Abstract
Background
Little is known about the incidence, location, etiologic organisms, and outcomes of infection in patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI).
Objectives
To address this knowledge gap using the database of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. We also assessed the association between serious infections and 90-day death or death/MI.
Methods
We analyzed data from 5745 STEMI patients enrolled in the APEX-AMI trial. Detailed information on infection was collected on all patients. We describe characteristics of patients according to infection and details of infection. Cox proportional hazards models were used to assess 90-day outcomes among patients with and without infections after adjusting for associated clinical variables and using infection as a time-dependent covariate.
Results
Overall, 138 patients developed a serious infection (2.4%), most of whom presented with a single-site infection. The median (25th, 75th percentile) time until diagnosis of infection was 3 (1, 6) days. The most commonly identified organism was Staphylococcus aureus, and the main location of infection was the bloodstream. These patients had more comorbidities and lower procedural success at index PCI than those without infections. Serious infection was associated with significantly higher rates of 90-day death (adjusted hazard ratio [HR] 5.6; 95% confidence interval [CI] 3.8-8.4) and death or MI (adjusted HR 4.9; 95% CI 3.4-7.1).
Conclusion
Infections complicating the course of patients with STEMI are uncommon but associated with markedly worse 90-day clinical outcomes. Mechanisms for early identification of these high-risk patients, as well as design of strategies to reduce their risk of infection, are warranted.
Keywords: ST-segment elevation myocardial infarction, percutaneous coronary intervention, infection, outcomes
Rates of infection complicating percutaneous coronary intervention (PCI) are very low (1-9), and reported rates of infection in patients undergoing cardiac catheterization are less than 1% (10-12). Cardiogenic shock complicating acute myocardial infarction (MI) is often accompanied by a systemic inflammatory response, manifested by high levels of interleukin-6 and associated with multi-organ failure and high mortality rates (13-15). The extent of overlap between cardiogenic shock and other conditions leading to a systemic inflammatory state, including serious infection, is unclear. In 1 report, among patients with cardiogenic shock complicating ST-segment elevation MI (STEMI), 21% died of non-cardiac causes, and, in 29%, the cause of death was sepsis (13).
In summary, little is known about the incidence, location, demographics, and etiologic organisms of serious infection in patients with acute MI, particularly STEMI, who are treated with primary PCI in the contemporary era. Thus, the primary objective of our study was to address this knowledge gap using the database of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. We also assessed the association between serious infection and 90-day death or death/MI.
Methods
We analyzed data from the APEX-AMI trial, a randomized clinical trial of 5745 STEMI patients treated with primary PCI. Briefly, the APEX-AMI trial was a multicenter (17 countries and 296 hospitals), randomized, double-blind trial of pexelizumab versus placebo in conjunction with primary PCI in STEMI. The trial was approved by the institutional ethics committee or institutional review board (IRB) of each participating site, and all enrolled patients provided written informed consent. The current analyses were approved by the IRB of Duke University Medical Center. The primary outcome of APEX-AMI was 30-day mortality. Secondary outcomes were 90-day mortality and the composite of death, cardiogenic shock, and congestive heart failure at 30 days. Additional outcomes included stroke, recurrent MI, and sepsis. In this large clinical trial, no difference in 30-day mortality was observed between the 2 randomized treatment groups (16).
Because pexelizumab is an inhibitor of C5 complement activation, resulting in formation of C5a (anaphylatoxin and pro-inflammatory substance) and C5b-9 or the membrane attack complex, patients with known or suspected active serious infection were excluded from the trial before randomization, and detailed information about the occurrence of infection during and post-treatment was collected (16). Data on serious infections were categorized in the case report form as follows: sepsis with and without shock, pneumonia, cellulitis, sternal wound, puncture site, and other. If a culture was obtained, the site of the culture (blood, urine, sputum, or wound), type of organism detected by the culture, date of infection, whether the investigator determined the infection was related to the study drug, and outcome were recorded. Infections were also collected as adverse events (serious or non-serious). These were categorized by MedDRA system organ class, high-level and preferred term. Both sources of infection diagnosis were used for our analysis.
“Serious infection” was determined by the investigator and was not centrally adjudicated. However, all in-hospital data from the case report forms were source data verified. Discrepant data regarding infection were reconciled, and extensive data queries were performed to verify the data.
While analysis of infection was not part of the formal statistical plan, we did plan to investigate events such as serious infection that were prospectively collected as yes or no variables on the case report form.
Statistical analysis
Continuous variables are presented as means with standard deviations, medians with first and third quartiles, and minima and maxima. Non-parametric p-values are given for comparing continuous variables. Categorical variables are presented as percentages and are compared with chi-square or exact p-values. Frequency tables are provided for number of infections, time from randomization to infection (in days), infection site (e.g., urine, bloodstream, and others), and infectious organism; index procedural characteristics are provided to describe the distributions of each.
