Abstract
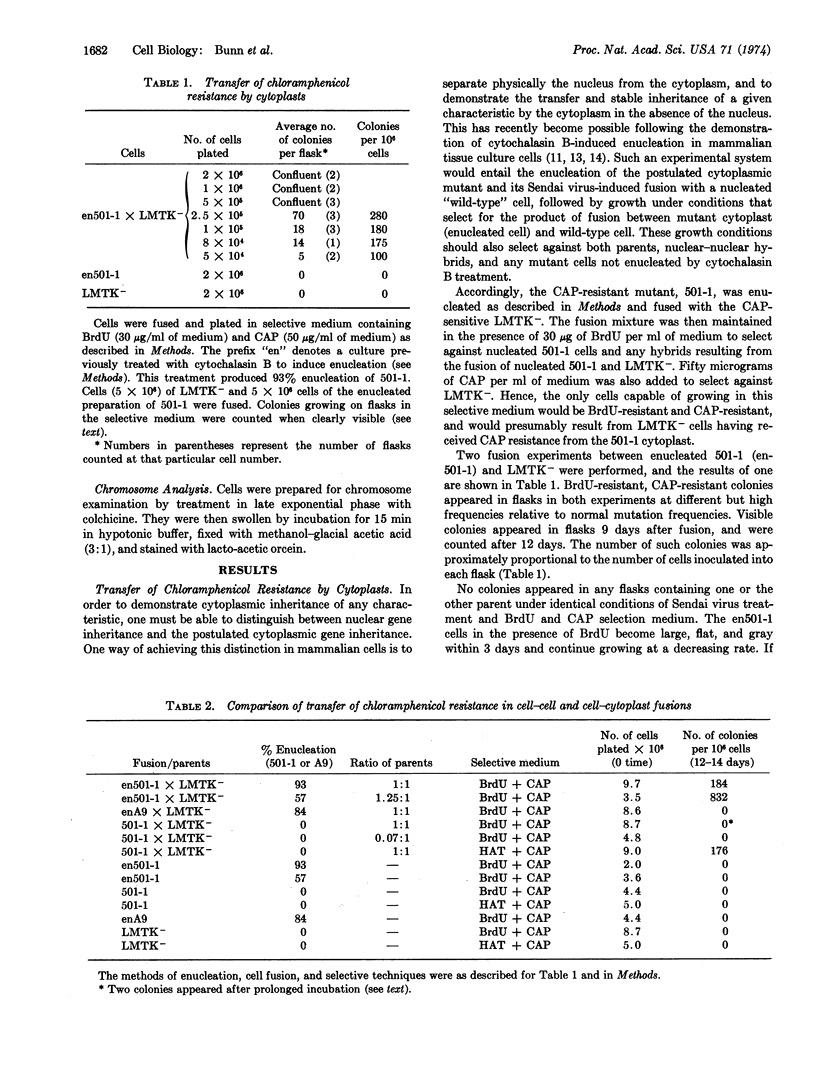
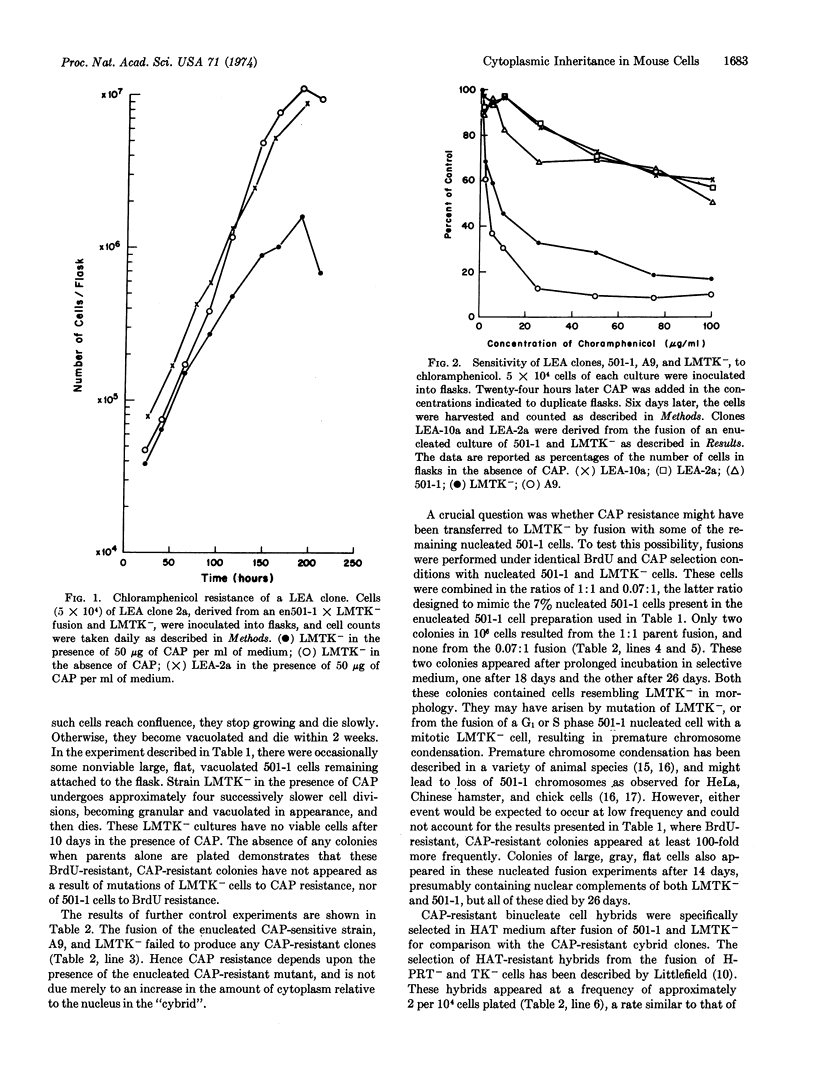
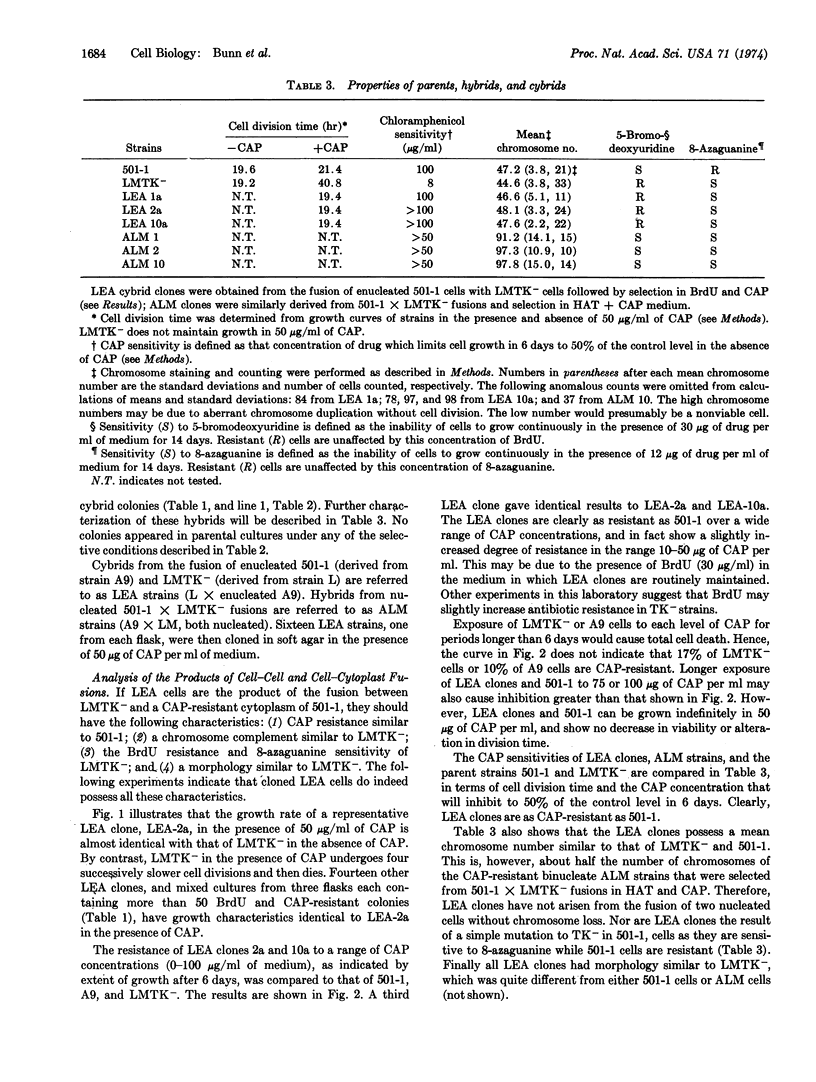
A chloramphenicol-resistant mutant, isolated from mouse A9 cells, was enucleated and fused with a nucleated chloramphenicol-sensitive mouse cell line. Resultant fusion products, cytoplasmic hybrids (or “cybrids”), were selected as resistant to chloramphenicol, and had the nuclear markers and chromosome complement of the chloramphenicol-sensitive parent. These cybrids appeared at the high frequency of 2-8 per 104 cells plated. Neither parent produced any colonies when plated under identical selective conditions. Fusion between enucleated chloramphenicol-sensitive cell fragments and the chloramphenicol-sensitive cell produced no resistant colonies, suggesting that chloramphenicol resistance is not due to an increase in the ratio of cytoplasm to nucleus. Furthermore, fusions between resistant and sensitive nucleated cells produced resistant hybrids at a frequency 100 times less than that of resistant cybrids. Thus, these stable chloramphenicol-resistant cybrids result from the fusion of a chloramphenicol-resistant cytoplasm with a chloramphenicol-sensitive cell. It is proposed, therefore, that chloramphenicol resistance is a cytoplasmically inherited characteristic in this mouse cell line.
