Abstract
Background
Risk stratification of atrial fibrillation patients with a CHADS2 score of < 2 remains imprecise, particularly in women. Our objectives were to validate the CHADS2 and CHA2DS2-VASc stroke risk scores in a healthy cohort of American women with atrial fibrillation and to determine whether CHA2DS2-VASc further risk stratifies individuals with a CHADS2 score of < 2.
Methods
We identified a cohort of 5,981 women with atrial fibrillation not on warfarin at baseline (mean age 65.9±7.2 years) enrolled in the Women's Health Initiative and followed for a median of 11.8 years. Univariate and multivariate proportional hazards analyses were used to examine these two risk scores with main outcome measures being annualized event rates of ischemic stroke or transient ischemic attack stratified by risk score.
Results
Annualized stroke/ transient ischemic attack rates ranged from 0.36-2.43% with increasing CHADS2 score (0-4+) (hazard ratio (HR) 1.57, 95% confidence interval (CI) 1.45-1.71 for each 1 point increase) and 0.20-2.02% with increasing CHA2DS2-VASc score (1-6+) (HR 1.50, 95% CI 1.41-1.60 for each 1 point increase). CHA2DS2-VASc had a higher c statistic than CHADS2: 0.67 (95% CI 0.65-0.69) vs. 0.65 (95% CI 0.62-0.67), p < 0.01. For CHADS2 scores < 2, stroke risk almost doubled with every additional CHA2DS2-VASc point.
Conclusions
Although both CHADS2, and CHA2DS2-VASc are predictive of stroke risk in post-menopausal women with atrial fibrillation, CHA2DS2-VASc further risk-stratifies patients with a CHADS2 score < 2.
Keywords: atrial fibrillation, CHADS2, CHA2DS2-VASc, stroke, women
Introduction
Reported stroke rates in patients with atrial fibrillation not treated with anticoagulation range from < 1% (low risk) to > 18% (high risk) per year3-5 and studies show that women are at higher risk.5-9 Although guidelines recommend treatment with anticoagulants in higher risk and aspirin in lower risk patients, the distinction between these two risk categories remains unclear.10,11
Of the many scoring systems developed to predict stroke risk in atrial fibrillation, CHADS23 and CHA2DS2-VASc8 are the most widely used. CHADS2, developed from a combination of Atrial Fibrillation Investigators (AFI) and Stroke Prevention in Atrial Fibrillation (SPAF), was validated using the National Registry of Atrial Fibrillation (NRAF) comprising 1,733 Medicare beneficiaries aged 65-95 with non-rheumatic atrial fibrillation not on warfarin at hospital discharge.3 In this score, while congestive heart failure (C), hypertension (H), age ≥ 75 (A) and diabetes (D) receive 1 point each, stroke or transient ischemic attack (S2) receives 2 points.
CHA2DS2-VASc, a modification of the 2006 Birmingham/NICE scheme,10 was validated in a cohort of 1,084 hospitalized, ambulatory patients not anticoagulated at baseline from the Euro Heart Survey on atrial fibrillation.8 CHA2DS2-VASc expands on CHADS2 by 1) including a history of systemic thromboembolism (S2) in the stroke category, and 2) adding vascular disease (defined as prior myocardial infarction, peripheral arterial disease or aortic plaque (V)), age (65-74 years (A)), and female gender (S) as risk factors. All risk factors receive 1 point, except age ≥ 75 and history of prior stroke/ transient ischemic attack / thromboembolism, which receive 2 points each.
As there are no validation studies comparing CHADS2 and CHA2DS2-VASc in an ambulatory US population of women with atrial fibrillation, our objectives were to 1) validate and compare the predictive power of these scores, 2) determine the annualized rates of stroke, and 3) clarify the discriminatory ability of CHA2DS2-VASc in such a population.
