Abstract
This exploratory secondary analysis examined whether the presence of six chronic health conditions moderated the effectiveness of a teleconference-delivered fatigue self-management education program for people with multiple sclerosis (MS). The longitudinal data used were from a randomized controlled trial involving 181 community-dwelling adults with MS. The primary outcome was fatigue impact, as measured by the Fatigue Impact Scale (FIS). Mixed-effects analysis of variance (ANOVA) models were used to determine the best-fitting model. Just under 65% (n = 112) of participants had at least one comorbid condition. Only diabetes and arthritis moderated all three FIS subscales over time. People with diabetes were slower to show improvement after intervention than people without diabetes. People with arthritis made much more dramatic initial gains compared with people without arthritis but had difficulty maintaining those gains over time. The results point to the need for greater attention to the impact of comorbidities on rehabilitation interventions. These exploratory findings suggest that fatigue self-management education protocols may need to be customized to people who are trying to incorporate MS fatigue self-management behaviors while simultaneously managing diabetes or arthritis.
Almost half of the US population lives with a chronic health condition.1 Unlike acute conditions, chronic ones tend to have a gradual onset, do not resolve on their own, and are rarely curable.2 Multiple sclerosis (MS) is one example of a chronic condition that tends to be diagnosed early in adult life and may not significantly reduce life expectancy.3 People with MS may also have other chronic conditions, such as arthritis, asthma, diabetes, or cardiovascular disease. These conditions may precede the MS diagnosis or be acquired later, as a person ages. From the perspective of the MS care team, these other conditions can be defined as comorbidities. According to Gijsen et al.,4 comorbidity refers to the total burden of illness beyond the disease of specific interest. For members of the MS care team, MS is the disease of interest. Other conditions experienced by the individual with MS (eg, hypertension, arthritis, diabetes, asthma) are comorbidities.
Over the past few years, several articles have been published about the prevalence and consequences of various comorbidities among people with MS.5–8 Both physical and mental comorbidities are common among people with MS. Using the North American Research Committee on Multiple Sclerosis (NARCOMS) database, one group of researchers found that 36.7% of 8983 respondents reported at least one physical comorbidity.9 The most common ones were hypercholesterolemia (37%), hypertension (30%), and arthritis (16%). Some of these same researchers also investigated mental comorbidities using NARCOMS, and found that 48% of 4264 respondents were affected. Depression (46%) was the most frequently reported, followed by anxiety (16.5%).
Comorbid conditions reduce quality of life, increase disability, and increase use and costs of health-care services.4,10 Recently, questions have been raised about whether comorbid conditions influence treatment outcomes among people with MS, either positively or negatively.8 These questions apply to traditional medical treatments (eg, pharmaceuticals) as well as symptomatic interventions delivered by rehabilitation professionals (eg, fatigue management, exercise). It may be that the presence of comorbidities influences who responds to treatment and who does not, as well as how well they respond. In other words, comorbidities may function as outcome moderators11 for MS interventions; if so, this would require providers to reconsider treatment goals, intervention protocols, or the general approach to service delivery. To begin to explore this issue, this longitudinal secondary analysis was conducted to examine whether the presence of certain physical comorbidities moderated the effectiveness of a teleconference-delivered fatigue self-management program for people with MS. While mental comorbidities may also moderate the effectiveness of rehabilitation interventions, they are beyond the scope of this article.
Methods
This study used existing data from a randomized controlled trial,12 which was reviewed and approved by the institutional review board of the authors' university. Because of the wait-list control design,12 all participants (N = 181) eventually received the intervention. Therefore, this secondary analysis is best described as a longitudinal study examining changes over time in a group of individuals receiving treatment.
