Photo-control using red light is highly desirable for biological applications since red wavelengths are the only part of the visible spectrum that can effectively penetrate tissue.[1] Efforts to develop optogenetic and optochemical genetic tools that are red-shifted range from exploring natural biodiversity in the search for red-shifted opsins[2] to the conjugation of chemical photoswitches to upconverting nanoparticles.[3] We recently reported that azobenzenes with bulky polar substituents in all four positions ortho to the azo group could undergo red-light driven photoisomerization.[4] However, the compounds require intense red light or long irradiation times to reach the photostationary state because the absorption coefficients for wavelengths >600 nm are very small.[4]
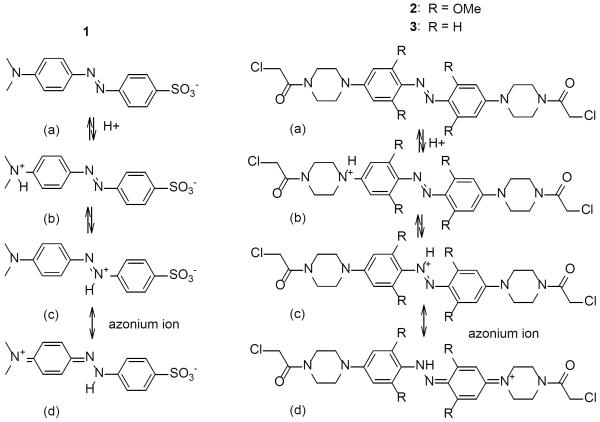
Azonium ions formed by amino-substituted azobenzenes (Fig. 1) are well known species that have strong absorbance in the red region of the spectrum.[5] Two features, however, make typical azonium ions difficult to use as photoswitches in a biological context. First, most azonium ions, such as protonated methyl orange (1), have pKas in the range of 1.5–3.5 [5a–c, 6] so that the azonium species is hardly present at the neutral pH normally encountered in vivo. Second, the cis-to-trans thermal isomerization rate of azonium ions is fast, with cis half lives in the μs range, so that production of a significant concentration of the cis isomer is difficult without very bright light sources.[7] The rapid thermal isomerization of the cis azonium species is attributed to the decreased double bond character of the N-N bond due to the contribution of resonance structure 1(d).[8]
Figure 1.
Neutral and protonated forms of methyl orange (1) and the ortho-substituted (2) and unsubstituted (3) aminoazobenzenes studied here. Di-protonated species can also form (see[5a]).
We report here that introduction of methoxy substituents to all four positions ortho to the azo group in an aminoazobenzene derivative has a remarkable effect on the photochemistry in that it enables photoswitching of the related azonium ion at neutral pH with red light. We synthesized the tetra-ortho-methoxy substituted aminoazobenzene derivative 2, a structure that permits two-point attached to a thiol containing target biomolecule. Synthesis of 2 was initiated from 1-bromo-3,5-dimethoxybenzene, which was transformed into 2,2',6,6'-tetramethoxy-4,4'-dibromoazobenzene as described previously.[4] Palladium-catalyzed two-fold amination at the 4 and 4' positions with tert-butyl piperazine-1-carboxylate led to di-tert-butyl-4,4'-(diazene-1,2-diylbis(3,5-dimethoxy-4,1-phenylene))bis-piperazine-1-carboxylate (see SI). Boc deprotection of amines followed by chloroacetylation using chloroacetyl chloride resulted in (2), which was then reacted with a test peptide AB15 (Ac-WGCAEAAAREAAAREAACRQ-NH2) having Cys residues at i and i+15 positions.[9] Attachment to a peptide ensures water solubility and prevents self-association of the dye as well as mimicking the target environment for the photoswitch. Azobenzene derivatives attached as cross-linkers to peptides and proteins have been used to drive conformational and functional changes in a variety of targets.[1a, 10]
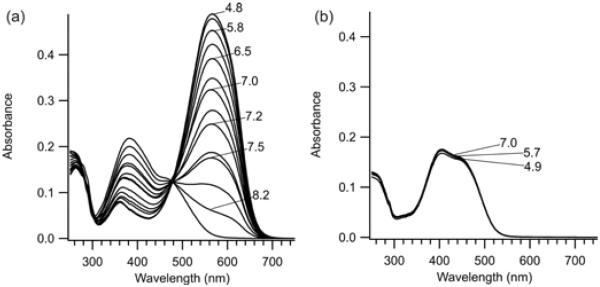
Figure 2 shows UV-Vis spectra in the range pH 5–9 of 2 and 3 (the non-methoxy substituted counterpart[9]) in their trans states after attachment to AB15. For the tetra-ortho-methoxy substituted compound 2, the azonium ion (λmax = 560 nm) is produced with an apparent pKa of 7.2. The corresponding non-ortho-substituted species 3 does not become protonated in this pH range (Fig. 2b). The azonium ion of 3 is only seen below pH 3 (Fig. S1).