Methods
Study Population
The study design has been described previously.12,13 Study participants were members of the Women's Health Initiative (WHI) cohort, a prospective, multi-arm clinical trial and observational study that focused on the causes and prevention of cardiovascular disease, cancer and osteoporosis in women. Major exclusion criteria were predicted survival < 3 years, alcohol or drug dependency, dementia, severe mental illness and participation in another clinical trial. WHI comprised an observational study and four randomized clinical trials: 1) estrogen plus progestin versus placebo, 2) estrogen alone versus placebo in hysterectomized women, 3) dietary modification trial, and 4) calcium/vitamin D versus placebo trial.
Beginning in 1993, 161,809 post-menopausal women aged 50-79 were prospectively enrolled in WHI. Events through September 2010 were used for this retrospective analysis. The initial study population consisted of women who reported a history of atrial fibrillation or had an electrocardiogram with documented atrial fibrillation at baseline (n=7,108). From this group, we excluded 291 with valvular heart disease or hyperthyroidism, 85 with missing values for either CHADS2 or CHA2DS2-VASc, and 790 on warfarin at WHI randomization or enrollment. 1,127 were excluded, leaving a final sample of 5,981, of whom 2,390 were participants in one of the clinical trials and 3,591 were enrolled in the observational study. 5,901 women with atrial fibrillation were identified by self-report, 24 by electrocardiogram, and 56 had both.
Definition of Variables
Congestive heart failure, diabetes mellitus and prior stroke or transient ischemic attack were defined by self-report at initial examination. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or use of any antihypertensive medication. Vascular disease was defined as self-report of any of the following: myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery or peripheral vascular disease. Information on aortic plaque and systemic thromboembolism, although included among the CHA2DS2-VASc risk factors,8 was not collected by WHI.
Follow-Up and End Point Determination
Intensity of follow-up visits varied based on enrollment arm, ranging from every 6 months (clinical trials) to every 3 years (observational study). When a potential outcome was identified, medical records were obtained and stroke (including self-reports) and transient ischemic attack (only the first event) were centrally adjudicated.14 No bleeding endpoints were collected. We lost 2.3% of our cohort to follow-up and 4.2% stopped follow-up early.
Statistical Analysis
We summarized baseline characteristics with means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Annualized percentages for each construct are presented, calculating the percentage for each CHADS2 and CHA2DS2-VASc level as the total number of events divided by the total at-risk follow-up time.
Proportional hazards modeling was used to evaluate the relationship between the two constructs and stroke. Both constructs were evaluated in a continuous and categorical form, modeling the stroke outcome by each construct, with non-stroke participants censored at death or when lost to follow-up. Hazard ratios (HR) and corresponding p-values are presented for each model.
For each model, Harrell's c statistic was calculated to quantify the discriminatory ability of the constructs, and 95% confidence intervals (CI) for each c statistic and the difference between c statistics using bootstrapping with 1,000 replications were computed.
Components of each construct as predictors of stroke were evaluated using both univariate and multivariate modeling. Events and annualized rates for each component level are presented with their corresponding univariate p-value from a model evaluating each component individually. All components were put into a single model with resulting HRs and corresponding p-values presented.
All proportional hazards models were adjusted for aspirin use and stratified within the model by WHI hormone trial arm (not randomized, active, placebo), dietary modification trial arm (not randomized, intervention, comparison), and calcium/vitamin D arm (not randomized, active, placebo). Analyses were completed using SAS version 9.1.
To compare CHADS2 and CHA2DS2-VASc on stroke risk prediction, we used the Net Reclassification Improvement Index (NRI), which identifies how many participants are correctly and incorrectly reclassified into different risk categories (upward for events, downward for non-events).15 Since 3.5% of our atrial fibrillation participants had a stroke/ transient ischemic attack event over the first 5 years of follow-up, we examined the < 3%, 3 to < 6%, 6 to < 9%, and ≥ 9% risk groups excluding participants censored prior to 5 years of follow-up.