Participants
Community-dwelling adults with MS were recruited through flyers, mailings, and other forms of advertising. Individuals interested in participating in the study contacted the study office. A trained research assistant conducted a telephone screening to determine eligibility. The following inclusion criteria were evaluated: living within the state of Illinois; self-reported diagnosis of MS; 18 years of age or older; functional English literacy (ie, able to read course materials and carry on telephone conversations in English); a Fatigue Severity Scale (FSS) score of 4 or greater (ie, moderate-to-severe fatigue)13; and a weighted score of at least 12 on the short version of the Blessed Orientation Memory Concentration Test (BOMCT).14 Both the FSS13 and BOMCT14 have documented validity and reliability. The FSS enabled us to target people for whom fatigue was a problem, and the BOMCT allowed us to screen out people who had significant memory and concentration deficits that may have impeded the success of an educational intervention. Eligible participants were mailed a study information sheet and a consent form. Upon return of the signed consent form, they were enrolled in the study.
Data Collection
A trained research assistant collected data by telephone a total of five times (before and immediately after the intervention and at 6 weeks, 3 months, and 6 months after the intervention). The research assistant was not involved in the delivery of the intervention.
Outcome Measure
The primary outcome of interest was fatigue impact, which was measured by the Fatigue Impact Scale (FIS).15 Each of the 40 items is rated using a 5-point scale ranging from 0 (no problem) to 4 (extreme problem). The instrument has documented validity and reliability, and is sensitive to changes as a consequence of intervention.15 A total score and three distinct subscale scores can be calculated (cognitive, physical, social). Since rehabilitation goals and interventions addressing fatigue need to be targeted and specific (eg, cognitive fatigue vs. physical fatigue), we chose to use the three FIS subscales for this analysis rather than the total score.
Covariates
Participants self-reported other health conditions using a simple yes/no format. The question asked participants whether they had previously been told by a physician that they had diabetes, arthritis, heart conditions, abnormal blood pressure, respiratory problems (eg, asthma, emphysema), previous stroke, or a thyroid condition. For the purpose of the original trial, this information was gathered to obtain a broader picture of the health status of the participants; no additional questions about the condition were asked, such as time since diagnosis, level of control, or severity. During analysis, previous stroke was excluded from the modeling process because of low frequency (n = 1).
Intervention
The intervention was a group-based, teleconference-delivered fatigue self-management education program facilitated by an occupational therapist.12,16 Sessions were held once a week for 6 weeks, with each session lasting 70 minutes. Each session provided opportunities for participants to learn about fatigue self-management strategies using a combination of teaching-learning strategies—for example, brief teaching sessions, discussions, and sharing of experiences.
The first session of the program focused on the fatigue cycle, the importance of self-monitoring fatigue, principles of rest, and ways to strategically apply those principles to manage fatigue. The second session addressed the role of communication and how communication skills can be used to elicit useful support from family, friends, and coworkers to manage fatigue. Through a discussion of body mechanics and the environmental setup of work spaces, the third session provided participants with knowledge and skills to make adaptations in order to use energy more efficiently during everyday tasks. The fourth session addressed activity analysis and modification in further detail, and the importance of making active choices and setting priorities for energy expenditures. The fifth session addressed planning days and weeks, combining and applying the contents of the previous sessions. The last session functioned as a review, and gave participants an opportunity to set short (1 week), intermediate (3 months), and long-term (6 months) goals for fatigue management.
Throughout the sessions, the occupational therapist facilitator employed methods to enhance self-efficacy development—for example, supporting social modeling and vicarious learning, and grading activities and content to promote mastery. At the end of each session, participants were given a practice activity based on the session's topic. These activities were completed by participants between sessions in order to apply the concepts being introduced.
Data Analysis
Mixed-effects analysis of variance (ANOVA) models with a combination of random effects (intercept and linear time trend) and variance-covariance structure (compound symmetry, Toeplitz band of size 4 and unstructured) were used to determine the best-fitting model.17 Model comparison was assessed using both the likelihood ratio test (for nested models only) and Akaike information criterion (AIC) score (lower AIC indicating better model). A quadratic time effect was considered based on previous analyses with these data.12 A quadratic time trend can be described as a sharp decrease (improvement) in the FIS score immediately after the intervention followed by maintenance over time.
Results
The 181 study participants were primarily middle-aged (mean [SD] age, 55.5 [8.8] years) at the beginning of the study and had been living with MS for just over 14 years (mean [SD], 14.6 [9.4] years). Ninety percent were white, and over three-quarters (79.1%) were female. Just over half (53.4%) reported having relapsing-remitting MS, 21.9% reported secondary progressive MS, and 8.9% reported primary progressive MS. The remaining participants did not know their MS type.