Tables of outcomes and concomitant medications are also provided. P-values are omitted from these tables because they include time-dependent covariates with unrecorded times, and it is unclear whether infection strictly preceded the outcome/medication dosing. A Cox proportional hazards model was fit for 90-day death and 90-day death or MI with infection as a time-dependent covariate. The model was adjusted for “sheath time” (the time a patient is undergoing catheterization/PCI and has an in-dwelling catheter) and other variables previously determined to be significant predictors of death in the APEX-AMI trial: age, baseline Killip class III or IV, baseline systolic blood pressure, baseline heart rate, and baseline creatinine level (17). Hazard ratios (HR) and corresponding 95% confidence intervals (CI) are reported. The model was fit both including and excluding early deaths (i.e., patients who died in the first 24 hours) to address survival bias such that a patient who died early had less opportunity to develop an infection.
A sensitivity analysis was performed dividing our infection patients into 2 groups: infection within 48 hours and infection after 48 hours of hospital admission. This analysis was done to assess eventual differences in clinical outcomes between early infection and nosocomial infection (or hospital-acquired infection), defined as any infection that occurs 48 hours after hospital admission.
All analyses were conducted using SAS v. 9.2 (SAS Institute, Cary, NC). The significance level was set at 0.05. No adjustments were made for multiple comparisons.
Results
Baseline characteristics
Of 5745 patients enrolled in the APEX-AMI trial, 138 (2.4%) developed at least 1 serious infection. The median (25th, 75th percentile) time until diagnosis of infection was 3 (1, 6) days, and, by day 8 after hospital admission, 80% of the infections had already occurred (Figure 1). The majority of patients (N=96, 69.6%) developed a serious infection 48 hours after hospital admission, and only 30.4% (N=42) developed infection before 48 hours. The rates of serious infections were similar between patients treated with pexelizumab (2.3%) and placebo (2.5%).
Figure 1.
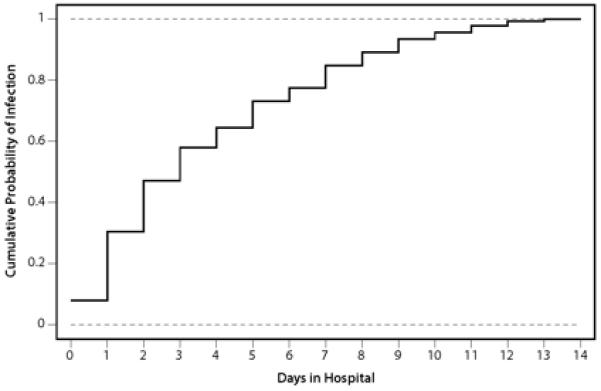
Rates of serious infection by days in the hospital. Infections are recorded to the day; infections from randomization to day 1 (exclusive) are considered as occurring at day 0.
Baseline characteristics of patients with and without infections are shown in Table 1. Patients with serious infections were older, more often had a history of a chronic inflammatory condition, chronic obstructive pulmonary disease, and diabetes, and more often had anterior MI, Killip class III and IV, higher heart rate, higher creatinine value, higher levels of peak creatine kinase (CK), and CK-MB levels with greater ST-segment deviation than patients without infections. In addition, they had lower success rates than those patients who did not develop serious infections (post-intervention TIMI flow grade 3, 75.4% vs. 90.3%, respectively).
Table 1. Baseline Demographics and Other Characteristics.
| Serious Infection (N=138) |
No Serious Infection (N=5606)* |
P-value | |
|---|---|---|---|
| Age, median years | 65 (57, 75) | 61 (52, 71) | <0.001 |
| Sex (female, %) | 26.1 | 23 | 0.394 |
| Enrolled in the U.S. (yes, %) | 32.6 | 30.4 | 0.583 |
| Transfer patient (yes, %) | 37 | 36.1 | 0.841 |
| Anterior MI (%) | 70.3 | 58.9 | 0.007 |
| Time from symptoms to | 2.3 (1.3, 3.8) | 2.2 (1.3, 3.3) | 0.581 |
| hospital admission (hrs)† | |||
| Time from symptoms to | 3.1 (2.1,4.2) | 2.8 (2.0, 3.9) | 0.116 |
| randomization (hrs)† | |||
| Time from hospital admission | 0.6 (0.3, 0.9) | 0.5 (0.2, 0.9) | 0.197 |
| to randomization (hrs)† | |||
| Systolic BP (mm Hg)† | 129 (110, 148) | 133 (117, 150) | 0.017 |