Results
Baseline Characteristics
In the WHI cohort with atrial fibrillation not on warfarin at baseline, 64.9% were hypertensive, 3.7% had congestive heart failure, 9.2% had diabetes mellitus, 2.6% had prior stroke, 4.9% had prior transient ischemic attack, 10.3% had prior coronary artery disease and 5.2% had peripheral vascular disease (Table 1). Warfarin users (not included in this analysis) had a significantly higher prevalence of CHADS2 and CHA2DS2-VASc risk factors than non-users. The WHI did not collect information regarding why patients were not on anticoagulation.
Table 1. Demographic and Clinical Characteristics of Participants with Baseline Atrial Fibrillation by Warfarin Use at Baseline.
| Baseline Characteristic | Non-Users (N =5981) |
Warfarin Users (N=753) |
p-value | ||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | ||
|
| |||||
| Congestive Heart Failure | 222 | 3.7 | 145 | 19.3 | <0.001 |
|
| |||||
| Hypertension (BP ≥ 140/90 or Meds Use) | 3879 | 64.9 | 615 | 81.7 | <0.001 |
|
| |||||
| Age, mean (SD) | 65.85 (7.18) | 69.03 (5.97) | <0.001 | ||
| <65 | 2469 | 41.3 | 161 | 21.4 | |
| 65 - 74 | 2789 | 46.6 | 447 | 59.4 | |
| ≥ 75 | 723 | 12.1 | 145 | 19.3 | |
|
| |||||
| Diabetes Mellitus | 553 | 9.2 | 102 | 13.5 | <0.001 |
|
| |||||
| Prior Stroke | 156 | 2.6 | 102 | 13.5 | <0.001 |
|
| |||||
| Prior TIA | 296 | 4.9 | 127 | 16.9 | <0.001 |
|
| |||||
| Clopidogrel (Plavix) Use | 1 | 0.0 | 0 | 0.0 | 0.723 |
|
| |||||
| Aspirin Use (≥ 80 mg / day) | 2041 | 34.1 | 49 | 6.5 | <0.001 |
|
| |||||
| Hormone Use | <0.001 | ||||
| Never | 2455 | 41.0 | 394 | 52.3 | |
| Past | 1184 | 19.8 | 147 | 19.5 | |
| Current | 2336 | 39.1 | 212 | 28.2 | |
|
| |||||
| History of Coronary Artery Disease (MI/CABG/PTCA) | 616 | 10.3 | 109 | 14.5 | <0.001 |
|
| |||||
| History of MI | 511 | 8.5 | 81 | 10.8 | 0.043 |
|
| |||||
| History of CABG/PTCA | 259 | 4.3 | 57 | 7.6 | <0.001 |
|
| |||||
| History of PVD | 312 | 5.2 | 59 | 7.8 | 0.012 |
