Abstract
This article reviews adherence to medication in multiple sclerosis (MS) patients from the perspective of nurse and social worker authors. It reviews data on patient adherence and offers practical, evidence-based strategies that health-care providers can use to facilitate adherence. In addition, it examines how emerging MS therapies may affect patient adherence and associated interventions. To promote adherence, interventions need to incorporate new and creative approaches. A proactive approach includes assessing patient needs and lifestyle before the start of medication and selecting the most appropriate disease-modifying therapy for each individual patient. Including multidisciplinary expertise and services in the treatment plan can be part of a comprehensive, holistic approach to helping patients and families. Optimization of health-care provider roles is likely to facilitate improved adherence.
Multiple sclerosis (MS) is a disease that often begins relatively early in adult life and progresses over time, exerting a profound effect on quality of life in most patients and often imposing a considerable economic burden.1 While available treatments for MS do not provide a cure, disease-modifying therapies (DMTs) have been shown effective in limiting the number of relapses and preventing new lesion formation on brain magnetic resonance imaging (MRI), as well as in reducing disability progression.2 These improvements in disease management are not a trivial achievement, given that this most common disabling neurologic disease of young people was, until the early 1990s, considered almost uniformly disabling.
As former US surgeon general C. Everett Koop noted, “drugs don't work in patients who don't take them.”3 It is important to make individualized treatment recommendations for each patient and provide education on the benefits of adherence to treatment. Suboptimal adherence to treatment has a negative impact on patient morbidity and mortality outcomes as well as on the overall cost of patient care.4
There are numerous benefits associated with treatment adherence that extend beyond a lower risk of clinical and radiographic relapses and reduced disability progression. Adherence to medication corresponds with lower rates of emergency room visits, hospital stays, and absences from work. These factors can significantly affect patient quality of life and treatment outcomes. Benefits for health-care providers, payers, and society at large, such as diminished costs and increased productivity, have also been associated with patient adherence (Figure 1). In a recent large (N = 2446) study on the impact of adherence to DMTs on clinical and economic outcomes in MS, the adherent group was significantly less likely to be hospitalized for MS-related reasons or to experience relapses than was the nonadherent group (with nonadherence defined as <80% medication possession ratio); adherent patients also incurred lower medical costs overall.6 Research in patients with MS has shown that higher rates of adherence are linked to a lower risk of severe relapse8 and that longer gaps (>90 days) during which individuals are nonadherent are linked to a higher risk of severe relapse.9 Post hoc analysis of cumulative dose and cumulative time on treatment in a long-term follow-up study of patients with relapsing-remitting MS (RRMS) who received subcutaneous (SC) interferon beta-1a (IFNb-1a) suggested that high exposure to this DMT may be associated with better clinical outcomes in MS patients than low exposure. The authors emphasized the importance of medication adherence in light of these findings.10
Figure 1.
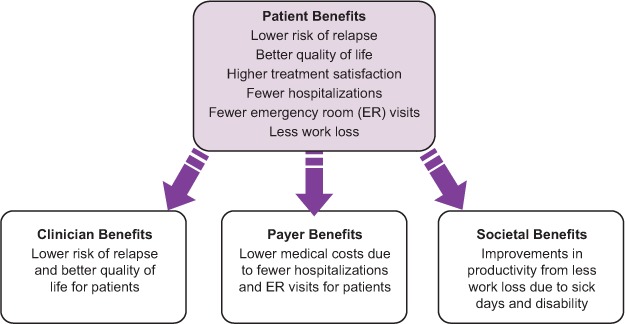
The benefits of adherence
Note: Based on Phillips et al.,5 Tan et al.,6 and Treadaway et al.7
Optimizing adherence to prescribed treatment is an ongoing challenge for clinicians who treat people with MS. Treatment is sometimes prescribed when the patient is feeling relatively healthy, so the benefits of treatment may not be easily recognized by the patient. This article reviews patient adherence—past, present, and future—from the perspective of nurse and social worker authors. It includes a review of studies on patient adherence, with an emphasis on MS-specific studies. The article offers practical, evidence-based strategies that clinicians can use to facilitate patient adherence. The article also examines how emerging MS therapies may affect patient adherence and associated interventions.