| Heart rate (bpm)† | 80 (65, 96) | 75 (65, 86) | 0.004 |
| Diastolic BP (mm Hg)† | 74.5 (65, 90) | 80 (70, 90) | 0.016 |
| Height (cm)† | 173 (165, 178) | 172.7 (166, 178) | 0.776 |
| Weight (kg)† | 80 (68, 90) | 80 (70, 91) | 0.436 |
| Body mass indext | 26.7 (24.5, 29.6) | 27.1 (24.5, 30.1) | 0.683 |
| CK valuet | 140 (87, 361.2) | 143.7 (90, 277) | 0.794 |
| Peak CK valuet | 3463 (1525.8,5850) | 1771.1 (843, 3261.5) | <0.001 |
| Creatinine valuet | 97.2 (79.6, 114.9) | 88.4 (79.6, 106.1) | 0.001 |
| CK-MB valuet | 6.8 (3.3, 84) | 4.6 (2.2, 14.8) | 0.016 |
| Peak CK-MB valuet | 268.9 (147.3,430) | 157.8 (68, 292.1) | 0.001 |
| ST deviation (mm)† | 15.5 (10, 21.3) | 13 (9, 18.5) | 0.004 |
| ECG anterior location (%) | 66.7 | 58 | 0.144 |
|
| |||
| Race (%) | 0.897 | ||
|
| |||
| White | 92.8 | 94.3 | |
| Black/African-American | 4.3 | 2.3 | |
|
| |||
| Smoking status (%) | 0.407 | ||
|
| |||
| Never | 37 | 33.2 | |
| Current | 37.7 | 43.4 | |
| Past | 25.4 | 23.4 | |
|
| |||
| Killip class (%) | <0.001 | ||
|
| |||
| Class I | 73.9 | 89.7 | |
| Class II | 15.9 | 8.3 | |
| Class III | 2.9 | 1.1 | |
| Class IV | 7.2 | 0.9 | |
|
| |||
| Medical history (%) | |||
|
| |||
| Atrial fibrillation | 6.5 | 4.1 | 0.156 |
| Angina | 26.1 | 24 | 0.568 |
| CABG | 2.2 | 2.2 | 1.000 |
| CAD | 21.7 | 16.3 | 0.087 |
| CHF | 9.4 | 3.5 | 0.001 |
| Chronic inflammatory | 6.3 | 1.7 | 0.001 |
| condition | |||
| Chronic liver disease | 1.4 | 0.7 | 0.259 |
| COPD | 11.6 | 4.7 | <0.001 |
| Current renal dialysis | 0.7 | 0.3 | 0.323 |
| Diabetes | 22.5 | 15.7 | 0.033 |
| Family history of CAD | 16.2 | 19 | 0.411 |
| ICH stroke | 0.0 | 0.3 | 1.000 |
| Hyperlipidemia | 48.6 | 49.7 | 0.820 |
| Hypertension | 57.2 | 49.3 | 0.063 |
|
| |||
| Post-intervention TIMI grade (%) | <0.001 | ||
|
| |||
| Grade 0 | 4.8 | 2.5 | |
| Grade 1 | 3.2 | 0.9 | |
| Grade 2 | 16.7 | 6.4 | |
| Grade 3 | 75.4 | 90.3 | |
One patient developed an infection prior to the randomization and thus is excluded from the denominator.
Median (25th, 75th percentile).
BP = blood pressure; bpm = beats per minute; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; ECG = electrocardiograph; ICH = intra-cerebral hemorrhage; TIMI = Thrombolysis in Myocardial Infarction.
In-hospital medications
In-hospital medications are listed in Table 2. Patients with serious infection had numerically less use of statins, angiotensin-converting enzyme inhibitors, beta-blockers, aspirin, and abciximab than patients without serious infection.
Table 2. In-hospital Medications Used by Patients With and Without Serious Infections.
| Medications | Serious Infection n/N (%) |
No Serious Infection n/N (%) |
|---|---|---|
| Antibiotics | 123/138 (89.1%) | 662/5606 (11.8%) |
| Aspirin | 128/138 (92.8%) | 5444/5601 (97.2%) |
| Thienopyridines | 115/117 (98.3%) | 5206/5249 (99.2%) |
| Abciximab | 50/84 (59.5%) | 2527/3903 (64.7%) |
| Eptifibatide | 25/84 (29.8%) | 1121/3903 (28.7%) |
| Tirofiban | 9/84 (10.7%) | 255/3903 (6.5%) |
| Statins | 109/138 (79%) | 5118/5602 (91.4%) |
| ACE inhibitors | 98/138 (71%) | 4459/5600 (79.6%) |
| Beta-blockers | 97/138 (70.3%) | 4770/5600 (85.2%) |
ACE = angiotensin-converting enzyme.
In-hospital procedures
In-hospital procedures are described in Table 3. In general, patients with serious infections had numerically more in-hospital procedures than patients without serious infections, including repeat diagnostic catheterization, additional PCI, cardiac surgery (coronary artery bypass grafting), automatic implantable cardiac defibrillator placement, use of mechanical support (intra-aortic balloon pump or left ventricular assist device), permanent pacemaker placement, mechanical ventilation, echocardiography, red blood cell transfusion, and dialysis. Similar results were observed when only serious infections that occurred after the procedures were analyzed (data not shown).