Figure 2.
UV-Vis spectra of the trans isomers of (a) 2 and (b) 3 cross-linked to the peptide AB15 in aqueous buffer at the indicated pHs (22°C).
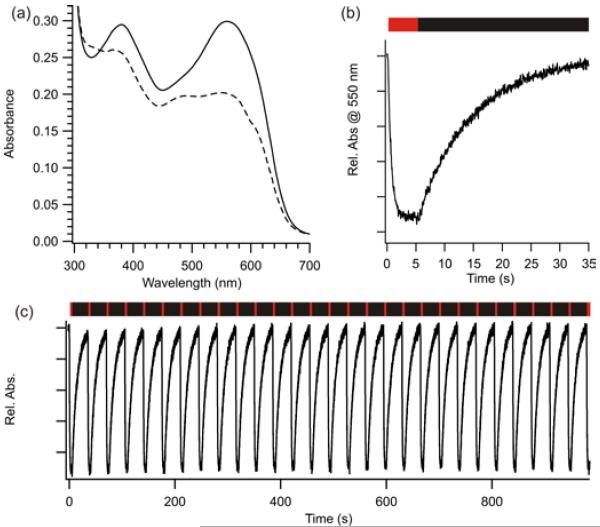
Irradiation of the azonium peak of 2 cross-linked to AB15 at pH 7.5 with red light (635 nm, 80 mW/cm2) produces a marked photochromism (Fig. 3a). The strong absorbance of the azonium species (~20,000 M−1cm−1 at 600 nm, pH 7.0, see SI) results in rapid (~1s) production of the photostationary state (Fig. 3b). After removing the red light irradiation, the dark state spectrum recovers thermally in a monoexponential manner with a half-life of ~10 s at pH 7.5 (Fig. 3b). This process can be repeated over many cycles with no apparent photobleaching (Fig. 3c).
Figure 3.
(a) UV-Vis spectra of 2 cross-linked to the peptide AB15: (—) dark-adapted, (---) with red light irradiation. (pH 7.5, 22°C) (b) photoisomerization under red light (indicated by the red bar) followed by thermal recovery in the dark (black bar). (c) Multiple photoswitching cycles show no evidence of photobleaching (pH 7.5, 22°C).
The thermal isomerization of methyl orange (1) has been studied in detail using laser flash photolysis techniques by Barra, Sanchez and colleagues.[7] These authors observed half-lives for thermal recovery via the azonium ion on the order of 2–3 μs. Thus, under comparable conditions, the thermal recovery rate of 2 is ~106 times slower. The consequence of this dramatically slowed thermal isomerization rate is that substantial populations of the thermally less stable cis species can be produced by red LED illumination under physiological conditions.
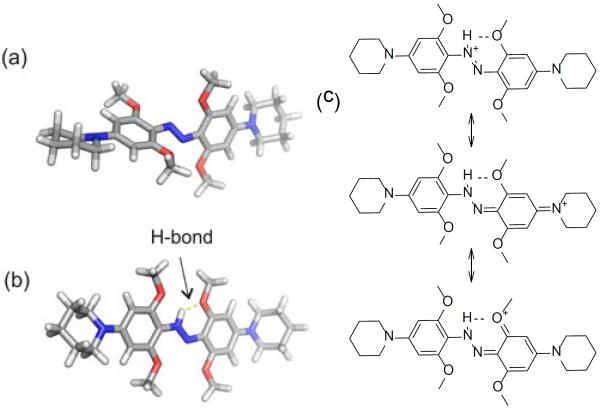
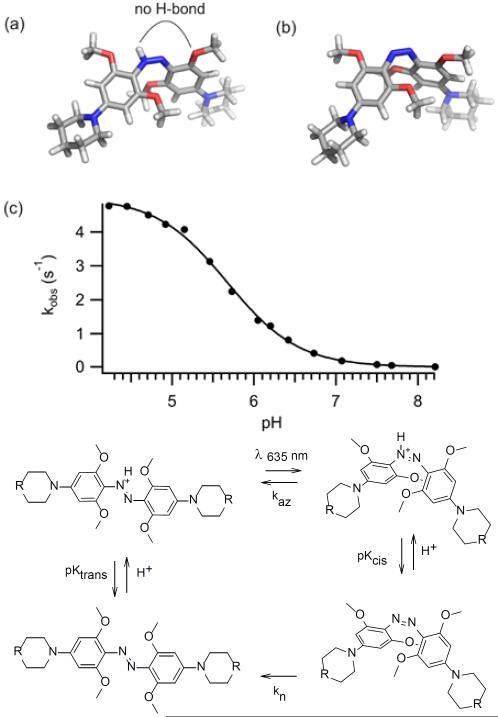
In an effort to understand the remarkable behaviour of 2, we carried out DFT (B3LYP, 6–311++G(d,p)) calculations to find minimum energy structures (See SI). We used piperidino analogues of 2 to simplify the calculations. As shown in Fig. 4, the neutral species 2a is non-planar (Fig. 4a) with substantial twisting of the rings as was observed in X-ray crystal structures of other neutral tetra-orthomethoxy species reported earlier.[4] Calculations predict the azonium species 2(c/d), however, is planar (Fig. 4b). Planarity facilitates resonance stabilization and H-bonding interactions as diagrammed in Fig. 4c. The presence of an H-bond as diagrammed in Fig. 4b was confirmed by an atoms-in-molecules analysis[11](see SI). Stabilization of azonium species by a single ortho substituent capable of H-bonding has been reported previously.[12] In 2, however, these effects apparently raise the pKa by ~ 4–5 pH units (compared to 3), so that the azonium species is the dominant species at physiological pHs.