Overview of Adherence Data: Literature Review
Adherence has been defined by the World Health Organization (WHO) as “the extent to which a person's behavior—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed recommendations from a healthcare provider.”11 It consists of three distinct categories: acceptance, persistence, and compliance. The patient must first accept the fact that he or she needs treatment, and then continue to take the treatment over time (persistence). Compliance refers to taking a medication as prescribed—the right dose at the right time and with the right frequency.12
It is essential that health-care providers understand data on patient adherence, which can help to inform appropriate initial selection of therapeutic agents for patients starting treatment, as well as for those who may need to switch treatment. A meta-analysis of adherence studies across many therapeutic areas found an average rate of adherence of 75%.13 However, the WHO estimates an average adherence rate of 50% among chronically ill patients in the developed world.11 Overall rates of patient adherence to DMTs for MS range from approximately 61% to 87%, with variations from study to study as discussed below. It is important to note that adherence may be overestimated, depending on the method used to measure it. In a recent study in patients with MS, patient reports tended to overestimate adherence as compared with electronic needle disposal monitoring.14
Several recent studies have compared patient adherence rates for the various injectable DMTs for MS—intramuscular (IM) and SC IFNβ-1a, IFNβ-1b, and glatiramer acetate (GA)—and identified the factors that most frequently influence adherence.7,15–18 Results of the largest of these studies are summarized in Table 1. A multicenter observational study of adherence to IM IFNβ-1a, SC IFNβ-1a, IFNβ-1b, and GA therapies over 2 months found overall rates of adherence by all patients for all treatments between 61% and 64%. Adherence rates for the individual injectable drugs ranged from 79% (IM IFNβ-1a) to 49% (IFNβ-1b and GA). Patient-reported reasons for nonadherence included (in order from highest to lowest percentage) forgetting to take medication, “didn't feel like it,” being “tired of shots,” and fatigue.7,15
Table 1.
Studies comparing adherence among DMTs
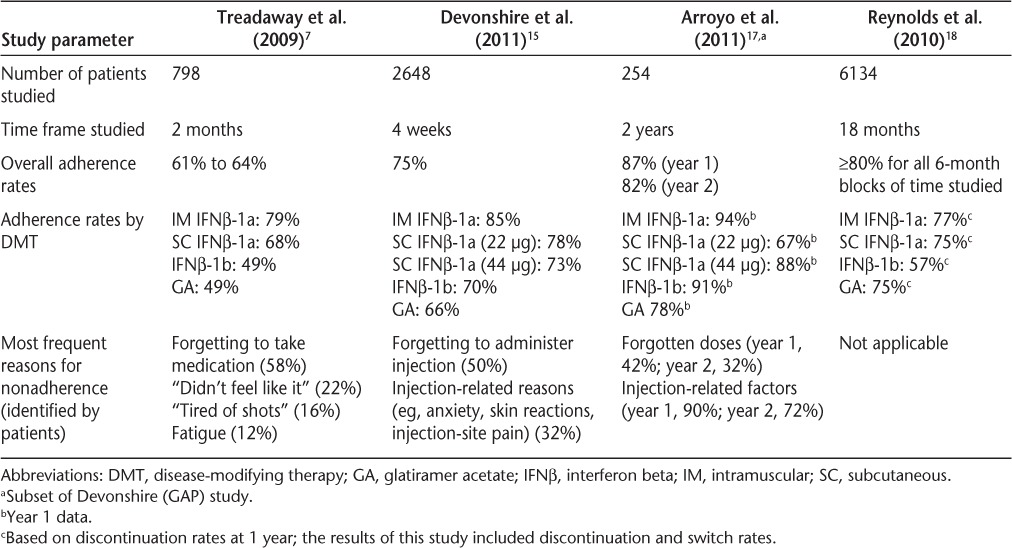
In another multicenter observational study of patients who had been treated with DMTs for at least 6 months, the international Global Adherence Project (GAP), approximately 75% of patients were adherent with injectable MS therapies over the 4-week study period. Adherence rates for the injectable drugs during this time period ranged from 85% (IM IFNβ-1a) to 66% (GA).15 Adherence rates were similar for longer-term treatment over 57 to 192 months, ranging from 87% (IM IFNβ-1a) to 65% (GA).16 Forgetting to administer the injection was the most common reason for nonadherence (affecting 50% of patients); more than 30% of patients cited injection-related reasons, such as anxiety regarding injections, adverse skin reactions, or injection-site pain.15 A recent article on interim (2-year) results in a Spanish subset of patients in a 5-year follow-up to the international GAP study also noted that the adherence rate for injectable DMTs, which was high overall (82% at 2 years for all patients), was highest at years 1 and 2 for those taking IM IFNβ-1a and significantly higher at year 1 for IM IFNβ-1a than for SC IFNβ-1a 22 μg (93.9% vs. 66.7%; P = .0251).17 The most common reasons for lack of adherence were forgotten doses and injection-related factors (especially patients' tiring of injections), which were noted at both baseline and follow-up visits. As in earlier studies, frequency of administration, medication side effects, and patient perceptions of treatment efficacy also affected nonadherence.