BP = blood pressure, SD = standard deviation, TIA = transient ischemic attack, MI = myocardial infarction, CABG = coronary artery bypass graft, PTCA = percutaneous transluminal coronary angioplasty, PVD = peripheral vascular disease
Stroke rate
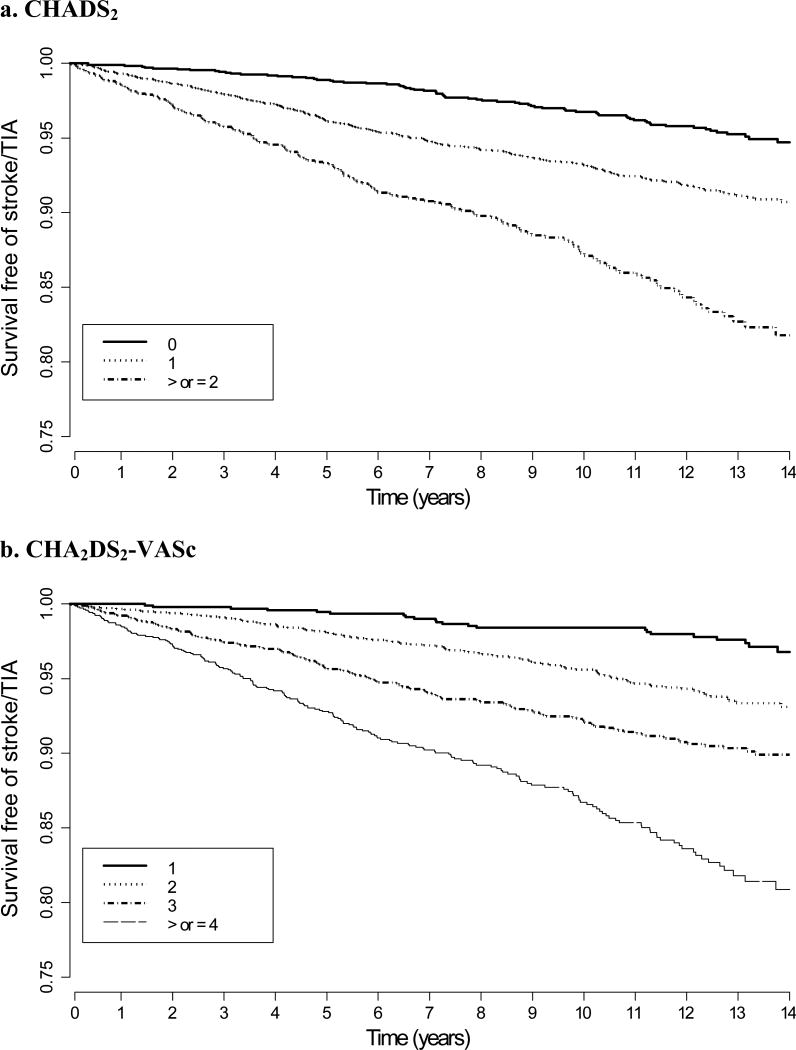
During a median of 11.8 years of follow-up (interquartile range 8.0-13.6), there were 457 stroke/ transient ischemic attack events (137 of which were transient ischemic attacks) among the 5,981 women. Annualized stroke/ transient ischemic attack rates ranged from 0.36-2.43% with increasing CHADS2 score (0-4+) and 0.20-2.02% with increasing CHA2DS2-VASc score (1-6+, since all were female) (Table 2). Kaplan-Meier curves for survival free from stroke/ transient ischemic attack, stratified by CHADS2 and CHA2DS2-VASc score, demonstrate a progressive decrease in event-free survival over time as the respective scores increase (Figure 1).
Table 2. Annualized Rates of Strokea / TIA by CHADS2 and CHA2DS2-VASc Scores.
| Construct, Level | N | Stroke1 / TIA | |
|---|---|---|---|
|
| |||
| Events | Annual % | ||
|
| |||
| CHADS2 Score | |||
| 0 | 1760 | 71 | 0.36 |
| 1 | 2879 | 219 | 0.72 |
| 2 | 922 | 106 | 1.27 |
| 3 | 299 | 38 | 1.45 |
| 4+ | 121 | 23 | 2.43 |
|
| |||
| CHA2DS2-VASc Score | |||
| 1 | 951 | 22 | 0.20 |
| 2 | 1794 | 96 | 0.48 |
| 3 | 1799 | 152 | 0.82 |
| 4 | 911 | 108 | 1.30 |
| 5 | 343 | 51 | 1.71 |
| 6+ | 183 | 28 | 2.02 |
Does not include hemorrhagic stroke
Figure 1. Kaplan-Meier curves of stroke/TIA-free survival stratified by CHADS2 and CHA2DS2-VASc score.
Stroke Scheme Performance
Although both risk scores were predictive of stroke risk according to univariate proportional hazards analysis (p < 0.001 for both), the discriminatory ability of CHA2DS2-VASc was higher than that of CHADS2 (p < 0.01). For every 1-point increase, while CHADS2 had a HR of 1.57 (95% CI 1.45, 1.71, c statistic: 0.65 (95% CI 0.62, 0.67)), CHA2DS2-VASc had a HR of 1.50 (95% CI 1.41, 1.60, c statistic: 0.67 (95% CI 0.65, 0.69)) (Table 3).
