Abstract
Fatigue and cognitive impairment are debilitating features of multiple sclerosis (MS). ENER-G was a 12-month, open-label, multicenter, single-arm observational study designed to evaluate changes in fatigue and cognition in MS patients treated with natalizumab. Adults with relapsing MS and initiating natalizumab were enrolled. The primary endpoint was change in Visual Analog Scale for Fatigue (VAS-F) score over 12 weeks. Changes in Modified Fatigue Impact Scale (MFIS) score, Fatigue Severity Scale (FSS) score, and cognitive performance, using Automated Neuropsychological Assessment Metrics (ANAM), were also assessed. Patients (N = 89) had a mean age of 41 years and a median Expanded Disability Status Scale score of 3.0, and 83% had used at least two prior MS therapies. Significant improvements were observed and maintained at 12 weeks in VAS-F (mean ± SD baseline score, 77.7 ± 10.2; mean ± SD change, −14.9 ± 17.1; P < .0001), MFIS (mean baseline score, 59.1 ± 12.2; mean change, −7.4 ± 11.8; P < .0001), and FSS (median baseline score, 6.3 [range, 3.9–7.0]; median change, −0.4 [range, −2.9–1.4]; P < .0001). Cognitive performance remained stable or improved (depending on the ANAM measure). Thus significant improvements in fatigue were maintained over time, and cognitive performance improved or remained stable up to 48 weeks after initiation of natalizumab in MS patients with some degree of fatigue.
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) caused by an aberrant immune response against CNS antigens and characterized by inflammation and progressive demyelination. Myelin damage ultimately leads to clinical manifestations of MS, including fatigue, cognitive impairment, pain, spasticity, and depression.1
Fatigue is common in MS and can severely limit daily activities. In a large registry study, severe fatigue was reported by more than 70% of respondents and was associated with reduced employment.2 MS patients consider fatigue one of their most disabling symptoms,3 likely because of its negative impact on daily work, family life, and social activities.2 It is associated with a perception of impaired general health, mental state, and quality of life (QOL) and may have a stronger effect on QOL than physical disability alone.4,5
Cognitive impairment, reported in up to 70% of MS patients, may result from fatigue and/or neurologic damage and can occur independently of physical disability.6,7 Aspects of cognitive functioning most commonly affected in MS patients include processing speed, learning, and memory.6–9 Characterization of the relationship between cognitive performance and fatigue is complicated by the subjective experience of patients.10 While patients often believe that fatigue contributes to poor cognitive performance, most research has not found a consistent association between fatigue and actual cognitive performance. However, some studies have found positive associations between increased fatigue and impairments in processing speed, attention, and other cognitive functions.11,12
Natalizumab (Tysabri, Biogen Idec Inc., Cambridge, MA), a monoclonal antibody against very-late-activation antigen 4, reduces leukocyte migration across the blood-brain barrier and has been shown to substantially reduce both clinical and magnetic resonance imaging (MRI) signs of central inflammatory activity in MS.13 In patients with highly active MS, natalizumab treatment has been shown to reduce fatigue using two fatigue outcome measures, the Modified Fatigue Impact Scale (MFIS) and the Fatigue Severity Scale (FSS).14 Fatigue and cognitive deficits are associated with disability.15,16 Although fatigue and cognition are not explicitly captured by the Expanded Disability Status Scale (EDSS), a reduction in sustained disability progression as measured by the EDSS is seen with natalizumab as compared with placebo.13 Here we report findings from the Evaluation of Natalizumab for thE Relief of MS Associated FatiGue (ENER-G) study, which was designed to evaluate changes in and correlations between fatigue and cognition in natalizumab-treated patients with relapsing MS.
Materials and Methods
Study Design and Patients
ENER-G was a multicenter, nonrandomized, open-label, single-arm, 12-month study conducted in 32 centers in the United States. It enrolled 89 patients aged 18 to 55 years with relapsing forms of MS who were naive to natalizumab and had an inadequate response to or were unable to tolerate other MS therapies. Enrollment criteria also required that patients exhibit fatigue (baseline average Visual Analog Scale for Fatigue [VASF] score ≥60, determined from three separate visits at least 7 days apart), have an EDSS score between 0.0 and 5.5, and be enrolled in the Tysabri Outreach: Unified Commitment to Health (TOUCH®) Prescribing Program. Exclusion criteria included primary progressive or secondary progressive MS without relapses, prior or current clinically significant medical illness or laboratory abnormality (including progressive multifocal leukoencephalopathy), and (in female patients) pregnancy, current breastfeeding, or plans to become pregnant. Patients unable to complete the fatigue (MFIS, FSS, and VAS-F) and cognition (Automated Neuropsychological Assessment Metrics [ANAM]) testing sessions were also excluded. Use of medications to treat fatigue was allowed; the protocol requested that fatigue medication use be optimized and stabilized 1 month before the screening visit and consistently maintained for the duration of the study. The study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guideline on Good Clinical Practice. All patients provided written informed consent. The study protocol and consent documents were approved by the institutional review boards of the participating centers.