Table 3. In-hospital Procedures in Patients With and Without Serious Infections.
| Procedures | Serious Infection n/N (%) |
No Serious Infection n/N (%) |
|---|---|---|
| Repeat diagnostic | 18/138 (13.0%) | 319/5598 (5.7%) |
| catheterization | ||
| Additional PCI (does not include primary PCI for the qualifying MI) |
12/138 (8.7%) | 383/5598 (6.8%) |
| Cardiac surgery (CABG) | 23/138 (16.7%) | 181/5599 (3.2%) |
| Automatic implantable cardioverter defibrillator |
1/138 (0.7%) | 28/5599 (0.5%) |
| Intra-aortic balloon pump | 55/138 (39.9%) | 389/5599 (7.0%) |
| Left ventricular assist device | 3/138 (2.2%) | 22/5599 (0.4%) |
| Dialysis | 6/138 (4.4%) | 21/5598 (0.4%) |
| Multi-uptake gated acquisition | 0/138 (0%) | 51/5598 (0.9%) |
| Stress test | 5/138 (3.6%) | 191/5598 (3.4%) |
| Cardiac MRI | 2/138 (1.5%) | 103/5598 (1.8%) |
| Permanent pacemaker | 2/138 (1.5%) | 47/5599 (0.8%) |
| Mechanical ventilation | 52/138 (37.7%) | 169/5599 (3.0%) |
| Echocardiography | 115/138 (83.3%) | 3866/5594 (69.1%) |
| Red blood cell transfusion | 48/138 (34.8%) | 295/5606 (5.3%) |
MRI = magnetic resonance imaging.
In-hospital complications
In-hospital complications are shown in Table 4. Patients with serious infections had numerically more in-hospital complications than patients without serious infections, including recurrent ischemia, atrial fibrillation, ventricular tachycardia, ventricular fibrillation, cardiac arrest, deep venous thrombosis, renal failure, and bleeding. Similar results were seen when only complications that occurred after a serious infection were considered (data not shown). Patients with versus without serious infection had longer length of hospital stay (12 vs. 5 days) and longer length of intensive care unit (ICU) stay (7 vs. 2 days).
Table 4. In-hospital Complications in Patients With and Without Serious Infections.
| Events | Serious Infection (N=138) |
No Serious Infection (N=5606) |
|---|---|---|
| Recurrent ischemia | 9.4% | 4.2% |
| Atrial fibrillation | 26.8% | 6.4% |
| Ventricular tachycardia | 12.3% | 2.3% |
| Ventricular fibrillation | 12.3% | 3.7% |
| Complete atrioventricular block |
3.6% | 1.6% |
| Electrical mechanical dissociation |
1.4% | 1.0% |
| Asystole | 7.2% | 2.1% |
| Cardiac arrest | 10.1% | 2.2% |
| Pericarditis | 2.9% | 1.2% |
| Cardiac tamponade | 0.7% | 0.3% |
| Acute mitral regurgitation | 1.4% | 0.2% |
| Acute ventricular septal defect | 2.9% | 0.1% |
| Ventricular rupture | 2.2% | 0.3% |
| Symptomatic hypotension | 36.2% | 8.8% |
| Pulmonary embolism | 2.2% | 0.0% |
| Deep venous thrombosis | 0.7% | 0.1% |
| Renal failure | 20.3% | 1.3% |
| Bleeding | 44.9% | 15.4% |
Serious infection features
Table 5 contains data about the location of infections, number of infections per patient, and organisms associated with infections. Most patients had only 1 type of infection, the most common location being the bloodstream and the most common organism being Staphylococcus aureus.
Table 5. Number of Infections, Location, Main Organisms, and Distribution of Treatment for Infected Patients.
| Frequency (n) | Percent | |
|---|---|---|
| Number of infections | ||
|
| ||
| 1 | 107 | 77.5 |
| 2 | 25 | 18.1 |
| 3 | 5 | 3.6 |
| 5 | 1 | 0.7 |
|
| ||
| Location of infection | ||
|
| ||
| Pleural effusion | 1 | 0.7 |
| Sputum | 35 | 25.4 |
| Tissue | 1 | 0.7 |
| Unlisted* | 28 | 20.3 |
| Urine | 20 | 14.5 |
| Blood | 56 | 40.6 |
| Wound | 6 | 4.3 |
|
| ||
| Organisms of infection | ||
|
| ||
| Culture contaminants | 2 | 1.4 |
| E. coli | 10 | 7.2 |
| Other | 50 | 36.2 |
| P. aeruginosa | 7 | 5.1 |
| Staph. aureus | 13 | 9.4 |
| Staph. epidermis | 5 | 3.6 |
| Staph. species | 8 | 5.8 |
| Strep. pneumoniae | 3 | 2.2 |
| Strep. species | 8 | 5.8 |
| Unlisted† | 28 | 20.3 |
|
| ||
| Distribution of treatment for infected patients | ||
|
| ||
| Placebo | 72 | 52.2 |
| Pexelizumab | 66 | 47.8 |
“Unlisted” is a patient who is infected but with no culture data.
“Unlisted” is a patient who is infected but with no organism data.
Clinical outcomes
Clinical outcomes are described in Table 6. Rates of death, congestive heart failure, shock, MI, and stroke were high among patients with serious infection compared to those without serious infection. Similar results were observed when only clinical outcomes that occurred after a serious infection were analyzed. Among patients with serious infections, there was no difference in clinical outcomes between patients treated with placebo or pexelizumab.