A large retrospective study of the PharMetrics claims database evaluated rates of switching and discontinuation as well as persistence and adherence rates for patients receiving IM IFNβ-1a, SC IFNβ-1a, IFNβ-1b, and GA. The overall rate of compliance for 6-month periods was at least 80% for each drug studied. Switching rates were similar between drugs; discontinuation rates were lowest for IM IFNβ-1a and highest for IFNβ-1b. Both formulations (IM and SC) of IFNβ-1a had similar rates of persistence, which were higher than the persistence rates for IFNβ-1b or GA.18 While it should be taken into account that studies of insurance claims databases may not be a complete representation of the population currently in treatment (types of MS are not always distinguished and diagnoses are not necessarily confirmed), these studies of large numbers of insured patients can help to identify patterns of adherence in conjunction with other studies.
Although individual therapies' adherence rates in different studies are shown in Table 1, it is inappropriate to make direct comparisons between injectable medications. The exact effect of missing a particular injection or series of injections on treatment efficacy is unknown.
Summary of Factors Influencing Patient Adherence
Adherence can be difficult to quantify, and the specific combination of factors that may affect a particular patient's adherence can be difficult to determine. As shown in Table 1, injection-related reasons and forgetting to take medication are factors frequently reported by patients to affect adherence.
The physical and cognitive decline that often accompanies chronic illness can impede patients' ability to obtain medication, report for follow-up, fully understand the need for medication, and remember scheduled medication times.4 Factors that generally influence adherence in chronic illness, particularly when cognition is a factor (as in MS), include the frequency and complexity of the dosing regimen,19 the need for specialized dosing or administration requirements (eg, the need for ongoing safety monitoring, office visits, or waiting time associated with dosing), and the presence or absence of active symptoms at the time of dosing.4
Data from clinical trials have identified myriad factors affecting adherence that are specific to patients with MS, including neuropsychological issues, patient self-efficacy, duration of disease and treatment (with shorter durations associated with greater adherence), issues associated with reduced quality of life, and patient perceptions of benefits and risks.20 The economic burden experienced by patients must also be evaluated on a case-by-case basis. Even seemingly subtle nuances of insurance plans can have a negative effect on adherence. One study found that the 10% increase in cost sharing required of patients who paid coinsurance (as opposed to copayments) for DMTs was associated with a decrease in adherence rates of nearly 9%.21 Table 2 summarizes potential interventions for factors commonly cited as affecting adherence.
Table 2.
Summary of interventions to promote adherence

Additional Factors Affecting Adherence
Patient Self-Efficacy
The patient's judgment that he or she is capable of organizing and implementing an intended action, known as self-efficacy, has been identified as a key factor supporting patient adherence. For example, a patient learning to properly self-administer injections must overcome any initial aversion to the injection process and continue to apply associated knowledge and skilled technique over the long term.20 Adherence rates in chronic illness tend to be lower over time than those in acute illness.3,19 In the case of MS, persistence drops precipitously after the first 6 months of therapy,3 and long-term adherence is more likely to be achieved if patients remain on treatment throughout the initial period. Adverse events (AEs), which may present a challenge at first, tend to diminish over time on treatment.22 In light of the risk of diminished adherence over time in MS, it is important that the development of patient self-efficacy be supported beginning with initial contact and maintained over the long term.
Patient self-efficacy and other factors potentially affecting adherence should be evaluated in an ongoing fashion in order to optimize adherence over the long term. The 18-item Multiple Sclerosis Self-Efficacy (MSSE) scale can be a useful tool to help ascertain the patient's level of self-efficacy. It is a patient-rated scale that has been found to be predictive of adherence and has been tested for reliability.30 The MSSE consists of two subscales, one of which measures the patient's belief in his or her ability to control the MS. The other subscale measures the patient's belief in his or her ability to function with MS.30
Patient-Clinician Relationships and Communication
Research has shown that patients who perceive themselves as having a high-quality relationship with their clinician are more likely to adhere to treatment recommendations. The clinician-patient relationship is a crucial factor affecting adherence, especially as treatment continues over the long term.4 Both the duration and the nature of the clinician-patient relationship can affect adherence. The Spanish GAP study noted that nurses tended to be more involved with treatment administration during treatment initiation. Study results suggested that ongoing nursing involvement can facilitate longer-term adherence.13
According to Becker's Health Belief Model,33 the tendency of patients to engage in treatment depends on four types of expectations: 1) the perceived severity of the illness, 2) the perceived vulnerability to that illness, 3) perceived benefits expected from the specific health behavior, and 4) perceived barriers to engaging in the specific health behavior. The clinician can work with the patient to explore each of these expectations and provide information that will help inform an accurate patient perspective.