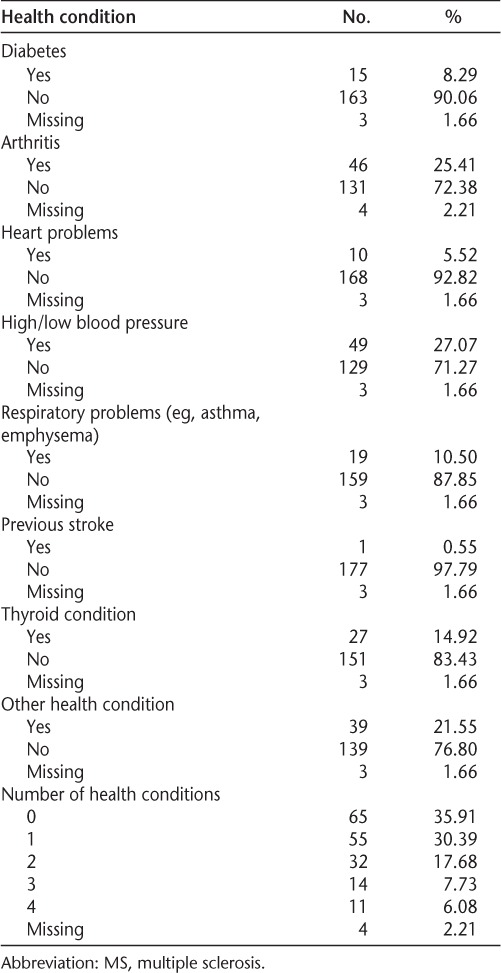
Just under 65% (n = 112) of participants indicated that they had at least one other health condition in addition to MS. Overall, abnormal blood pressure was the most prevalent condition (27%), followed by arthritis (26%). Among those with a chronic condition, just over half reported having at least two conditions (Table 1).
Table 1.
Distribution of participants' health conditions other than MS (N = 181)
For all three FIS subscales, a random intercept and random linear trend model was the best fit for the data, which indicates that participants with and without the condition in question started with different baseline scores and had their own score trends over time. While baseline differences were found between people with and without heart conditions on all three FIS subscales and between people with and without respiratory problems on the FIS-physical subscale, the differences persisted over time. For both conditions, people with the condition consistently scored significantly higher (ie, worse; greater fatigue impact).
Only diabetes and arthritis moderated all three FIS subscales over time (Table 2). For all FIS subscales, people without diabetes tended to have a much steeper initial improvement and maintained the improvements through the 6-month follow-up. In comparison, people with diabetes improved more gradually and steadily over time (Figure 1).
Table 2.
Results from final mixed-effects models for the three FIS subscales (N = 181)
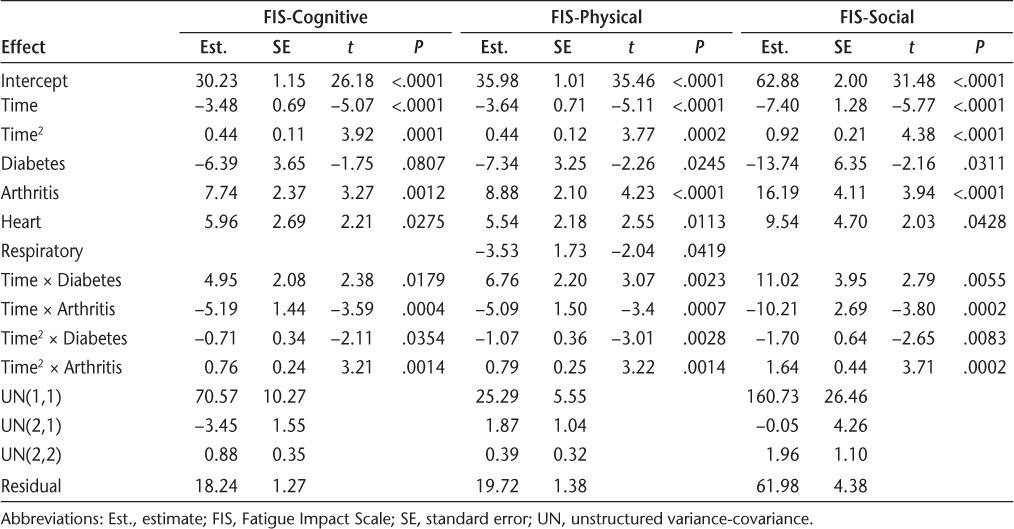
Figure 1.