Table 6. Clinical Outcomes in Patients With and Without Serious Infections.
| End Points | Serious Infection n/N (%) |
No Serious Infection n/N (%) |
|---|---|---|
| Death | 40/138 (29.0%) | 278/5604 (5.0%) |
| CHF | 32/138 (23.2%) | 245/5606 (4.4%) |
| Shock | 28/138 (20.3%) | 168/5606 (3.0%) |
| MI | 16/138 (11.6%) | 174/5601 (3.1%) |
| Stroke | 9/138 (6.5%) | 68/5601 (1.2%) |
After multivariable adjustment, serious infection was significantly associated with 90-day death (adjusted HR 5.3; 95% CI 3.5-7.8) and death or MI (adjusted HR 4.6; 95% CI 3.2-6.6, p≤0.001). After excluding deaths that occurred in the first 24 hours after acute MI presentation (N=0 in the infection group and N=60 in the no-infection group), serious infection remained significantly associated with 90-day death (adjusted HR 5.6; 95% CI 3.8-8.4) and death or MI (adjusted HR 4.9; 95% CI 3.4-7.1, p≤0.001). Similar results were observed in patients who developed a serious infection within 48 hours of (N=42, 30.4%) and 48 hours after (N=96, 69.6%) hospital admission (data not shown).
Patients who presented in hospital with serious infection were also more likely to be re-admitted within 90 days for another serious infection when compared with patients who did not develop a serious infection during index hospitalization for acute MI (5.1% versus 0.7%, respectively). Overall, when compared with patients who did not have an infection, those with any serious infection were also more likely to die or be readmitted for any cause within 30 days (23.0% versus 11.0%) and within 90 days (41.0% versus 20.4%).
Discussion
We demonstrated that, in a contemporary cohort of STEMI patients undergoing primary PCI, serious infection was rare, occurred at a median of 3 days after presentation, and was more frequent among sicker patients with prior history of inflammatory disease, chronic obstructive pulmonary disease, and diabetes and among those with worse prognostic markers such as higher creatinine level at baseline, more advanced Killip class, higher heart rate and larger infarcts. These patients also had worse angiographic results at the index PCI. Patients undergoing procedures (e.g., coronary artery bypass grafting, intra-aortic balloon pumping, dialysis) after the index PCI also more frequently developed serious infections. Patients with infection also had more in-hospital complications other than infection and had longer ICU and hospital stays. The most common site of infection was the bloodstream, and the most commonly identified organism was Staphylococcus aureus. Finally, serious infection was associated with 5-fold higher rates of 90-day death and death or MI.
Fever and systemic inflammation in conjunction with MI
Patients with MI, particularly large MIs, can manifest fever at presentation or during their hospital course, but it is particularly common in the first 24 hours after presentation and does not necessarily mean that an infection is present (18-21). However, it can often be a clinical challenge to determine whether the fever is due to the MI, to a complicating infection, or to other causes of systemic inflammation. Regardless of the etiology, because fever has a strong influence on oxygen consumption, it is important to recognize preventable or treatable underlying causes of fever that might predispose a patient to infarct extension and greater infarct size (19,20).
The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial investigators explored the systemic inflammatory state in patients with cardiogenic shock complicating STEMI (22,23). They concluded that inappropriate vasodilation in patients with systemic inflammatory response syndrome may play an important role in the pathogenesis and persistence of shock, with or without infection (22). While Ben-Dor and colleagues found that fever was frequent after reperfusion, they concluded that it was due to infarct size and not to a nonspecific systemic inflammatory response (19). In their study, fever was correlated with high-sensitivity C-reactive protein but not with fibrinogen levels or white blood cell count. Subsequently, Naito and colleagues found that fever after acute MI was associated with worse clinical outcome and with infarction expansion, suggesting a relationship between systemic inflammatory response and left ventricular remodeling in the post-infarction period (20). Further, the rates of death due to pump failure, malignant arrhythmias, and cardiac failure increased significantly with increasing quartile of body temperature (20).
Clinical infection after MI
Although fever tends to occur most commonly in the first 24 hours after MI and may be related to infarct size, serious infection as a complication of MI seems to occur later in the hospital course. Although previous literature is scarce regarding primary PCI-treated acute MI patients, in a cooperative study involving over 12,000 cardiac catheterizations, only 0.10% of patients had serious infection complications documented (9). Similar to our results, 1 case-control study in stable patients undergoing elective PCI found that infection after PCI was most prevalent on day 3 after presentation and that Staphylococcus aureus was the most common organism (24). Another study in elective PCI found Staphylococcus epidermidis was the most frequently identified organism associated with post-PCI infection. Together, these studies suggest that infection may be most related to instrumentation (25). As with our study, previous studies have suggested that congestive heart failure, multiple punctures in the same site, difficult vascular access, duration of sheath placement lasting more than 1 day, and longer duration of procedures are important risk factors for bacteremia associated with cardiac catheterization or PCI (10,24).