Interventions and Assessments
Enrolled patients received natalizumab (300 mg intravenously every 4 weeks) and completed evaluations of fatigue and cognition at three visits at least 7 days apart, including the baseline visit, prior to initiating natalizumab therapy and at weeks 4, 8, 12, 24, and 48 after initiating natalizumab treatment. The primary endpoint was change in VAS-F score 12 weeks after initiating treatment with natalizumab. The VAS-F is a visual tool in which the patient is asked to place an arrow on a line that reads left to right from “no fatigue” to “severe fatigue.” Entries are graded on a corresponding scale of 0 to 100.17 The VAS-F was chosen as the primary outcome measure because it is a global assessment of overall fatigue, in contrast to MFIS and FSS, which measure the impact of fatigue on functioning or quality of life.
Secondary endpoints included the change in VAS-F score 24 and 48 weeks after initiating natalizumab treatment, as well as the change in MFIS and FSS scores 12, 24, and 48 weeks after initiating natalizumab treatment. The MFIS is a valid and reliable 21-question scale (score range, 0–84), with lower total scores indicating lower impact of fatigue on patient function. Subscales within the MFIS evaluate the impact of fatigue on physical functions (range, 0–36), cognitive functions (range, 0–40), and psychosocial functions (range, 0–8).18 The FSS is a validated scale containing nine statements concerning the severity, frequency, and impact of fatigue on daily life. Individuals rate their agreement with each statement on a scale of 1 (do not agree) to 7 (agree). Lower scores indicate less severity, frequency, and impact of fatigue.19
Cognitive function was assessed as a tertiary endpoint using computerized ANAM, a reliable and reproducible screening instrument designed for repeat evaluations.20 ANAM is sensitive and specific in identifying patients with cognitive difficulties and in evaluating cognitive function changes in individual patients.21 From the battery of cognitive tests within ANAM, the ENER-G study analyzed data for the Index of Cognitive Efficiency (ICE), Procedural Reaction Time (PRO), and Code Substitution Delayed Memory (CDD). These tests were selected a priori based on a previous study assessing MRI and cognitive test findings in patients with MS.22 Several scores are available for individual tests within ANAM, including task accuracy, mean response time for accurate responses, and a throughput score, defined as the number of correct responses per usable minute.
PRO is a choice reaction time measure that requires the participant to differentiate between two sets of characters on a computer screen. In the CDD, participants must recall, following a 25-minute delay, how symbols and digits have been paired in a “key” that they are shown.20,23 ICE is a weighted summary score capturing the entire ANAM battery performance. The ICE score is computed by weighting the throughput scores from five ANAM tests (PRO, CDD, Matching-to-Sample, Logical Relations, and Mathematical Processing) so that each score contributes equally to the total ICE score.
Statistical Analyses
Baseline demographic and disease characteristics including age, gender, disease duration, EDSS score, number of relapses in the past year, time since most recent relapse, and therapy prior to enrollment were summarized using descriptive statistics. Baseline VAS-F, MFIS, and FSS fatigue scores were calculated as an average of scores from three visits within the 45 days prior to the first natalizumab infusion and at least 7 days apart from one another. Week 12 VAS-F, MFIS, and FSS fatigue scores were calculated as an average of the scores from the week 4, 8, and 12 visits. Average scores, which were calculated using all available data without imputation of missing data, were used to maximize the consistency, accuracy, and stability of fatigue scores. Using average scores also increased the number of patients included in the analysis, thus increasing statistical power.
Changes from baseline in VAS-F and MFIS scores were analyzed using a paired t test, and changes from baseline in FSS scores were analyzed using a nonparametric Wilcoxon signed rank test. Analysis of covariance was used to assess associations between patient baseline characteristics and changes from baseline in fatigue scores at 12 weeks. Changes in fatigue measures over time up to week 48 were assessed using a linear mixed model with random effects for the intercept and time. The effect of the medications used to treat fatigue was also evaluated in the linear mixed models.
For cognitive data, a linear mixed-effects model was also employed, with random effects for intercept and slope to investigate time effects on ICE, CDD, and PRO throughout the 48 weeks of natalizumab treatment. To assess relationships between fatigue data (VASF, MFIS, and FSS) and ICE, CDD, and PRO cognitive data, Spearman correlation coefficients were determined for baseline data and for the average of all postbaseline measurements. Use of averaged data was meant to provide more reliable scores given the variability of these measures. Analyses were performed using the intent-to-treat population; all patients with at least one prespecified postbaseline assessment were included.