One tool that can enable improved communication and education is motivational interviewing, which has been associated with improved adherence in MS as well as other therapeutic areas.20 A principle of this approach is that the relationship between clinician and patient must be such that it enables the patient, who may be feeling ambivalent about treatment, to positively change his or her behavior in a collaborative relationship with the clinician.34 In motivational interviewing, the clinician uses a structured, goal-oriented approach to actively listen, reinforce positive behavior, and help the patient explore his or her ambivalence and determine an action plan.35
Several studies in MS have reported improved adherence and a lower discontinuation rate when telephone-based counseling is offered by nurses,20 paralleling findings of studies in therapeutic areas such as cardiovascular disease and diabetes.4 The same principle of employing technology to offer timely support and reminders to patients has been applied with the more recent use of text-messaging in other therapeutic areas, including infectious disease.36 Collaboration with a social worker can also help to optimize communication with and education of the patient and his or her family, as well as facilitate interdisciplinary communication regarding the patient.37 The patient's quality of life can be further improved by social worker assistance with practical matters, including identifying available resources and obtaining disability insurance and financial assistance that may be needed to help cover treatment and medication costs.38
Case Studies
Note: The following cases represent experiences with actual patients and preceded the availability of an oral treatment for MS.
Case 1: The Recently Diagnosed, Nonadherent College Student
Alana is a 20-year-old female college student recently diagnosed with RRMS. She was prescribed SC IFNβ-1a injections upon diagnosis but was not able to adhere to therapy. She lives in a dormitory setting and feels self-conscious about injections in the presence of her roommate.
Following titration of SC IFNβ-1a, Alana reported flu-like symptoms, and premedication with ibuprofen was offered. If flu-like symptoms persist, she may benefit from sustained-release naproxen pretreatment, as well as a repeat dose the next morning. The DMT can also be tried at night, as for many patients flu-like symptoms can be mitigated with evening dosing.39 Alternatively, given Alana's history of nonadherence, it may be prudent to consider a switch to a DMT option that requires less frequent dosing, such as IM IFNβ-1a, if other strategies prove unsuccessful.
On a follow-up visit, Alana reports trouble remembering injections, mentioning that she has missed at least 1 to 2 doses per week of SC IFNβ-1a since starting about a month ago. She goes to parties on weekends and often forgets her Friday evening doses. She also reports no relief of flu-like symptoms with the ibuprofen. She has been resistant to her mother's offer to call her with medication reminders. It is recommended that Alana schedule her medication to coincide with her Monday/Wednesday/Friday morning class schedule, since the structured routine she follows to get ready for class can facilitate her remembering her medication. She is switched to sustained-release naproxen pretreatment to prevent flu-like symptoms. Alana agrees to let her mother remind her with text messages rather than phone calls.
Alana returns to the clinic for a follow-up visit 1 month later. Her switch to a Monday/Wednesday/Friday morning medication schedule has been helpful, and the naproxen has helped to alleviate the flu symptoms before she goes out with friends on Friday evenings. The text-message alerts from her mother have helped to remind her about her medication without causing unnecessary interruption or embarrassment in social situations. She is also encouraged to bring her roommate, to whom she is close, to a clinic visit to allow the roommate to learn more about MS and Alana's required injections.
Case 2: The “Frequent Flyer” Nonadherent Patient
Jacob is a 43-year-old technical writer diagnosed with RRMS. He has been taking daily GA injections for approximately 1 year. Recently, he admits to noncompliance, mainly due to the requirements of his new job: he travels half of each week and often travels internationally. He often misses doses when he is en route to another country, forgets to take the injection entirely, or mistakenly leaves his GA in the hotel room or at home when he has to rush to the airport. He reports that he does not mind injecting himself but does mind having to remember to do it every day.
Jacob's 6-month follow-up visit with his nurse practitioner (NP) reveals that he is missing three or four injections per week. He asks if there is a therapy that he can take less frequently, and IM IFNβ-1a is recommended. The NP explains that this is an intramuscular rather than a subcutaneous injection as with GA. She also reviews the potential for side effects such as flu-like symptoms (including fever, chills, fatigue, and body aches) and injection-site reactions. They discuss how he can help mitigate flu-like symptoms by taking one or two doses of a nonsteroidal anti-inflammatory drug (NSAID) 1 to 2 hours prior to the injection and, if necessary, repeating the dose of NSAID the next day. They also go over the need to allow the injection to come to room temperature prior to administration and to ensure proper drug storage, and the clinician recommends routine laboratory work (complete blood count, complete metabolic profile, and thyroid-stimulating hormone) every 6 months to monitor liver function, white blood cell count, and thyroid function while on interferon therapy. Jacob decides it will work well with his schedule to take his injection after returning home from business travel each week. Injection training is arranged so that a nurse can visit Jacob at home and teach him how an IM injection differs from a SC one. Jacob is also instructed to gradually titrate the dose of IFNβ-1a by taking one-quarter of the dose the first week, one-half of the dose the next week, three-quarters the third week, and finally the full dose the fourth week and thereafter.