In a retrospective case-control study of 1227 acute MI patients admitted during the previous 47 months, 5% had infectious complications (26). Similar to our findings, patients with infections were older (67.5 vs. 62.6 years), had longer length of hospital stay (26.7 vs. 12 days), and had higher mortality (45 vs. 12%) compared with patients without infections. The most common site of infection was the lungs (63%), followed by the urinary tract (37%). Heart infections, such as purulent pericarditis and myocardial abscess, following acute MI have been reported but are very infrequent (2,27-34).
Association of infection with clinical outcomes and length of stay
Mortality associated with the presence of infection in patients undergoing elective PCI with drug-eluting stents has been estimated at 1% (35). However, mortality has reached over 40% in some studies of infection after MI (24,26). In 1 prior study, the most common causes of death in acute MI patients with infections were cardiogenic shock (41%) and septic shock (30%) (26).
In our study of patients with STEMI treated with primary PCI in the contemporary era, we demonstrated that serious clinical infection was independently associated with 5-fold worse clinical outcomes, including mortality and death or MI at 90 days. Importantly, these STEMI patients with serious infection during the index hospitalization were more likely to be re-admitted to the hospital with another serious infection within 90 days from discharge when compared with those patients who never developed an infection. Our findings illustrate the importance of serious infection as a marker of worse subsequent clinical outcomes in patients with STEMI treated with primary PCI.
In addition to increased mortality and morbidity associated with serious infection in acute MI patients, serious infections also appear to be associated with measures of resource use. Whereas the length of stay after uncomplicated STEMI in the United States is approximately 4-5 days, length of stay in complicated STEMI has been shown to average 11 days (36,37). In our study, we demonstrated that patients with clinically diagnosed serious infections had longer length of stay than patients without infections, mirroring previous data on uncomplicated and complicated MIs. Patients with serious infections also had lower rates of post-intervention TIMI grade 3 flow compared with patients without infection. It is possible that longer procedure times, more bleeding, and vascular access complications in these patients could have contributed to a longer length of stay. Conversely, these complications or low TIMI flow grade itself may have resulted in the use of invasive support devices like intra-aortic balloon pumps and other in-dwelling lines or catheters that may have not only increased the likelihood of developing a serious infection during the course of hospitalization but also resulted in longer hospital stay.
Another important related issue is the underlying definition of hospital infection. In general, a new infection occurring in a patient during hospitalization at least 48 hours following admission is suspected to be nosocomial, or hospital-acquired; the rate of nosocomial infection has been proposed as a measure of quality in patient care (38,39). In our study, 96 (69.6%) patients who presented with a serious infection did so 48 hours after hospital admission. Interestingly, in our overall cohort of patients who developed serious infection, there were no differences in 90-day clinical outcomes between those patients who developed a serious infection within 48 hours and those who did so after this time window. These results highlight the importance of identifying patients who are at risk for infection following PCI for STEMI, as well as seeking effective strategies for prevention, both to improve clinical outcomes and to reduce resource use. In addition, vigilance for early diagnosis and treatment of those who develop infection is essential to minimize serious complications. In particular, if a patient develops a fever more than 24 hours after presentation, this fever may not be due to infarct size or systemic inflammatory response to the infarction, but rather may be an early sign of a serious infection that could lead to worse clinical outcomes and greater resource use.
Limitations
First, this was an observational study; therefore, a causal relationship between serious infection and clinical outcomes cannot be established. In addition, it is likely that in our study the majority of serious infections were hospital-acquired and related to instrumentation. Despite our efforts and extensive statistical adjustments for important confounders, we cannot fully tease out the influence of pre-existing conditions predisposing a patient to infection or of the infection itself that led to worse clinical outcomes. Second, our population with infection was small, although the larger APEX-AMI population with STEMI from which they were drawn allowed us to define the contemporary incidence of infection. Third, serious infection was determined by the investigator and was not centrally adjudicated. However, all in-hospital data from the case report forms were source data-verified. In addition, discrepant data regarding infection were reconciled, and extensive data queries were performed to verify the accuracy of the data. Finally, we did not collect information on the specific treatments that were used for serious infection in our patient population.
Conclusion
In the contemporary era, serious infections complicating the course of patients with STEMI treated with primary PCI are uncommon (2.4%) but are associated with worse 90-day clinical outcomes and longer hospital stays. The most commonly identified organism was Staphylococcus aureus, and the main location was the bloodstream. The majority of patients developed a serious infection 48 hours after hospital admission, and patients who developed any infection were more likely to be admitted with another serious infection within 90 days of hospital discharge. Further studies to identify these high-risk patients, as well as to design strategies to reduce their risk of infection, are warranted.
Acknowledgments
The APEX-AMI trial was jointly funded by Procter & Gamble Pharmaceuticals and Alexion Pharmaceuticals. This analysis was supported by the Duke Clinical Research Institute. Kyle White’s efforts were funded by NIH training grant T32 HL079896. The trial sponsors played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Lopes had full access to all data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The APEX-AMI trial was jointly funded by Procter & Gamble Pharmaceuticals and Alexion Pharmaceuticals. This analysis was supported by the Duke Clinical Research Institute. Kyle White’s efforts were funded by NIH training grant T32 HL079896.