Results
Patient Characteristics
Of the 179 patients who were screened, a total of 89 patients were enrolled in ENER-G and were treated with natalizumab. Reasons for elimination at the screening stage included degree of fatigue too low (n = 49), EDSS score out of range (n = 12), withdrawal of consent (n = 9), age out of range (n = 6), other (n = 5), insurance issues (n = 4), previous natalizumab exposure (n = 3), and loss to follow-up (n = 2). Fifteen enrolled patients (16.9%) discontinued natalizumab and withdrew from the study. The reasons for study withdrawal included “other” (n = 9), withdrawal of consent (n = 2), voluntary discontinuation for reasons other than adverse event (n = 2), subject lost to follow-up (n = 1), and subject noncompliant (n = 1).
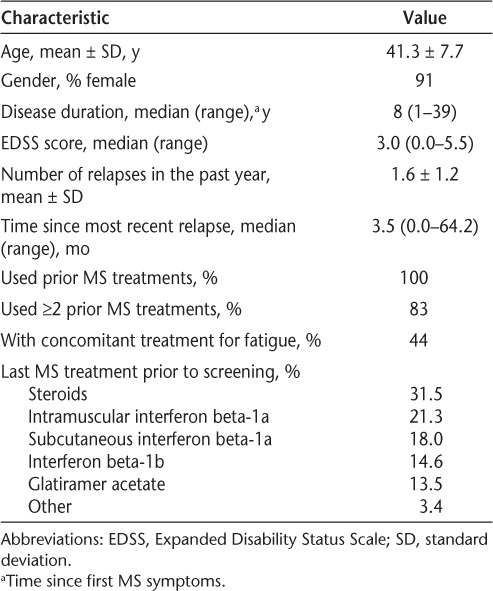
Patient demographics and disease characteristics are shown in Table 1. Enrolled patients received a median of 13 natalizumab doses (range, 1–13).
Table 1.
Demographic and disease characteristics of the study population (N = 89)
Changes in Fatigue During Treatment with Natalizumab
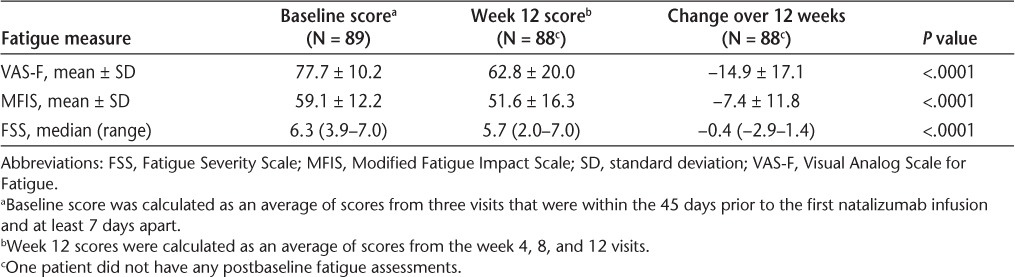
Patients in the study had evidence of fatigue at baseline, with average scores of 77.7, 59.1, and 6.3 (median) on the VAS-F, MFIS, and FSS, respectively (Table 2). Over 3 months of natalizumab therapy, there were significant improvements in the primary fatigue measure of change in mean VAS-F score from baseline to week 12 (−14.9; P < .0001) and in change in the total MFIS and FSS scores from baseline to week 12 (P < .0001 for both parameters) (Table 2). Significant improvements were also observed in all MFIS subscales from baseline to week 12. The mean changes (± standard deviation) in the physical, cognitive, and psychosocial subscale scores were −3.7 ± 5.29, −3.1 ± 5.76, and −0.7 ± 1.52, respectively (n = 88; P ≤ .0001 for all comparisons). No significant associations were found between change in fatigue measures over time (baseline to week 12) and baseline characteristics, including age, gender, EDSS score, disease duration, previous relapses, time since last relapse, and most recent therapy.
Table 2.
Changes in fatigue scale scores from baseline to week 12
All three fatigue measures showed significant improvements (P < .0001) with natalizumab that were maintained over time (Table 3; Figure 1). Improvement was seen both in patients with a recent relapse (≤3 months) prior to initiation of natalizumab and in patients whose most recent relapse was more than 3 months prior to initiation of natalizumab (data not shown). There was no statistically significant difference in the score changes from baseline to any postbaseline time point for any of the three fatigue measures when comparing patients with and without a recent relapse.
Table 3.
Summary statistics from mixed-effects regression analyses of fatigue measures in patients receiving natalizumab for 48 weeks
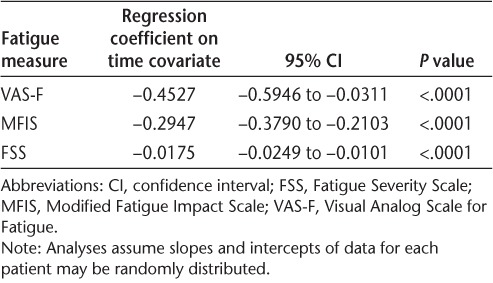
Figure 1.