Table 3. Univariate Proportional Hazards Analysesa of Stroke/ TIA by CHADS2 and CHA2DS2-VASc Constructs.
| Construct, Level | Stroke / TIA (N =5981) | ||
|---|---|---|---|
| HR (95% CI) | p-value | c-statistic (95% CI) | |
| CHADS2 Score | |||
| Continuous - 1 pt increase | 1.57 (1.45, 1.71) | <0.001 | 0.65 (0.62, 0.67) |
| Categorical | <0.001 | 0.65 (0.62, 0.67) | |
| 0 | 1.00 (ref) | ||
| 1 | 2.00 (1.53, 2.61) | ||
| 2 | 3.55 (2.62, 4.81) | ||
| 3 | 4.02 (2.70, 5.99) | ||
| 4+ | 6.69 (4.16, 10.78) | ||
| CHA2DS2-VASc Score | |||
| Continuous - 1 pt increase | 1.50 (1.41, 1.60) | <0.001 | 0.67 (0.65, 0.69) |
| Categorical | <0.001 | 0.67 (0.65, 0.69) | |
| 1 | 1.00 (ref) | ||
| 2 | 2.45 (1.54, 3.90) | ||
| 3 | 4.16 (2.65, 6.52) | ||
| 4 | 6.67 (4.20, 10.60) | ||
| 5 | 8.92 (5.38, 14.78) | ||
| 6+ | 10.34 (5.87, 18.23) | ||
All models are adjusted for aspirin use and stratified within the model by WHI hormone trial arm (not randomized, active, placebo); WHI dietary modification arm (not randomized, intervention, comparison); WHI calcium / vitamin D arm (not randomized, active, placebo)
All individual construct components of the risk scores were predictive of stroke in univariate analysis but congestive heart failure was not significant in multivariate analysis (HR 1.05, 95% CI 0.68, 1.64, p=0.827) (Table 4). The most predictive variables by multivariate analysis were age ≥ 75 (HR 2.91, 95% CI 2.19, 3.86, p <0.001) and history of stroke or transient ischemic attack (HR 2.13, 95% CI 1.60, 2.82, p <0.001).
Table 4. Univariate and Multivariate Proportional Hazards Analyses of Stroke / TIA by Risk Factor.
| Risk Factor | Group Event (Ann %) | Univariate p-value | Multivariate HR (95% CI) | Multivariate p-value |
|---|---|---|---|---|
|
| ||||
| Hx of CHF | 22 (1.14) | 0.042 | 1.05 (0.68, 1.64) | 0.827 |
|
| ||||
| Hypertension | 351 (0.90) | <0.001 | 1.55 (1.24, 1.94) | <0.001 |
|
| ||||
| Age (ref=<65) | <0.001 | <0.001 | ||
| 65 - 74 | 248 (0.88) | 1.95 (1.56, 2.45) | ||
| 75 + | 93 (1.46) | 2.91 (2.19, 3.86) | ||
|
| ||||
| Hx of Diabetes | 58 (1.15) | <0.001 | 1.38 (1.04, 1.83) | 0.027 |
|
| ||||
| Hx of Stroke/TIA | 59 (1.80) | <0.001 | 2.13 (1.60, 2.82) | <0.001 |
|
| ||||
| Hx of CAD/PVD | 99 (1.29) | <0.001 | 1.46 (1.15, 1.85) | 0.002 |
To determine the possible effect of hormone replacement therapy on the prognostic ability of these risk scores, we evaluated a subgroup interaction between both the continuous CHADS2 and CHA2DS2-VASc scores and hormone therapy, defined by intervention assignment if enrolled in the hormone therapy trial and by baseline hormone use if not. For both CHADS2 (p=0.70) and CHA2DS2-VASc (p=0.43), the association of the score was not significantly modified by hormone use.