Three months after initiation of therapy with IFNβ-1a, Jacob returns to the MS clinic for a follow-up visit. He reports dramatically improved DMT adherence after switching to weekly IM IFNβ-1a, having found it easier to take one injection after returning home from business travel than to travel with multiple injections and inject in hotel rooms. He has also worked out a system to remember to take the shot. He takes it every Saturday evening before bedtime, because that is always the end of his workweek, even when he travels. Because he takes his injection at bedtime, he reports that he does not experience fatigue as a side effect. He initially had some problems with flu-like symptoms after he titrated to full dose, but a prescription called in by the clinic for sustained-release naproxen sodium (one to two tablets 1 hour prior to injection) has helped to prevent any fever, chills, or body aches.
Case 3: A New Mother at Risk for Complete Nonadherence
Stephanie is a 32-year-old married woman who was diagnosed with RRMS at age 22 and started treatment with IM IFNβ-1a shortly thereafter. She has been stable on therapy for 10 years and currently uses no assistance to walk. She discontinued treatment with IM IFNβ-1a before becoming pregnant. She reports flu-like symptoms (despite the use of the same premedication she used before pregnancy) and depression since restarting IM IFNβ-1a following delivery of her son. She describes having a more difficult time being adherent to therapy because she is feeling overwhelmed, cannot seem to remember if or when she took her injection each week, and is expending more energy in taking care of her new baby without getting adequate sleep. She questions the efficacy of the medication, declaring that if she “feels this bad, it's probably not working and not worth dealing with the side effects.” Brain MRI reveals two small new T2 lesions without enhancement.
Stephanie is at increased risk for complete nonadherence, which can put her at risk for a decline in her health and quality of life. It is important for her to recognize that if she slows the progression of her disease, she will be better able to care for her son. The clinician educates Stephanie on the expected course and manifestations of MS as well as medication side effects. She is given positive feedback for starting therapy so soon after being diagnosed, which helps set the stage for motivational interviewing. The clinician recognizes that Stephanie's psychological state may also have a negative effect on adherence and that Stephanie should be evaluated for depression, including postpartum depression. In a recent study, memory difficulties, anxiety, depression, neuroticism, and low levels of conscientiousness were all associated with poor adherence; patients with MS who had current mood or anxiety disorders were nearly five times as likely as those with no psychiatric diagnoses to have problems adhering to prescribed DMTs.40 These findings are consistent with reports on the negative effect of depression on adherence, both in patients with MS and in other chronically ill populations.7 Stephanie is referred to a counselor with experience in treating patients with MS for psychiatric evaluation. Depression may also be associated with fatigue,41 so interventions that target fatigue may have some positive effect. Stephanie is reassured that psychosocial assessment is recommended for all patients and that intervention may be required only temporarily.
Stephanie is reminded that flu-like symptoms may diminish over time; however, despite trials of premedication with an oral corticosteroid and NSAIDs and a change in her medication administration time, Stephanie continues to complain of flu-like symptoms. She discontinues IFNβ-1a and starts GA, which is less likely to be associated with flu-like symptoms or depressive symptoms. The weekly IFNβ-1a injections were hard for Stephanie to keep track of; she feels that she will benefit from picking a daily activity that helps remind her to inject GA each day (eg, she could inject after taking a shower or before a daily walk outside with her son), which would reduce the complexity of the dosing regimen. Since she self-injects, she can easily rotate injection sites and may be able to use an autoinjector. Stephanie is also reminded of the importance of site rotation and the need to report any injection-site reactions.
Three months after the initial discussion regarding nonadherence, Stephanie returns to the clinic with her healthy baby and reports improved adherence on the new regimen. She has also found her new therapist to be helpful, but she worries about the effect of her illness on her family.
This patient is in need of support that extends to her family as well, so that they, in turn, can support her adherence with her medication. Research has found that married patients with MS who receive greater perceived support from their spouses tend to adhere more to treatment.7 Stephanie is given a referral for a counselor who may be helpful for her husband. Children of parents with MS can also be incorporated into the care plan. Stephanie and her clinician will speak further about how to incorporate her MS diagnosis into her son's life as he grows up so that he can better understand how to support her. The National Multiple Sclerosis Society (NMSS) and the Multiple Sclerosis Association of America (MSAA) provide programs and camps specifically designed for families and children/teens. This can be an ideal way to meet other parents with MS. In the future, her son may want to attend clinic visits with her to ask questions, or her clinic may host educational programs for the children of patients.