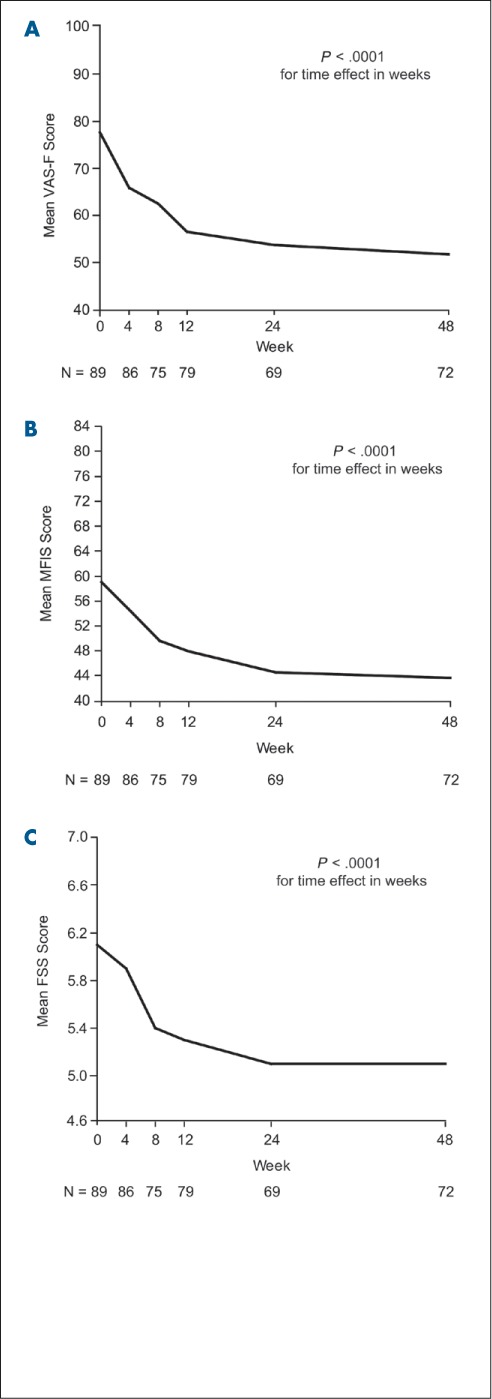
VAS-F (A), MFIS (B), and FSS (C) scores from baseline to week 48
Negative (downward) slope indicates improvement. FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; VAS-F, Visual Analog Scale for Fatigue.
Both patients using fatigue medications and patients not using fatigue medications showed an improvement in fatigue measures over time (Figure 2). Analyses of the use of fatigue medications and fatigue outcomes (VASF, FSS, and MFIS) showed a significant effect of fatigue medications on outcomes over time for VAS-F scores only (regression coefficient for the interaction between time and use of fatigue medications = 0.2991, P = .0336). Both patient groups showed a significant reduction in VAS-F scores over time. No effect of fatigue medication was seen for FSS or MFIS scores.
Figure 2.
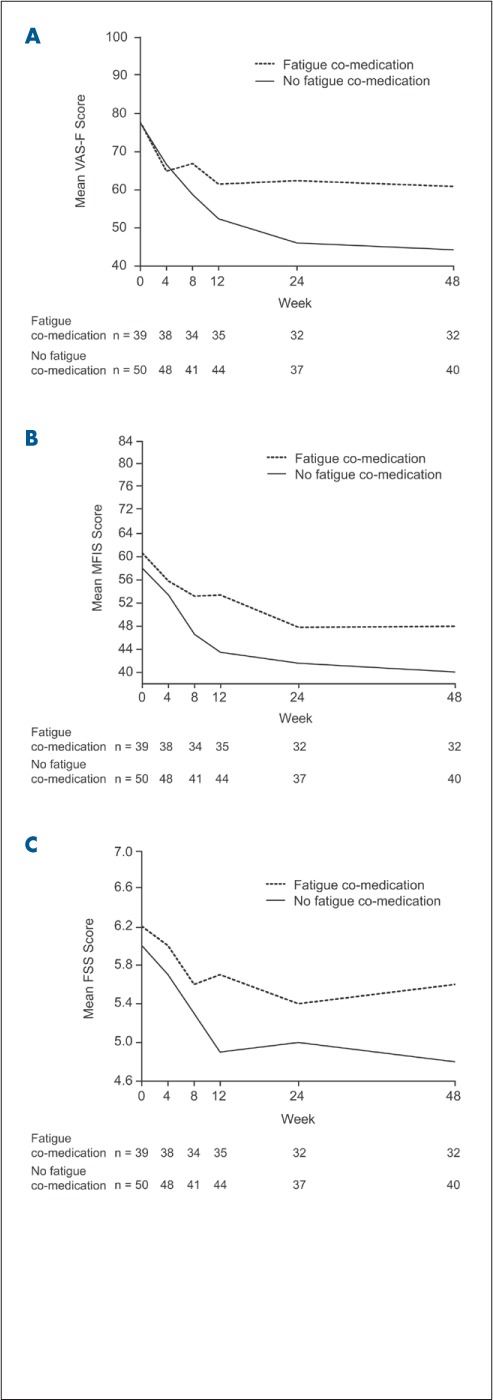
VAS-F (A), MFIS (B), and FSS (C) scores from baseline to week 48 stratified by use of co-medication for fatigue
Negative (downward) slope indicates improvement. FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; VAS-F, Visual Analog Scale for Fatigue.