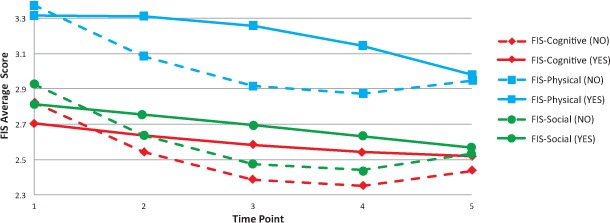
Predicted Fatigue Impact Scale (FIS) scores over time, by diabetes status
For the FIS-cognitive and FIS-physical subscales, people with arthritis started off higher (worse) than people without arthritis, experienced a sharp improvement (decline) in scores by the first post-intervention time point, and then continued to improve to the second post-intervention time point. By month 6, some improvements had been lost, but scores were still better than they were at time 1. People without arthritis also improved, but their improvements tended to be more gradual over time (Figure 2).
Figure 2.
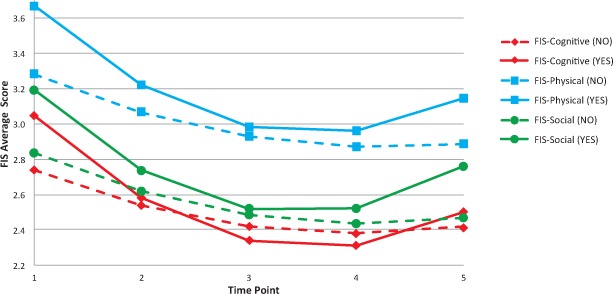
Predicted Fatigue Impact Scale (FIS) scores over time, by arthritis status
Discussion
Chronic illness management involves monitoring medical aspects of the condition (eg, symptoms, treatments), coping with emotions, and making adaptations to roles and responsibilities.18,19 Each of these management tasks alone can be challenging. When multiple conditions are involved, management becomes even more complex, particularly if the adjustments needed for each condition are not complementary or easily coordinated.20,21 Progressive conditions that vary over time, such as MS, add another dimension to this situation.
Fatigue is one of the most disabling symptoms of MS22 and is also common in other chronic conditions.23 Fatigue self-management interventions enable people with MS to develop the knowledge and skills necessary to evaluate their rest-activity ratios, plan for and modify activities and environments to reduce energy demands, and make active choices about energy use based on personal values and goals.12,24 Despite the growing evidence in support of MS fatigue self-management interventions,12,25–27 the results of this study indicate that the protocols may need to be customized to people who are trying to incorporate fatigue self-management behaviors to manage MS while simultaneously managing diabetes or arthritis.
The findings suggest that people with MS who also have diabetes may need more support to start incorporating fatigue management strategies into their lives, as their initial improvement was much slower than that of people without diabetes. Diabetes management is complex because it requires following schedules for glucose monitoring and medication administration, synchronizing meals and medications, restricting diet, keeping physically active, and so on.28 People with diabetes have self-management habits and routines that are already very full. Consequently, it may be more challenging and take more time for them to integrate additional fatigue self-management strategies for their MS into their lives. Examining this issue through future qualitative research could provide important insights for MS self-management interventions and possible guidance for customization. From a practice perspective, the findings raise questions about whether greater collaboration and synergy between MS and diabetes care providers could facilitate self-management support for those people coping with both conditions.