The Future of Adherence to MS Care
The recently approved drugs fingolimod and teriflunomide, along with anticipated oral drugs such as BG-12, offer new options that may be particularly appealing to patients who have needle phobia or regularly experience injection-related AEs.28 Experts suggest that oral therapies may improve long-term adherence. Adherence data on new oral therapies are still limited and reflect adherence in a controlled clinical trial setting. Although oral therapies may prove convenient for some patients, it is also recognized that the safety and tolerability profiles of these drugs must be monitored over the long term before informed risk-benefit profiles can be fully developed.42
While research in other therapeutic areas (eg, oncology and diabetes) has shown that patients generally prefer oral medications, it is important that other factors affecting adherence be evaluated when considering any change in medication.22 Such factors as the complexity of the individual regimen, requirements for safety monitoring, and patient forgetfulness have the potential to negatively affect adherence to any therapy.43 Research in chronic illness has shown that adherence rates are higher with less frequent medication schedules.19 However, medication schedules must be considered on a case-by-case basis that takes into account patient preferences and patterns. For example, the recommended dosing for alemtuzumab (not yet approved in the United States for MS) is unusual in nature, consisting of 3- to 5-day courses of daily infusion treatment followed by a period of approximately 1 year before retreatment.44 This dosing regimen could either promote or detract from adherence depending on individual patient factors.
Another treatment for MS currently in development is SC peginterferon. This new therapy is being developed to reduce dosing frequency (with administration by injection either once every 2 weeks or once a month) in an effort to improve patient convenience, while maintaining the proven efficacy, safety, and tolerability of interferon therapy.45
It is important that new MS drugs be evaluated based not only on their efficacy and safety profiles but also on potential benefits or barriers to adherence. Over time, it will become more apparent how safety and tolerability issues and drug-specific requirements affect patient adherence to newer drugs in comparison with current platform therapies. The need for additional safety monitoring with certain treatments may affect patient adherence and should be considered prior to initiating therapy. Safety concerns can become apparent at any time during the use of any drug. For example, the US Food and Drug Administration (FDA) has recently recommended additional cardiovascular monitoring after the first dose of fingolimod as a result of a further safety evaluation following the death of a patient within 24 hours of the first dose of the drug.46
Discussion
Studies have shown that suboptimal adherence is linked to poor patient outcomes in MS as well as a higher cost of care. Discontinuation of therapy has been associated with an increased proportion of patients experiencing relapse and an increased level of disability. Improvements in adherence are likely to have a positive impact on patient outcomes. A wide range of factors influence adherence, and these factors should be evaluated on a case-by-case basis with an eye toward practical interventions to support the patient.
It is important that interventions promoting adherence incorporate new and creative approaches beyond traditional education and monitoring. A proactive approach on the part of the clinician includes assessment of patient needs and lifestyle even before the start of medication, which will, in turn, support selection of the most appropriate DMT for the individual patient. Incorporation of multidisciplinary expertise and services into the treatment plan can help ensure a comprehensive, holistic approach to helping patients and families. Clinicians, especially those with regular patient contact, are in a unique position to evaluate, provide support to, and educate the patient with MS on an ongoing basis. Optimization of these roles is likely to facilitate improved adherence.
PracticePoints.
Factors that can influence adherence to disease-modifying therapies (DMTs) include medication tolerability, patient physical and cognitive decline, the frequency and complexity of the dosing regimen, the duration of disease and treatment, patient perceptions of medication benefits and risks, and the economic burden associated with medication.
Interventions aimed at optimizing medication adherence by the patient with MS need to incorporate new and creative approaches that take individual patient needs and lifestyle into account.
When considering a DMT, it is important to evaluate the safety and tolerability profile of the drug, the individual patient's needs and lifestyle, and how the specific requirements and characteristics of the drug intersect with the individual patient's profile.
Acknowledgments
The authors would like to thank Elliot Frohman, MD, PhD, of the University of Texas Southwestern Medical Center in Dallas, TX, who served as an expert adviser. Medical writing assistance was provided by Katherine Hauswirth, RN, MSN, and editorial support was provided by Joshua Safran, both of Infusion Communications. Their work was funded by Biogen Idec Inc.
Footnotes
Financial Disclosures: Ms. Remington has received honoraria from the Consortium of Multiple Sclerosis Centers (CMSC), Genzyme, Biogen Idec, Acorda, and Teva. Ms. Logan has received consulting fees from Teva, Biogen Idec, Bayer, and Acorda. Ms. Treadaway has received honoraria for speaking from Teva. Ms. Rodriguez and Ms. Williamson have no conflicts of interest to disclose.
Funding/Support: Funding for this manuscript was provided by Biogen Idec Inc.
Note: Supplementary material for this article (a list of additional references (53.1KB, pdf) and two tables (134.5KB, pdf) containing additional information on disease-modifying therapies for the treatment of multiple sclerosis and resources to support patients) is available on IJMSC Online at ijmsc.org.