Changes in Cognition During Treatment with Natalizumab
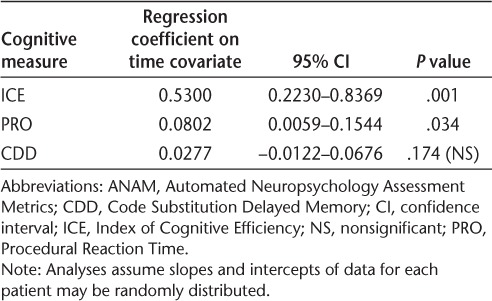
Baseline values and changes in ANAM measures of cognitive function over the 48 weeks of natalizumab treatment are shown in Figure 3. The cognitive measures ICE and PRO showed significant improvement across the 48 weeks of treatment (P = .001 and P = .034, respectively), while CDD scores remained stable (Table 4). ICE scores showed improvements starting at week 8 and PRO scores showed improvements starting at week 4; both ICE and PRO scores stabilized by week 12. CDD scores increased over time, starting at week 4, but the change was not significant (P = .174) (Figure 3).
Figure 3.
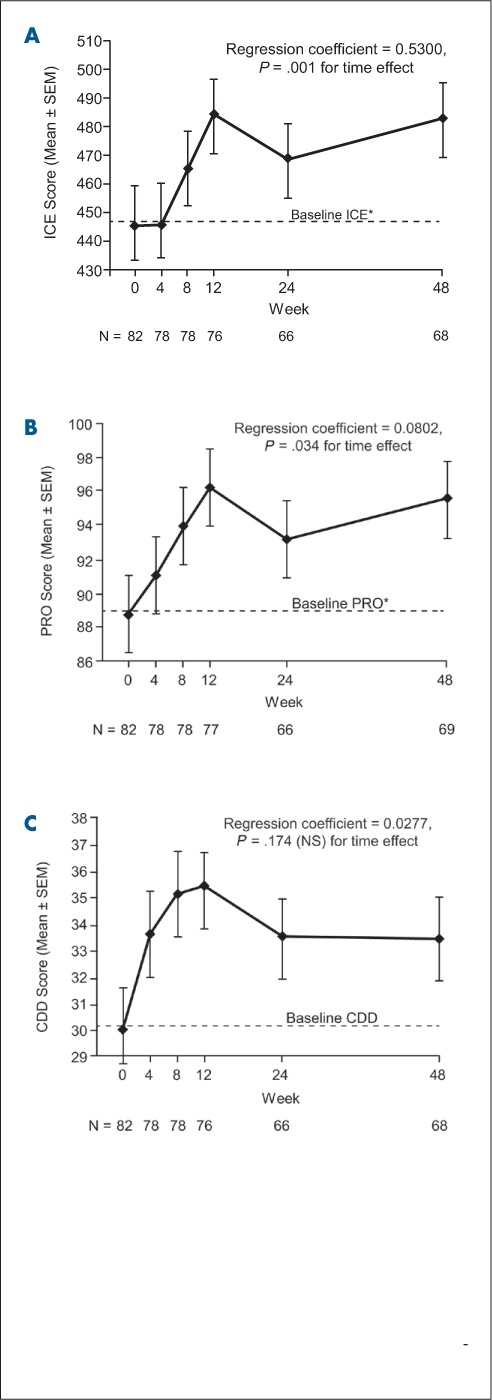
ICE (A), PRO (B), and CDD (C) scores from baseline to week 48 in patients receiving natalizumab
*Average over three visits within the 45 days prior to the first natalizumab infusion. Positive (upward) slope indicates improvement. CDD, Code Substitution Delayed Memory; ICE, Index of Cognitive Efficiency; NS, not significant; PRO, Procedural Reaction Time; SEM, standard error of the mean.
Table 4.