Risk Score Comparison
Although CHADS2 is helpful for scores ≥ 2, clinical decision-making becomes more ambiguous when the score is < 2. Since the two highest frequency CHADS2 scores in our population were 1 (n=2,879, 48%) and 0 (n=1,760, 29%), we compared the classification of these patients in both schemes to see if CHA2DS2-VASc would have an added benefit (Table 5). Table 5 demonstrates that within any CHADS2 score column, a higher CHA2DS2-VASc score corresponds to a higher event rate but the same is not true when stratifying risk within any given CHA2DS2-VASc score row. For CHADS2 scores < 2, stroke risk almost doubles with every additional CHA2DS2-VASc point. Further, when using CHA2DS2-VASc at 5-year follow-up, the NRI was 21.1%, z-stat = 4.70, p < 0.001: 65 of 212 patients with a stroke/thromboembolic event (30.7%) and 897 of 5,372 patients without an event (16.6%) were reclassified to a higher risk category.
Table 5. Event Totals/Participant Totals and (Annualized Rates of Stroke/TIA) by Risk Score.
| CHADS 2 | ||||||
|---|---|---|---|---|---|---|
| Total Participants | 0 | 1 | 2 | 3 | ≥4 | |
| CHA2DS2-VASc | 1 | 22/951 (0.20) | ||||
| 2 | 44/755 (0.53) | 52/1039 (0.44) | ||||
| 3 | 5/54 (0.91) | 136/1601 (0.82) | 11/144 (0.77) | |||
| 4 | 31/239 (1.43) | 71/610 (1.29) | 6/62 (0.98) | |||
| 5 | 24/168 (1.68) | 26/169 (1.73) | 1/6 (1.61) | |||
| ≥6 | 6/68 (1.19) | 22/115 (2.49) | ||||
Discussion
This study validated CHADS2 and CHA2DS2-VASc for predicting stroke risk in a US population of post-menopausal women with atrial fibrillation followed for a median of 11.8 years. While both scores have modest but similar discriminative accuracy, CHA2DS2-VASc appears to be useful in further risk-stratifying women with CHADS2 < 2.
In the original validation study, CHADS2 had a c statistic of 0.82 (95% CI, 0.80-0.84), while AFI and SPAF had c statistics of 0.68 (95% CI, 0.65-0.71) and 0.74 (95% CI, 0.71-0.76) respectively.3 Largely based on this study, current US guidelines16 recommend risk-stratification and anticoagulation treatment as follows: aspirin 81-325 mg daily, CHADS2 = 0; aspirin or anticoagulation with warfarin or dabigatran, CHADS2 = 1; and anticoagulation, CHADS2 ≥ 2.
Although CHADS2 is a useful risk-stratification scheme that has been adopted by the ACC, AHA, ESC, and Heart Rhythm Society (HRS), 11,16,17_ENREF_18 one drawback is that it places a large percentage of patients (up to 47% by some estimates; 48% of our cohort)18 in an intermediate risk category (CHADS2 = 1) with subsequent ambiguity as to the appropriate anticoagulation strategy. If aspirin, dabigatran, rivaroxaban, apixaban and warfarin all offered equivalent protection from stroke, this recommendation would be uncontroversial. However, while warfarin decreases stroke risk in atrial fibrillation patients by approximately 68%, aspirin has been shown to provide only a 20-36% reduction in ischemic stroke risk.19-22 Moreover, aspirin is often used even when warfarin is indicated, because of its ease of administration and the widely-held view that it provides a decreased risk of major bleeding, studies demonstrating equivalent risk notwithstanding.19,23,24
Lip et al. (2010)8 suggested that a significant advantage of CHA2DS2-VASc was that it classified very few women as intermediate risk (score of 1 = 15.1%) compared to CHADS2 (1 score = 34.9%). Also, there was a very low event rate in the CHA2DS2-VASc 0-1 group (0 score = 0%; 1 score = 0.6%). In their analysis, CHADS2 and CHA2DS2-VASc had c statistics of 0.586 and 0.606 respectively. Based on these findings, the ESC guidelines recommend that CHA2DS2-VASc be used when CHADS2 ≤ 1 and that anticoagulation be prescribed when CHA2DS2-VASc ≥ 2, aspirin 75-325 mg or anticoagulation when CHA2DS2-VASc = 1 (anticoagulation preferred), and aspirin only when CHA2DS2-VASc = 0.17