References
- 1.Brandes DW, Shaya FT, Pill MW. Quantifying the role of natalizumab in health and economic outcomes in multiple sclerosis. Am J Manag Care. 2010;16(suppl):S171–S177. [PubMed] [Google Scholar]
- 2.Trojano M, Paolicelli D, Tortorella C. Natural history of multiple sclerosis: have available therapies impacted long-term prognosis? Neurol Clin. 2011;29:309–321. doi: 10.1016/j.ncl.2010.12.008. et al. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Bubalo J, Clark RK Jr, Jiing SS. Medication adherence: pharmacist perspective. J Am Pharm Assoc. 2010;50:394–406. doi: 10.1331/JAPhA.2010.08180. et al. [DOI] [PubMed] [Google Scholar]
- 5.Phillips AL, Ivanova JI, Bergman RE. Toronto, Ontario, Canada: Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis. et al. Poster presented at: 62nd Annual Meeting of the American Academy of Neurology; April 10–17, 2010. [Google Scholar]
- 6.Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28:51–61. doi: 10.1007/s12325-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 7.Treadaway K, Cutter G, Salter A. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256:568–576. doi: 10.1007/s00415-009-0096-y. et al. [DOI] [PubMed] [Google Scholar]
- 8.Meletiche D, Dickson M, Kozma C. Association between adherence with multiple sclerosis disease modifying therapy and severe relapses using three measures of medication adherence. J Neurol. 2008a;255(suppl 2):P717. et al. [Google Scholar]
- 9.Al-Sabbagh A, Bennet R, Kozma C, Dickson M, Meletiche D. Medication gaps in disease modifying therapy for multiple sclerosis are associated with an increased risk of relapse: findings from a national managed care database. J Neurol. 2008;255(suppl 2):S79. [Google Scholar]
- 10.Uitdehaag B, Constantinescu C, Cornelisse P. Impact of exposure to interferon beta-1a on outcomes in patients with relapsing-remitting multiple sclerosis: exploratory analyses from the PRISMS long-term follow-up study. Ther Adv Neurol Disord. 2011;4:3–14. doi: 10.1177/1756285610391693. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 12.Devonshire V, Pfohl D, Perrin Ross A, Poplear L. Adherence to Disease-Modifying Therapy: Recognizing the Barriers and Offering Solutions. Ridgewood, NJ: Delaware Media Group; 2007. [Google Scholar]
- 13.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 14.Bruce JM, Hancock LM, Arnett P, Lynch S. Objective adherence monitoring in multiple sclerosis: initial validation and association with self-report. Mult Scler. 2010a;16:112–120. doi: 10.1177/1352458509351897. [DOI] [PubMed] [Google Scholar]
- 15.Devonshire V, Lapierre Y, Macdonell R. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77. doi: 10.1111/j.1468-1331.2010.03110.x. et al. [DOI] [PubMed] [Google Scholar]
- 16.Devonshire V, Lapierre Y, Macdonell R. Madrid, Spain: The Global Adherence Project (GAP): a multicentre observational study on adherence to disease-modifying therapies in patients suffering from relapsing-remitting multiple sclerosis. et al. Poster presented at: 22nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis; September 27–30, 2006. [Google Scholar]
- 17.Arroyo E, Grau C, Ramo-Tello C, Parra J, Sánchez-Soliño O. Adherence to disease-modifying therapies in patients with relapsing multiple sclerosis: two-year interim results of the Global Adherence Project. Eur Neurol. 2011;65:59–67. doi: 10.1159/000323216. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin. 2010;26:663–674. doi: 10.1185/03007990903554257. [DOI] [PubMed] [Google Scholar]
- 19.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:E22–E33. [PubMed] [Google Scholar]
- 20.Caon C, Saunders C, Smrtka J, Baxter N, Shoemaker J. Injectable disease-modifying therapy for relapsing-remitting multiple sclerosis: a review of adherence data. J Neurosci Nurs. 2010;42(5 suppl):S5–S9. doi: 10.1097/jnn.0b013e3181ee1240. [DOI] [PubMed] [Google Scholar]
- 21.Dor A, Lage MJ, Tarrants ML, Castelli-Haley J. Cost sharing, benefit design, and adherence: the case of multiple sclerosis. In: Dor A, editor. Pharmaceutical Markets and Insurance Worldwide. Bingley, UK: Emerald Group Publishing Limited; 2010. [DOI] [PubMed] [Google Scholar]
- 22.Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adher. 2010;4:1–9. doi: 10.2147/ppa.s8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matson MA, Zimmerman TR, Tuccillo D, Tang Y, Deykin A. Dose titration of intramuscular interferon beta-1a reduces the severity and incidence of flu-like symptoms during treatment initiation. Curr Med Res Opin. 2011;27:2271–2278. doi: 10.1185/03007995.2011.630720. [DOI] [PubMed] [Google Scholar]