Summary statistics from mixed-effects regression analyses of cognitive measures from ANAM in patients receiving natalizumab for 48 weeks
Correlations Between Fatigue and Cognitive Measures
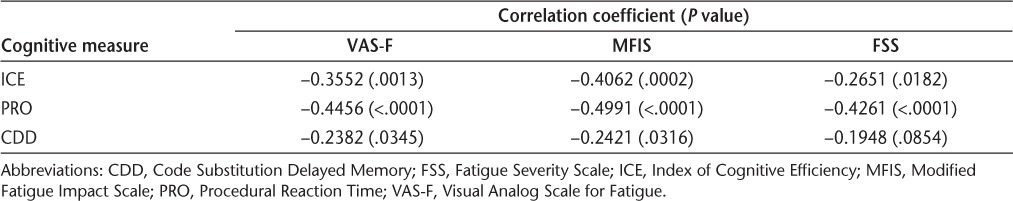
At baseline, no significant correlations between cognitive measures and VAS-F score were seen. In contrast, all cognitive measures were significantly correlated with MFIS score, such that cognitive function declined as MFIS score increased (Table 5). PRO and CDD cognitive data showed significant correlations with FSS at baseline, while the weighted summary score ICE, which includes contributions from match to sample, logical relations, and mathematical processing scores in addition to PRO and CDD, failed to show a significant correlation with FSS (Table 5). When analyses were performed for the averages of all postbaseline data, significant correlations were observed for all pairs except CDD with FSS (Table 6). CDD showed the weakest correlations with all fatigue measures, possibly reflecting in part the absence of significant improvement in CDD, as seen in the regression analysis.
Table 5.
Spearman correlation coefficients between cognitive measures (ICE, PRO, and CDD) and fatigue measures (VAS-F, MFIS, and FSS) at baseline
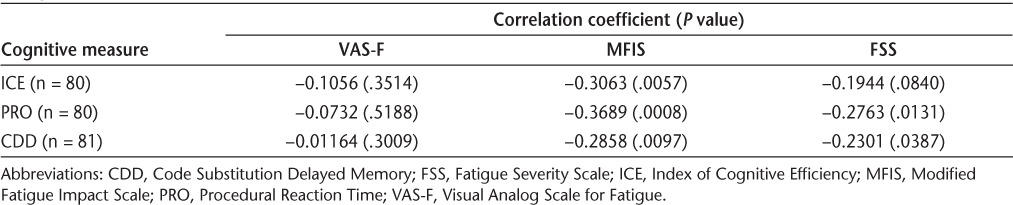
Table 6.
Spearman correlation coefficients between averages of all postbaseline cognitive measures (ICE, PRO, and CDD) and fatigue measures (VAS-F, MFIS, and FSS) in patients receiving natalizumab for 48 weeks (n = 79)
Discussion
Natalizumab has shown clinical benefits for MS patients, including reduced relapse rate, slowed disability progression, fewer MRI lesions, and reduced fatigue.13,14,24,25 ENER-G is the largest prospective study to evaluate fatigue and cognition during natalizumab treatment, and to examine correlations between the two. Both fatigue and cognitive decline can be debilitating for MS patients. In this study, we provide evidence that fatigue and cognitive decline were reduced in MS patients on natalizumab therapy.
With natalizumab therapy, patients experienced significant improvements in fatigue, as measured by three patient-reported scales, and the improvements that were observed during the first 12 weeks of treatment were maintained for up to 48 weeks of treatment. These results are consistent with those of a smaller study that found reduced fatigue with natalizumab at 6 months compared with baseline.14 Natalizumab has also been found to be associated with reduced fatigue as compared with other disease-modifying therapies after longer-term use.25 In the current study, improvement in fatigue scores was seen in patients with and without a recent relapse and in patients who were and were not using co-medication for fatigue, suggesting that the effect was not due to recovery from a relapse or use of new fatigue medications but possibly a result of natalizumab therapy. Test scores measuring reaction time (PRO) and pooled scores measuring numerous cognitive skills (including recall, matching, logical relations, mathematical processing, and reaction time) (ICE) showed statistically significant improvements over the 48 weeks of natalizumab treatment. In contrast, CDD, a measure of memory function, remained stable during the 1-year study.
Previous studies have reported improved cognitive test scores during natalizumab therapy.26–30 A large Swedish study demonstrated a significant increase in mean Symbol Digit Modalities Test score at 2 years over baseline in natalizumab-treated relapsing-remitting MS patients.26 A follow-up analysis comparing AFFIRM patients treated with natalizumab with those treated with placebo showed a significant improvement in Paced Auditory Serial Addition Test score at 2 years.30 In addition, an open-label study showed that after 6 months of natalizumab therapy, patients demonstrated improved test scores on four of six different cognitive tests, with no deterioration in any test, despite disease duration of more than 10 years.27 Our results using ANAM, a sensitive measure of multiple cognitive skills, support and extend these observations that cognitive function may be maintained and potentially improved with natalizumab. Because of potential practice effects with cognitive tests, and in the absence of a control group, it cannot be definitively determined whether cognitive function improved in ENER-G patients. However, there was no evidence of worsening cognitive status, and the improvement in test scores suggested, at minimum, that cognition remained stable and that patients retained the ability to learn and benefit from practice.