Although people with diabetes were slower to benefit from the fatigue management intervention, people with arthritis were less likely to maintain their initial improvements compared with people without arthritis. There are two possible explanations for this finding. First, arthritis symptoms (eg, pain, swelling) are often variable over time.29,30 Simultaneously managing two chronic conditions, both of which have variable symptoms, may add a layer of complexity in the application of fatigue management strategies that was not adequately addressed in the intervention. Second, because we did not ask participants to identify type of arthritis or extent of involvement (eg, single vs. multiple joints), the analysis may be masking variability in response to intervention among individuals with arthritis. For example, if the majority of our participants had a form of arthritis for which fatigue is a symptom (eg, rheumatoid arthritis29,30), it may have been more difficult for them to maintain improvements over time. Certainly future researchers will need to explore these issues in greater depth in subsequent studies. Despite this limitation, the current analysis does suggest that people who are living with both MS and arthritis may need more frequent follow-up for fatigue self-management in order to reinforce and maintain improvements they experience.
Limitations
This study was an exploratory secondary analysis of existing data. Therefore, participants were not screened for comorbid conditions, and the consequence is low frequency for most conditions. Low frequency limits statistical power and ability to draw firm conclusions. Because rehabilitation trials often use small samples, overcoming this limitation in future research will require pooling of data across multiple sites in order to increase the prevalence of comorbid conditions within a given analytic sample.
A second important limitation of the current work is that all comorbid conditions were self-reported using a simple yes/no format. For the purpose of description, which was the original intent in collecting these data, this format was adequate. Yet, for an examination of moderators, the format was limiting because it did not provide information about condition subtype (eg, rheumatoid arthritis vs. osteoarthritis; type 1 versus type 2 diabetes), severity, degree of control (eg, well vs. poorly controlled), or temporal relationship with the MS diagnosis (eg, before or after MS). These nuances may be important in selecting fatigue management strategies to introduce to a person with MS, as well as in how these strategies are conveyed during intervention, monitored over time, or linked to other life routines. On a related note, Marrie and colleagues9 suggested that reporting of diabetes and heart conditions is likely accurate among people with MS, while accuracy of self-reported arthritis is unknown. Consequently, the current study may also be limited by the self-report of arthritis. Clearly, collaborative efforts to accurately document and then examine the impact of comorbidities on rehabilitation outcomes over time will be needed in the future. Greater understanding of the temporal aspects of multiple chronic condition management over time could provide important insights for MS rehabilitation care.
Conclusion
Given the high likelihood that people with MS have or will acquire other chronic conditions during their disease course, studies like this one are important for informing goal setting and treatment planning in MS care and rehabilitation. This study explored the potential moderating effects of comorbid conditions on the effectiveness of fatigue self-management education. Managing MS fatigue requires increased knowledge of available strategies and a commitment to behavior changes that can be challenging for some people to integrate into daily routines. Future research should examine whether providing fatigue management intervention early in the course of MS makes it easier for individuals to incorporate additional self-management tasks as additional chronic illnesses emerge later in life. Moreover, this study should be replicated with other rehabilitation interventions that target other important MS symptoms (eg, related to cognition, pain, balance) and activity limitations (eg, in walking, transfers, communication). Without examining potential moderators of intervention effectiveness, it will be difficult for members of the MS care team to accurately target treatment goals, select and sequence intervention protocols, choose appropriate outcomes, and determine the right timing for follow-up care.
PracticePoints.
A large percentage of people with MS have comorbid physical or psychological conditions.
People with MS who have comorbid conditions may respond differently to fatigue management interventions than those without such conditions.
Supporting the behavior changes necessary for fatigue management requires efforts tailored to the challenges of managing multiple chronic conditions simultaneously.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This study was supported in part by a Field-Initiated research grant from the National Institute of Disability and Rehabilitation Research (grant H133G070006).
References
- 1.Centers for Disease Control. Chronic diseases and health promotion. Centers for Disease Control website. 2010. http://www.cdc.gov/chronicdisease/overview/index.htm. Accessed March 23, 2011.