Figure 4.
Calculated minimum energy structures of trans neutral (a) and protonated (b) forms of a model compound representative of 2. (c) Resonance representations showing effects that stabilize the azonium ion.
The red light induced photochromism observed (Fig. 3) implies the trans azonium species can absorb red light and isomerize to produce a cis species that has a lower absorbance coefficient. Figure 5 shows calculated minimum energy structures for the protonated and neutral cis species (Fig. 5a,b). Unlike the trans azonium species, the proton on the azo moiety of the cis azonium ion is too far from the ortho methoxy substituents to form an effective H-bond, suggesting the pKa of the cis azonium species is lower than the trans. DFT calculations also indicate the proton affinity of the cis isomer is lower than the trans (see SI). Thus, photoisomerization to the cis azonium species may be accompanied by conversion to the neutral cis state. Time-dependent DFT calculations predict that both neutral and protonated cis species have lower absorbance coefficients in the red region than the trans azonium ion (see SI).
Figure 5.
A neutral cis isomer would be expected to undergo thermal relaxation to the trans state much more slowly than the cis azonium species.[7–8] We measured the rate of thermal recovery as a function of pH (Fig. 5c) and found a sigmoidal dependence to the observed rate constant. This behavior is consistent with the simple kinetic scheme shown in Fig. 5d in which protonation/deprotonation rates are fast compared to rates of thermal relaxation and the neutral cis species undergoes relaxation (kn) ~1000 times more slowly than the cis azonium ion (kaz). From these data the cis azonium ion is estimated to have a pKa of 5.7, lower by 1.5 pH units than the trans azonium ion. The million fold decrease in the rate of thermal relaxation of 2 compared to typical azonium ions therefore appears due to two factors, (i) an intrinsic effect of the tetra-ortho substitution pattern on the thermal barrier, and (ii) deprotonation accompanying conversion to the cis isomer at physiological pHs.
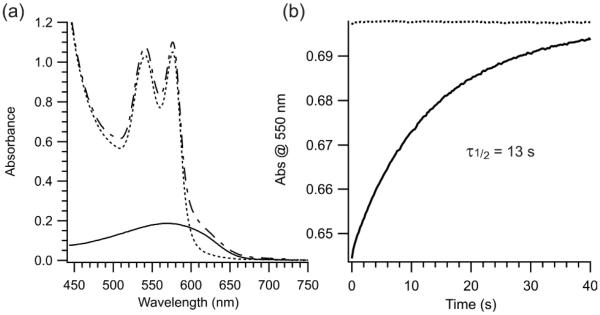
Red light has significantly higher penetration through biological tissue than other parts of the visible spectrum primarily because wavelengths >~600 nm can avoid absorbance by hemoglobin.[13] To test directly the possibility of using 2 as a photoswitch for controlling conformational changes and ultimately bioactivity in vivo, we mixed 2-crosslinked peptide directly with whole blood. The azonium absorption band extends beyond the hemoglobin absorbance (Fig. 6a). Irradiation of the sample at 635 nm produced photoswitching that was stable for at least 12 h (Fig. 6b).
Figure 6.
(a) UV-vis spectra of whole blood (…), 2-cross-linked AB15 alone (—) and 2-cross-linked AB15 (1 mM)(–˙–) in whole blood. Each sample was diluted 1000 fold in tris buffered saline, pH 7.4 before scanning (b) Thermal relaxation after red light irradiation (80 mW/cm2, 635 nm) of blood only (…) and undiluted blood containing 2-crosslinked AB15 (—) (after incubation at room temperature for 12h).