While fatigue and cognitive decline in MS have great clinical relevance, the underlying etiologies remain unclear. Fatigue in MS may be related to impaired interactions among functionally related cortical and sub-cortical areas, it could be a side effect of depression, or both.15,31,32 MS-related cognitive dysfunction could be a function of fatigue, central inflammation, neurodegeneration, neuroendocrine dysregulation, and/or depression.33
This study is the first to document moderate correlations between measurements of fatigue and cognitive function at baseline and during natalizumab treatment. The associations between measurements of fatigue and cognitive function observed in this study further support linkage between the two MS symptoms, but the specific interactions and causality remain unclear. It has been proposed that cognitive impairment may increase fatigue in MS and that both outcomes are closely linked to the regional brain atrophy and lesions characteristic of MS pathology. Fatigue may also exacerbate cognitive impairment.34,35
A limitation of the ENER-G trial is the lack of a placebo group or active comparator, which precludes definitive conclusions about the effects of natalizumab on the studied endpoints. In addition, results from patients using medications to treat fatigue or recovering from recent relapse may have had confounding effects, although our data suggest otherwise. In patients with MS, fatigue and cognition may worsen with time.36,37 Given the long duration of this trial, if natalizumab had little or no effect, one might expect an average increase (worsening) in fatigue scores and evidence of increasing cognitive dysfunction. In contrast, ENER-G data demonstrate a sustained positive effect of natalizumab on measures of fatigue and cognition in MS patients. The results also reinforce the growing role of patient-reported outcomes in providing important measures of treatment and contributing to the overall understanding of natalizumab for the treatment of MS. When making treatment decisions, health-care providers should consider data from other, nonstandard outcome measures as well.
PracticePoints.
Fatigue and cognitive impairment are common and debilitating features of MS.
MS patients treated with natalizumab experienced significant improvements in fatigue within the first 12 weeks of therapy, and improvements were maintained for up to 48 weeks.
Cognitive performance improved or remained stable for up to 48 weeks in patients initiating natalizumab.
Correlations observed between cognitive and fatigue scores support a linkage between fatigue and cognitive impairment in MS.
Acknowledgments
Medical writing assistance was provided by Britt Anderson, PhD, and editorial support was provided by Jackie Cannon and Joshua Safran, all from Infusion Communications in Haddam, CT. Their work was funded by Biogen Idec Inc. and Elan Pharmaceuticals, Inc.
Footnotes
Financial Disclosures: Dr. Wilken has been a consultant for Bayer and Biogen Idec and has received research support from Biogen Idec and Genzyme. Dr. Kane has been a consultant for Biogen Idec. Dr. Sullivan has been a consultant for Genzyme and has received research support from Biogen Idec. Dr. Gudesblatt has received consulting fees from Biogen Idec, Medtronic, and Teva Neuroscience. Dr. Lucas has been a consultant for Bayer, Biogen Idec, and EMD Serono; has received honoraria or speaker's fees from Biogen Idec, EMD Serono, Genzyme, and Novartis; and has received research support from Biogen Idec and Sanofi-Aventis. Dr. Fallis has been a consultant for Biogen Idec and Teva Neuroscience. Dr. You and Dr. Foulds are employees of Biogen Idec.