- 2.Dowrick C, Dixon-Woods M, Holman H, Weinman J. What is chronic illness? Chronic Illn. 2005;1:1–6. doi: 10.1177/17423953050010010901. [DOI] [PubMed] [Google Scholar]
- 3.Kantarci OH, Weinshenker BG. Natural history of multiple sclerosis. Neurol Clin. 2005;23:17–38. doi: 10.1016/j.ncl.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GAM. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, Horwitz R, Cutter G, Tyry T. Cumulative impact of comorbidity on quality of life in MS. Acta Neurol Scand. 2012;125:180–186. doi: 10.1111/j.1600-0404.2011.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Mult Scler. 2009;15:385–392. doi: 10.1177/1352458508099477. [DOI] [PubMed] [Google Scholar]
- 7.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72:117–124. doi: 10.1212/01.wnl.0000333252.78173.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010;9:820–828. doi: 10.1016/S1474-4422(10)70135-6. [DOI] [PubMed] [Google Scholar]
- 9.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler. 2008;14:1091–1098. doi: 10.1177/1352458508092263. [DOI] [PubMed] [Google Scholar]
- 10.Marengoni A, Angleman S, Melis R. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. et al. [DOI] [PubMed] [Google Scholar]
- 11.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson M, Preissner K, Cho C, Plow M. Randomized trial of a teleconference-delivered fatigue management program for people with multiple sclerosis. Mult Scler J. 2011;17:1130–1140. doi: 10.1177/1352458511404272. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 14.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 15.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 16.Finlayson M. Pilot study of an energy conservation education program delivered by telephone conference call to people with multiple sclerosis. NeuroRehabilitation. 2005;20:267–277. [PubMed] [Google Scholar]
- 17.Chakroborty H, Gu H. A Mixed Model Approach for Intent-to-Treat Analysis in Longitudinal Clinical Trials with Missing Values. Research Triangle Park, NC: RTI Press, Inc; 2009. [PubMed] [Google Scholar]
- 18.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 19.Ryan P, Sawin K. The individual and family self-management theory: background and perspectives on context process and outcomes. Nurs Outlook. 2009;57:217–225. doi: 10.1016/j.outlook.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevick M, Trauth J, Ling B. Patients with complex chronic diseases: perspectives on supporting self-management. J Gen Intern Med. 2007;22:438–444. doi: 10.1007/s11606-007-0316-z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noël P, Parchman M, Williams J. The challenges of multimorbidity from the patient perspective. J Gen Intern Med. 2007;22:419–424. doi: 10.1007/s11606-007-0308-z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler. 2003;9:219–227. doi: 10.1191/1352458503ms904oa. [DOI] [PubMed] [Google Scholar]
- 23.Jason LA, Evans M, Brown M, Porter N. What is fatigue? pathological and nonpathological fatigue. PM&R. 2010;2:327–331. doi: 10.1016/j.pmrj.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Matuska K, Mathiowetz V, Finlayson M. Use and perceived effectiveness of energy conservation strategies for managing multiple sclerosis fatigue. Am J Occup Ther. 2007;61:62–69. doi: 10.5014/ajot.61.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Kos D, Duportail M, D'hooghe MB, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler. 2007;13:996–1003. doi: 10.1177/1352458507078392. [DOI] [PubMed] [Google Scholar]
- 26.Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler. 2005;11:592–601. doi: 10.1191/1352458505ms1198oa. [DOI] [PubMed] [Google Scholar]
- 27.Sauter C, Zebenholzer K, Hisakawa J, Zeitlhofer J, Vass K. A longitudinal study on effects of a six-week course for energy conservation for multiple sclerosis patients. Mult Scler. 2008;14:500–505. doi: 10.1177/1352458507084649. [DOI] [PubMed] [Google Scholar]
- 28.Tamir O, Wainstein J, Abadi-Korek I, Horowitz E, Shemer J. The patient-perceived difficulty in diabetes treatment (PDDT) scale identifies barriers to care. Diabetes Metab Res Rev. 2012;28:246–251. doi: 10.1002/dmrr.1300. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda YL. Rheumatoid arthritis, osteoarthritis and fibromyalgia. In: Radomski MV, Trombly Latham CA, editors. Occupational Therapy for Physical Dysfunction. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 1214–1243. [Google Scholar]
- 30.Orchanian DP. Rheumatoid arthritis. In: Achison BJ, Dirette DK, editors. Conditions in Occupational Therapy: Effect on Occupational Performance. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 275–310. [Google Scholar]