- 24.Nadjar Y, Coutelas E, Prouteau P. Injection of interferon-beta in the morning decreases flu-like syndrome in many patients with multiple sclerosis. Clin Neurol Neurosurg. 2011;113:316–322. doi: 10.1016/j.clineuro.2010.12.013. et al. [DOI] [PubMed] [Google Scholar]
- 25.Singer B, Lucas S, Kresa-Reahl K, Ross AP, Blake P. Optimizing adherence to multiple sclerosis therapies. Int J MS Care. 2008;10:113–126. [Google Scholar]
- 26.Frohman EM, Brannon K, Alexander S. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302–307. doi: 10.1191/1352458504ms1002oa. et al. [DOI] [PubMed] [Google Scholar]
- 27.McEwan L, Brown J, Poirier J. Best practices in skin care for multiple sclerosis patients receiving injectable therapies. Int J MS Care. 2010;12:177–189. et al. [Google Scholar]
- 28.Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Exper Opin Drug Deliv. 2009;6:995–1002. doi: 10.1517/17425240903134769. [DOI] [PubMed] [Google Scholar]
- 29.Buhse M. Efficacy of EMLA cream to reduce fear and pain associated with interferon beta-1a injection in patients with multiple sclerosis. J Neurosci Nurs. 2006;38:222–226. doi: 10.1097/01376517-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Fraser C, Polito S. A comparative study of self-efficacy in men and women with multiple sclerosis. J Neurosci Nurs. 2007;39:102–106. doi: 10.1097/01376517-200704000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Grossman P, Kappos L, Gensicke H. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75:1141–1149. doi: 10.1212/WNL.0b013e3181f4d80d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtney SW. Imagine the possibilities: an introduction to guided imagery and its potential benefits for individuals with MS. Motivator. 2008. http://www.msassociation.org/publications/winter08/cover.story.asp. Accessed June 8, 2011.
- 33.Ogden J. Health psychology. In: Hewstone M, Fincham F, Foster J, editors. Psychology. Hoboken, NJ: Wiley-Blackwell; 2005. [Google Scholar]
- 34.Falvo DR. Effective Patient Education: A Guide to Increased Adherence. 4th ed. Sudbury, MA: Jones and Bartlett Publishers; 2010. [Google Scholar]
- 35.Gance-Cleveland B. Motivational interviewing: improving patient education. J Pediatr Health Care. 2007;21:81–88. doi: 10.1016/j.pedhc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Pop-Eleches C, Thirumurthy H, Habyarimana JP. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–834. doi: 10.1097/QAD.0b013e32834380c1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz C, Frohner R. Contribution of demographic, medical, and social support variables in predicting the mental health dimension of quality of life among people with multiple sclerosis. Health Soc Work. 2005;30:203–212. doi: 10.1093/hsw/30.3.203. [DOI] [PubMed] [Google Scholar]
- 38.Jiwa TI. The social worker as advocate. 2003. http://www.mscare.org/cmsc/images/pdf/SWADVOCATE.pdf. Accessed April 25, 2011.
- 39.Lublin FD, Whitaker JN, Eidelman BH, Miller AE, Arnason BG, Burks JS. Management of patients receiving interferon beta-1b for multiple sclerosis: report of a consensus conference. Neurology. 1996;46:12–18. doi: 10.1212/wnl.46.1.12. [DOI] [PubMed] [Google Scholar]
- 40.Bruce JM, Hancock LM, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med. 2010b;33:219–227. doi: 10.1007/s10865-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 41.Bakshi R, Shaikh ZA, Miletich RS. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler. 2000;6:181–185. doi: 10.1177/135245850000600308. et al. [DOI] [PubMed] [Google Scholar]
- 42.Gold R. Oral therapies for multiple sclerosis: a review of agents in phase III development or recently approved. CNS Drugs. 2011;25:37–52. doi: 10.2165/11539820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, Bouvy ML, de Bakker DH. Improving medication adherence in diabetes type 2 patients through real time medication monitoring: a randomised controlled trial to evaluate the effect of monitoring patients' medication use combined with short message service (SMS) reminders. BMC Health Serv Res. 2011;11:5. doi: 10.1186/1472-6963-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early MS. N Engl J Med. 2008;359:1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 45.Hu X, Miller L, Richman S. A novel pegylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol. 2012;52:798–808. doi: 10.1177/0091270011407068. et al. [DOI] [PubMed] [Google Scholar]
- 46.Gilenya (fingolimod) US prescribing information. http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf. Accessed June 8, 2012.