Funding/Support: This study was funded by Biogen Idec Inc. and Elan Pharmaceuticals, Inc.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100. doi: 10.1186/1477-7525-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 4.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7:340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- 5.Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. 2002;205:51–58. doi: 10.1016/s0022-510x(02)00312-x. [DOI] [PubMed] [Google Scholar]
- 6.Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. 2002;16:445–455. doi: 10.2165/00023210-200216070-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 8.Benedict RH, Bruce JM, Dwyer MG. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63:1301–1306. doi: 10.1001/archneur.63.9.1301. et al. [DOI] [PubMed] [Google Scholar]
- 9.Benedict RH, Shucard JL, Zivadinov R, Shucard DW. Neuropsycho-logical impairment in systemic lupus erythematosus: a comparison with multiple sclerosis. Neuropsychol Rev. 2008;18:149–166. doi: 10.1007/s11065-008-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinsinger SW, Lattie E, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsycho-logical functioning in patients with multiple sclerosis. Neuropsychology. 2010;24:573–580. doi: 10.1037/a0019222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23:189–199. doi: 10.1016/j.acn.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, Elkins LE. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology. 2000;55:934–939. doi: 10.1212/wnl.55.7.934. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, O'Connor PW, Havrdova E. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. et al. [DOI] [PubMed] [Google Scholar]
- 14.Putzki N, Yaldizli O, Tettenborn B, Diener HC. Multiple sclerosis associated fatigue during natalizumab treatment. J Neurol Sci. 2009;285:109–113. doi: 10.1016/j.jns.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Kroencke DC, Lynch SG, Denney DR. Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler. 2000;6:131–136. doi: 10.1177/135245850000600213. [DOI] [PubMed] [Google Scholar]
- 16.Lynch SG, Parmenter BA, Denney DR. The association between cognitive impairment and physical disability in multiple sclerosis. Mult Scler. 2005;11:469–476. doi: 10.1191/1352458505ms1182oa. [DOI] [PubMed] [Google Scholar]
- 17.Rudick RA, Miller D, Hass S. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–346. doi: 10.1002/ana.21163. et al. [DOI] [PubMed] [Google Scholar]
- 18.Téllez N, Río J, Tintoré M, Nos C, Galán I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler. 2005;11:198–202. doi: 10.1191/1352458505ms1148oa. [DOI] [PubMed] [Google Scholar]
- 19.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 20.Reeves DL, Winter KP, Bleiberg J, Kane RL. ANAM genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol. 2007;22(suppl 1):S15–S37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Kane RL, Roebuck-Spencer T, Short P, Kabat M, Wilken J. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch Clin Neuropsychol. 2007;22(suppl 1):S115–S126. doi: 10.1016/j.acn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Pellicano C, Kane RL, Gallo A. Cognitive impairment and its relation to imaging measures in multiple sclerosis: a study using a computerized battery. J Neuroimaging. 2013;23:445–452. doi: 10.1111/j.1552-6569.2011.00687.x. et al. [DOI] [PubMed] [Google Scholar]
- 23.Wilken JA, Kane R, Sullivan CL. The utility of computerized neuropsychological assessment of cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2003;9:119–127. doi: 10.1191/1352458503ms893oa. et al. [DOI] [PubMed] [Google Scholar]
- 24.Rudick RA, Stuart WH, Calabresi PA. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. et al. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz M, Tettenborn B, Putzki N. Multiple sclerosis-associated fatigue during disease-modifying treatment with natalizumab, interferon-beta and glatiramer acetate. Eur Neurol. 2011;65:231–232. doi: 10.1159/000324028. [DOI] [PubMed] [Google Scholar]
- 26.Holmen C, Piehl F, Hillert J. A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler. 2011;17:708–719. doi: 10.1177/1352458510394701. et al. [DOI] [PubMed] [Google Scholar]
- 27.Lang C, Reiss C, Maurer M. Natalizumab may improve cognition and mood in multiple sclerosis. Eur Neurol. 2012;67:162–166. doi: 10.1159/000334722. [DOI] [PubMed] [Google Scholar]
- 28.Mattioli F, Stampatori C, Bellomi F, Capra R. Natalizumab efficacy on cognitive impairment in MS. Neurol Sci. 2011;31(suppl 3):321–323. doi: 10.1007/s10072-010-0351-0. [DOI] [PubMed] [Google Scholar]
- 29.Morrow SA, O'Connor PW, Polman CH. Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler. 2010;16:1385–1392. doi: 10.1177/1352458510378021. et al. [DOI] [PubMed] [Google Scholar]
- 30.Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(suppl 3):S8–S15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese M, Rinaldi F, Mattisi I. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74:321–328. doi: 10.1212/WNL.0b013e3181cbcd03. et al. [DOI] [PubMed] [Google Scholar]
- 32.Filippi M, Rocca MA, Colombo B. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage. 2002;15:559–567. doi: 10.1006/nimg.2001.1011. et al. [DOI] [PubMed] [Google Scholar]
- 33.Heesen C, Schulz KH, Fiehler J. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav Immun. 2010;24:1148–1155. doi: 10.1016/j.bbi.2010.05.006. et al. [DOI] [PubMed] [Google Scholar]
- 34.Bradshaw J, Rose A. Cognition, depression and fatigue in multiple sclerosis. Adv Clin Neurosci Rehabil. 2008;8:15–17. [Google Scholar]
- 35.Winkelmann A, Engel C, Apel A, Zettl UK. Cognitive impairment in multiple sclerosis. J Neurol. 2007;254(suppl 2):II35–II42. doi: 10.1007/s00415-007-2010-9. [DOI] [PubMed] [Google Scholar]
- 36.Johansson S, Ytterberg C, Hillert J, Widen HL, von Koch L. A longitudinal study of variations in and predictors of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:454–457. doi: 10.1136/jnnp.2007.121129. [DOI] [PubMed] [Google Scholar]
- 37.Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis: a controlled 3-year follow-up. Brain. 1997;120(pt 2):289–297. doi: 10.1093/brain/120.2.289. [DOI] [PubMed] [Google Scholar]