Abbreviations
- APEX-AMI
Assessment of Pexelizumab in Acute Myocardial Infarction
- CI
confidence interval
- CK
creatine kinase
- HR
hazard ratio
- ICU
intensive care unit
- IRB
institutional review board
- PCI
percutaneous coronary intervention
- SHOCK
Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock
- STEMI
ST elevation myocardial infarction
Footnotes
Trial Registration: clinicaltrials.gov identifier NCT00091637
REFERENCES
- 1.Ziakas A, Konstantinou V, Giannoglou G, Gemitzis K, Louridas G. Enoxaparin-induced psoas hematoma complicated by Staphylococcus aureus infection after cardiac catheterization. Thromb Res. 2006;118:535–7. doi: 10.1016/j.thromres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Alkan M, Cristal N, Paran E. Infectious complications of acute myocardial infarction. Infect Control. 1986;7:220–2. doi: 10.1017/s0195941700083983. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso F, Moreno R, Vergas J. Fatal infection after rapamycin eluting coronary stent implantation. Heart. 2005;91:e51. doi: 10.1136/hrt.2005.061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh YH, Chang CJ, Hsu TS, Ko YS, Hsu LA, Kuo CT. Cardiac catheterization complicated by iliacus abscess: a rare complication of transfemoral approach—a case report. Angiology. 2005;56:623–5. doi: 10.1177/000331970505600515. [DOI] [PubMed] [Google Scholar]
- 5.Khumri TM, Joslin NB, Nayyar S, Main ML. Transesophageal echocardiographic diagnosis of Aspergillus fumigatus aortitis after percutaneous coronary intervention. J Am Soc Echocardiogr. 2006;19(1072):e9–11. doi: 10.1016/j.echo.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Perez RE. Endocarditis with Moraxella-like M-6 after cardiac catheterization. J Clin Microbiol. 1986;24:501–2. doi: 10.1128/jcm.24.3.501-502.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizos M, Falagas ME, Tsiodras S, Betsou A, Foukas P, Michalopoulos A. Usual interstitial pneumonia associated with cytomegalovirus infection after percutaneous transluminal coronary angioplasty. Eur J Clin Microbiol Infect Dis. 2004;23:848–50. doi: 10.1007/s10096-004-1220-7. [DOI] [PubMed] [Google Scholar]
- 8.Rubin SJ, Lyons RW, Murcia AJ. Endocarditis associated with cardiac catheterization due to a Gram-positive coccus designated Micrococcus mucilaginosus incertae sedis. J Clin Microbiol. 1978;7:546–9. doi: 10.1128/jcm.7.6.546-549.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason JW, Rossen RM, Colby T, Harrison DC. Bacterial endocarditis after cardiac catheterization. Chest. 1976;70:293–6. doi: 10.1378/chest.70.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Samore MH, Wessolossky MA, Lewis SM, Shubrooks SJ, Jr, Karchmer AW. Frequency, risk factors, and outcome for bacteremia after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1997;79:873–7. doi: 10.1016/s0002-9149(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 11.Ricci MA, Trevisani GT, Pilcher DB. Vascular complications of cardiac catheterization. Am J Surg. 1994;167:375–8. doi: 10.1016/0002-9610(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 12.Banai S, Selitser V, Keren A, et al. Prospective study of bacteremia after cardiac catheterization. Am J Cardiol. 2003;92:1004–7. doi: 10.1016/s0002-9149(03)00990-1. [DOI] [PubMed] [Google Scholar]
- 13.Geppert A, Steiner A, Zorn G, et al. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit Care Med. 2002;30:1987–94. doi: 10.1097/00003246-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Jeger RV, Assmann SF, Yehudai L, Ramanathan K, Farkouh ME, Hochman JS. Causes of death and re-hospitalization in cardiogenic shock. Acute Card Care. 2007;9:25–33. doi: 10.1080/17482940601178039. [DOI] [PubMed] [Google Scholar]
- 15.Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong PW, Granger CB, Adams PX, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Stebbins A, Mehta RH, Armstrong PW, et al. Assessment of Pexelizumab in Acute Myocardial Infarction (APEX AMI Investigators). A model for predicting mortality in acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: results from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. Circ Cardiovasc Interv. 2010;3:414–22. doi: 10.1161/CIRCINTERVENTIONS.109.925180. [DOI] [PubMed] [Google Scholar]
- 18.Lofmark R, Nordlander R, Orinius E. The temperature course in acute myocardial infarction. Am Heart J. 1978;96:153–6. doi: 10.1016/0002-8703(78)90078-9. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Dor I, Haim M, Rechavia E, et al. Body temperature—a marker of infarct size in the era of early reperfusion. Cardiology. 2005;103:169–73. doi: 10.1159/000084589. [DOI] [PubMed] [Google Scholar]
