurol. 1999;246:1027–1032. doi: 10.1007/s004150050508. [DOI] [PubMed] [Google Scholar]
- 4.Betts CD, D'Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:245–250. doi: 10.1136/jnnp.56.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Sèze M, Ruffion A, Denys P et al. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007;13:915–928. doi: 10.1177/1352458506075651. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer DR. The cervical cord in multiple sclerosis. Neuropathol Appl Neurobiol. 1978;4:151–162. doi: 10.1111/j.1365-2990.1978.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 7.Blaivas JG, Kaplan SA. Urologic dysfunction in patients with multiple sclerosis. Semin Urol. 1988;8:159–164. doi: 10.1055/s-2008-1041371. [DOI] [PubMed] [Google Scholar]
- 8.Wyndaele JJ, Castro D, Madersbacher H . Neurogenic and faecal incontinence. In: Abrams P, editor. Incontinence. Paris: Health Publications; 2005. pp. 1059–1162. [Google Scholar]
- 9.Nicholas RD, Friede T, Hollis S et al. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009;1:CD004193. doi: 10.1002/14651858.CD004193.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Neel KF, Soliman S, Salem M et al. Botulinum-A toxin: solo treatment for neuropathic noncompliant bladder. J Urol. 2007;178:2593–2598. doi: 10.1016/j.juro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Hay-Smith J, Ellis G, Herbison GP. Which anticholinergic drug for overactive bladder symptoms in adults (review) Cochrane Database Syst Rev. 2005;3:CD005429. doi: 10.1002/14651858.CD005429. [DOI] [PubMed] [Google Scholar]
- 12.Tsui JKC. Botulinum toxin as a therapeutic agent. Pharmacol Ther. 1996;72:13–24. doi: 10.1016/s0163-7258(96)00091-5. [DOI] [PubMed] [Google Scholar]
- 13.Simpson LL. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 14.Schiavo G, Shone CC, Rosetto O et al. Botulinum neurotoxin serotype-F is a zinc endopeptidase specific for VAMP/synaptobrevin. J Biol Chem. 1993;268:11516–11519. [PubMed] [Google Scholar]
- 15.Foran PG, Mohammed N, Lisk GO et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A: basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- 16.Schurch B, Stöhrer M, Kramer G et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? preliminary results. J Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Schurch B, Schmid DM, Stöhrer M. Treatment of neurogenic incontinence with botulinum toxin A. N Engl J Med. 2000;342:665. doi: 10.1056/NEJM200003023420918. [DOI] [PubMed] [Google Scholar]
- 18.Reitz A, Stöhrer M, Kramer G et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004;45:510–515. doi: 10.1016/j.eururo.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Kalsi V, Popat RB, Apostolidis A et al. Cost-consequence analysis evaluating the use of botulinum neurotoxin-A in patients with detrusor overactivity based on clinical outcomes observed at a single UK center. Eur Urol. 2006;49:519–527. doi: 10.1016/j.eururo.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Giannantoni A, Mearini E, Del Zingaro M et al. Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: clinical and urodynamic results. Eur Urol. 2009;55:705–712. doi: 10.1016/j.eururo.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Ehren I, Volz D, Farrelly E et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomized, placebo-controlled, double-blind study. Scand J Eurol Nephrol. 2007;41:335–340. doi: 10.1080/00365590601068835. [DOI] [PubMed] [Google Scholar]
- 22.Gamé X, Castel-Lacanal E, Bentaleb Y et al. Botulinum toxin A detrusor injections in patients with neurogenic detrusor overactivity significantly decrease the incidence of symptomatic urinary tract infections. Eur Urol. 2008;53:613–619. doi: 10.1016/j.eururo.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Grosse J, Kramer G, Stöhrer M. Success of repeat detrusor injections of botulinum A toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur Urol. 2005;47:653–659. doi: 10.1016/j.eururo.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Meguid TA. Botulinum toxin-A injections into neurogenic overactive bladder—to include or exclude the trigone? a prospective, randomized, controlled trial. J Urol. 2010;184:2423–2428. doi: 10.1016/j.juro.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–943. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- 26.Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sui GP, Rothery S, Dupont E et al. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–120. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V, Cross RL, Chess-Williams R et al. Recent advances in basic science for overactive bladder. Curr Opin Urol. 2005;15:222–226. doi: 10.1097/01.mou.0000172393.52857.92. [DOI] [PubMed] [Google Scholar]
- 29.Chapple C. Patel A: Botulinum toxin—new mechanisms, new therapeutic directions? Eur Urol. 2006;49:606–608. doi: 10.1016/j.eururo.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Apostolidis A, DasGupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in treatment of human detrusor overactivity. Eur Urol. 2006;49:644–650. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Herschorn S, Gajweski J, Ethans K et al. Efficacy of botulinum toxin A injections for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J Urol. 2011;185:2229–2235. doi: 10.1016/j.juro.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Bach P, Wormland RT, Möhring C et al. Electromotive drug-administration: a pilot study for minimal-invasive treatment of therapy-resistant idiopathic detrusor overactivity. Neurourol Urodynam. 2009;28:209–213. doi: 10.1002/nau.20624. [DOI] [PubMed] [Google Scholar]
- 33.Schurch B, Reitz A, Tenti G. Electromotive drug administration of lidocaine to anesthetize the bladder before botulinum-A toxin injections into the detrusor. Spinal Cord. 2004;42:338–341. doi: 10.1038/sj.sc.3101593. [DOI] [PubMed] [Google Scholar]
- 34.Kajbafzadeh AK, Montaser-Kouhsari L, Ahmadi H. Intravesical electromotive botulinum toxin type A administration: Part I—experimental study. J Urol. 2011;77:1460–1464. doi: 10.1016/j.urology.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Kajbafzadeh AK, Montaser-Kouhsari L, Ahmadi H et al. Intravesical electromotive botulinum toxin type A administration: Part II—clinical application. J Urol. 2011;77:439–445. doi: 10.1016/j.urology.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Ruffion A, Capelle O, Paparel P et al. What is the optimum dose of type A botulinum toxin for treating neurogenic bladder overactivity? BJU Int. 2006;97:1030–1034. doi: 10.1111/j.1464-410X.2006.06091.x. [DOI] [PubMed] [Google Scholar]
- 37.Wyndaele JJ, Van Dromme SA. Muscular weakness as side effect of botulinum toxin injection for neurogenic detrusor overactivity. Spinal Cord. 2002;40:599–600. doi: 10.1038/sj.sc.3101318. [DOI] [PubMed] [Google Scholar]
- 38.Haferkamp A, Krengel U, Reitz A et al. Are botulinum-A toxin injections into the detrusor of patients with neurogenic detrusor overactivity safe? ultrastructural data of detrusor biopsies. Neurourol Urodynam. 2003;22:499–500. [Google Scholar]