The tetra-ortho-methoxy substitution pattern could be used to develop a class of azo compounds whose isomerization can be triggered by long wavelength light. Azonium species are known with absorbance maxima >660 nm [5b, 5d] and with further tuning of the pKa difference between cis and trans isomers, azo compounds might be developed for manipulating biomolecules in vivo with near infrared light. As might be anticipated based on previous work,[4] there is some sensitivity to reduction by high concentrations of glutathione, however (the compound is reduced by 10 mM GSH with a half-life of ~1.4 h, see SI), so that use may be restricted to extracellular and/or oxidizing environments in vivo. For example, blood-borne peptide hormones and growth factors could be coupled to an azonium based photoswitch and manipulated non-invasively with a high degree of spatiotemporal control.
Experimental Section
The peptide AB15 was prepared using standard Fmoc-based solid-phase synthetic methods. Intramolecular cross-linking of cysteine residues on AB15 with (2) (freshly prepared, see details in the SI) was performed in 50% DMSO as follows: a solution of 0.5 mM peptide (freshly purified) and 2 mM cross-linker 2 in 50 mM Tris buffer at pH 8 was stirred at 40°C under a nitrogen atmosphere for 16–20 h. The completion of the reaction was judged by MALDI mass spectra. The reaction was dried under high vacuum, and the cross-linked peptide was purified by reverse-phase HPLC on a semipreparative RX-C8 column (Zorbax, 9.4 mm ID × 255 mm) with a linear gradient of 10–65% acetonitrile/water (containing 0.1% trifluoroacetic acid) over a period of 25 min. Cross-linked AB15 was eluted at 47% acetonitrile. ESI-MS: m/z calcd for C114H173N38O34S2: 2683.9 [M+], obs'd 2682.4
Supplementary Material
Footnotes
We are grateful to NSERC and NIH (R01 MH086379) for financial support of this work.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1].(a) Szymanski W, Beierle JM, Kistemaker HA, Velema WA, Feringa BL. Chem. Rev. 2013 doi: 10.1021/cr300179f. [DOI] [PubMed] [Google Scholar]; (b) Shcherbo D, Shemiakina, Ryabova AV, Luker KE, Schmidt BT, Souslova EA, Gorodnicheva TV, Strukova L, Shidlovskiy KM, Britanova OV, Zaraisky AG, Lukyanov KA, Loschenov VB, Luker GD, Chudakov DM. Nat. Methods. 2010;7:827–829. doi: 10.1038/nmeth.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu J, Bu W, Pan L, Shi J. Angew. Chem. Int. Ed. Engl. 2013 [Google Scholar]
- [4].Samanta S, Beharry AA, Sadovski O, McCormick TM, Babalhavaeji A, Tropepe V, Woolley GA. J. Am. Chem. Soc. 2013;135:9777–9784. doi: 10.1021/ja402220t. [DOI] [PubMed] [Google Scholar]
- [5].(a) Stoyanov S, Antonov L, Stoyanova T, Petrova V. Dyes Pigments. 1996;32:171–185. [Google Scholar]; (b) Sawicki E. J. Org. Chem. 1957;22:1084–1088. [Google Scholar]; (c) Sawicki E. J. Org. Chem. 1957;22:621–625. [Google Scholar]; (d) Yagupolskii L, Gandelsman L. J. Gen. Chem. USSR. 1965;35:1259–1266. [Google Scholar]
- [6].Zenhausern A, Zollinger H. Helv. Chim. Acta. 1962;45:1890–1898. [Google Scholar]
- [7].Sanchez AM, Barra M, de Rossi RH. J. Org. Chem. 1999;64:1604–1609. doi: 10.1021/jo982069j. [DOI] [PubMed] [Google Scholar]
- [8].Azuki M, Morihashi K, Watanabe T, Takahashi O, Kikuchi O. J. Mol. Struct. Theochem. 2001;542:255–262. [Google Scholar]
- [9].Beharry AA, Sadovski O, Woolley GA. Org. Biomol. Chem. 2008;6:4323–4332. doi: 10.1039/b810533b. [DOI] [PubMed] [Google Scholar]
- [10].Beharry AA, Woolley GA. Chem. Soc. Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- [11].(a) Koch U, Popelier PLA. J. Phys. Chem. 1995;99:9747–9754. [Google Scholar]; (b) Fuster F, Grabowski SJ. J. Phys. Chem. A. 2011;115:10078–10086. doi: 10.1021/jp2056859. [DOI] [PubMed] [Google Scholar]
- [12].(a) Gregory P, Thorp D. J. Chem. Soc. Perkin 1. 1979:1990–2000. [Google Scholar]; (b) Ross WCJ, Warwick GP. J. Chem. Soc. 1956:1719–1724. [Google Scholar]
- [13].Cheong WF, Prahl SA, Welch AJ. IEEE J. Quantum Electronics. 1990;26:2166–2185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.