- 20.Naito K, Anzai T, Yoshikawa T, et al. Increased body temperature after reperfused acute myocardial infarction is associated with adverse left ventricular remodeling. J Card Fail. 2007;13:25–33. doi: 10.1016/j.cardfail.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Risoe C, Kirkeby OJ, Grottum P, Sederholm M, Kjekshus JK. Fever after acute myocardial infarction in patients treated with intravenous timolol or placebo. Br Heart J. 1987;57:28–31. doi: 10.1136/hrt.57.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohsaka S, Menon V, Lowe AM, et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. 2005;165:1643–50. doi: 10.1001/archinte.165.14.1643. [DOI] [PubMed] [Google Scholar]
- 23.Hochman JS, Sleeper LA, Godfrey E, et al. The SHOCK Trial Study Group Should we emergently revascularize occluded coronaries for cardiogenic shock: an international randomized trial of emergency PTCA/CABG-trial design. Am Heart J. 1999;137:313–21. doi: 10.1053/hj.1999.v137.95352. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann BA, Kaiser C, Pfisterer ME, Bonetti PO. Coronary stent infection: a rare but severe complication of percutaneous coronary intervention. Swiss Med Wkly. 2005;135:483–7. doi: 10.4414/smw.2005.11142. [DOI] [PubMed] [Google Scholar]
- 25.Shea KW, Schwartz RK, Gambino AT, Marzo KP, Cunha BA. Bacteremia associated with percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn. 1995;36:5–9. doi: 10.1002/ccd.1810360103. discussion 10. [DOI] [PubMed] [Google Scholar]
- 26.Grandini LC, Jr, Caramelli B. Infection complication portends poor prognosis in acute myocardial infarction. Arq Bras Cardiol. 2006;87:267–74. doi: 10.1590/s0066-782x2006001600007. [DOI] [PubMed] [Google Scholar]
- 27.Klacsmann PG, Bulkley BH, Hutchins GM. The changed spectrum of purulent pericarditis: an 86 year autopsy experience in 200 patients. Am J Med. 1977;63:666–73. doi: 10.1016/0002-9343(77)90150-4. [DOI] [PubMed] [Google Scholar]
- 28.Silliman RA, Peters JD, Ginsberg MB. Infections of the heart complicating acute myocardial infarction. South Med J. 1984;77:934–6. doi: 10.1097/00007611-198407000-00040. [DOI] [PubMed] [Google Scholar]
- 29.Canning BS, Mulcahy R, Towers R. Abscess formation in an acute cardiac infarct. Br Med J. 1969;1:164. doi: 10.1136/bmj.1.5637.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCue MJ, Moore EE. Myocarditis with microabscess formation caused by Listeria monocytogenes associated with myocardial infarct. Hum Pathol. 1979;10:469–72. doi: 10.1016/s0046-8177(79)80052-0. [DOI] [PubMed] [Google Scholar]
- 31.Katz A. Abscess of the myocardium complicating infarction: report of 2 cases. Can Med Assoc J. 1964;91:1225–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Korns ME. Suppuration within an acute myocardial infarct with rupture and tamponade. Am J Cardiol. 1966;18:124–6. doi: 10.1016/0002-9149(66)90205-0. [DOI] [PubMed] [Google Scholar]
- 33.Schatz JW, Wiener L, Gallagher HS, Eberly RJ. Salmonella pericarditis: an unusual complication of myocardial infarction. Chest. 1973;64:267–9. doi: 10.1378/chest.64.2.267. [DOI] [PubMed] [Google Scholar]
- 34.Berman DA, Burgess JB, Steeper TA. Myocardial abscess due to Fusobacterium following acute myocardial infarction. Clin Cardiol. 1988;11:575–7. doi: 10.1002/clc.4960110812. [DOI] [PubMed] [Google Scholar]
- 35.Lee MS, Canan T, Perlowski A, Bhatia R, Jurewitz D, Tobis JM. Causes of death in patients undergoing percutaneous coronary intervention with drug-eluting stents in a real-world setting. J Invasive Cardiol. 2009;21:441–5. [PubMed] [Google Scholar]
- 36.Skoulas A, Alloo RG, Weisbart MH, Manelis MA. Duration of hospital stay and post-myocardial infarction morbidity and mortality: a Kaiser-Permanente experience. Angiology. 1981;32:509–15. doi: 10.1177/000331978103200708. [DOI] [PubMed] [Google Scholar]
- 37.Spencer FA, Lessard D, Gore JM, Yarzebski J, Goldberg RJ. Declining length of hospital stay for acute myocardial infarction and postdischarge outcomes: a community-wide perspective. Arch Intern Med. 2004;164:733–40. doi: 10.1001/archinte.164.7.733. [DOI] [PubMed] [Google Scholar]
- 38.Larson E, Horan T, Cooper B, Kotilainen HR, Landry S, Terry B. Study of the definition of nosocomial infections (SDNI). Research Committee of the Association for Practitioners in Infection Control. Am J Infect Control. 1991;19:259–67. doi: 10.1016/0196-6553(91)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]