Keywords: enucleation, 5-bromodeoxyuridine resistance, cell fusion, mitochondria, somatic cell genetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Attardi G. Fate of mitochondrial DNA in human-mouse somatic cell hybrids (density gradient centrifugation-ethidium bromide-karyotype). Proc Natl Acad Sci U S A. 1972 Jan;69(1):129–133. doi: 10.1073/pnas.69.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. I-DNA: Its packaging into I-somes and its relation to protein synthesis during differentiation. Nature. 1969 Oct 25;224(5217):326–328. doi: 10.1038/224326a0. [DOI] [PubMed] [Google Scholar]
- Bond H. E., Cooper J. A., 2nd, Courington D. P., Wood J. S. Microsome-associated DNA. Science. 1969 Aug 15;165(3894):705–706. doi: 10.1126/science.165.3894.705. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Teplitz R. L., Nabholz M., Dovey H., Bodmer W. Mitochondrial DNA of human-mouse cell hybrids. Nature. 1971 Dec 31;234(5331):560–562. doi: 10.1038/234560a0. [DOI] [PubMed] [Google Scholar]
- Coen D., Deutsch J., Netter P., Petrochilo E., Slonimski P. P. Mitochondrial genetics. I. Methodology and phenomenology. Symp Soc Exp Biol. 1970;24:449–496. [PubMed] [Google Scholar]
- Coon H. G., Horak I., Dawid I. B. Propagation of both parental mitochondrial DNAs in rat-human and mouse-human hybrid cells. J Mol Biol. 1973 Dec 15;81(3):285–298. doi: 10.1016/0022-2836(73)90142-3. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Enucleation of cells made simple and rescue of SV40 by enucleated cells made even simpler. Virology. 1973 Jan;51(1):227–229. doi: 10.1016/0042-6822(73)90382-6. [DOI] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Differential effects of chloramphenicol on the growth and respiration of mammalian cells. Biochem Biophys Res Commun. 1968 Aug 13;32(3):398–402. doi: 10.1016/0006-291x(68)90674-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Rao P. N., Hughes H. D. Mammalian cell fusion. 3. A HeLa cell inducer of premature chromosome condensation active in cells from a variety of animal species. J Cell Physiol. 1970 Oct;76(2):151–157. doi: 10.1002/jcp.1040760204. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kislev N., Spolsky C. M., Eisenstadt J. M. Effect of chloramphenicol on the ultrastructure of mitochondria in sensitive and resistant strains of HeLa. J Cell Biol. 1973 May;57(2):571–579. doi: 10.1083/jcb.57.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THE INOSINIC ACID PYROPHOSPHORYLASE ACTIVITY OF MOUSE FIBROBLASTS PARTIALLY RESISTANT TO 8-AZAGUANINE. Proc Natl Acad Sci U S A. 1963 Sep;50:568–573. doi: 10.1073/pnas.50.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A., Kohne D. E., Lerner R. A. Physical properties of cytoplasmic membrane-associated DNA. J Mol Biol. 1973 Jun 25;78(1):43–56. doi: 10.1016/0022-2836(73)90427-0. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Premature chromosome condensation: a mechanism for the elimination of chromosomes in virus-fused cells. J Cell Sci. 1972 Mar;10(2):495–513. doi: 10.1242/jcs.10.2.495. [DOI] [PubMed] [Google Scholar]
- Schneider W. C., Kuff E. L. The association of deoxyribonucleic acid with liver microsomes. J Biol Chem. 1969 Sep 25;244(18):4843–4851. [PubMed] [Google Scholar]
- Schwartz A. G., Cook P. R., Harris H. Correction of a genetic defect in a mammalian cell. Nat New Biol. 1971 Mar 3;230(1):5–8. doi: 10.1038/newbio230005a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- WELSHONS W. J., GIBSON B. H., SCANDLYN B. J. Slide processing for the examination of male mammalian meiotic chromosomes. Stain Technol. 1962 Jan;37:1–5. doi: 10.3109/10520296209114560. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]