Several studies have validated and compared CHA2DS2-VASc to CHADS2 in recently hospitalized populations not on anticoagulation. In a cohort of Danish patients, Olesen et al. (2011) found that both were predictive of stroke risk (CHADS2: c statistic of 0.812; CHA2DS2-VASc: c statistic of 0.885).25 Friberg et al. (2012) evaluated stroke risk schemes in a cohort from the National Swedish Drug registry over a median of 1.4 years and found that both schemes were predictive of stroke risk (CHADS2: c statistic of 0.62; CHA2DS2-VASc: c statistic of 0.67).26 In both studies, CHA2DS2-VASc was better at identifying patients assessed as truly low-risk for thromboembolic stroke (0 score). Olesen et al. (2012) validated CHA2DS2-VASc in a population with a CHADS2 score < 2 and, as in our study, found that CHA2DS2-VASc improved on the predictive ability of CHADS2 (NRI= 14.2; p < 0.001).27
Our study offers unique data in a large prospective cohort of American women by validating CHADS2 and CHA2DS2-VASc in a population of healthy, ambulatory, post-menopausal women with more than 11 years of follow-up. This is particularly important since all validations to date of CHA2DS2-VASc have been performed in recently hospitalized, non-US cohorts. Second, it provides additional evidence that the risk factors of age 65-74 and history of vascular disease included in CHA2DS2-VASc may help to further risk-stratify those patients who would otherwise fall into either a low or intermediate risk category (CHADS2 ≤ 1).
A first limitation of this study involves the inherent confines of the WHI cohort. Because it did not include men, no conclusions can be made regarding the effect of female gender on stroke risk and because information on aortic plaque and systemic thromboembolism was not available, these variables were not able to be included in the evaluation of CHA2DS2-VASc.
Another limitation is that the designation of atrial fibrillation (and of many of the risk factors) was made by self-report in the majority of participants (5,901 out of 5,981), which could have led to participant misclassification and lower than expected event rates (Table 2).3,8 However, a recent study suggests that self-report of atrial fibrillation is as predictive of stroke risk as documentation by electrocardiogram.28 An alternative explanation for the relatively low stroke/ transient ischemic attack rates may be the relative health of our cohort. A recent comparison of stroke risk schemes in a cohort of 13,559 patients with atrial fibrillation by Fang et al. found similar annual stroke rates in low and intermediate-risk patients.4 It is also possible that some of the women may have started warfarin during the follow-up period, which may have influenced (presumably by decreasing) the event rate of stroke/ transient ischemic attack. However, at year 3, only 340 of the 5,021 women on whom we have medication data had started warfarin. Further, re-analysis after excluding these women did not substantially change the stroke rates. It is also possible that patients in our cohort died of causes other than a stroke, since the annualized death rates in our cohort more closely approximate the annualized stroke/ transient ischemic attack rates in the original studies (Table 6).3,8
Table 6. Annualized Rates of Death by CHADS2 and CHA2DS2-VASc Scores.
| Construct, Level | N | Death | |
|---|---|---|---|
|
| |||
| Events | Annual % | ||
|
| |||
| CHADS2 Score | |||
| 0 | 1760 | 175 | 0.87 |
| 1 | 2879 | 494 | 1.57 |
| 2 | 922 | 309 | 3.53 |
| 3 | 299 | 97 | 3.49 |
| 4+ | 121 | 63 | 6.13 |
|
| |||
| CHA2DS2-VASc Score | |||
| 1 | 951 | 56 | 0.50 |
| 2 | 1794 | 204 | 1.00 |
| 3 | 1799 | 345 | 1.80 |
| 4 | 911 | 307 | 3.53 |
| 5 | 343 | 133 | 4.18 |
| 6+ | 183 | 93 | 6.28 |
A further finding of our study that does not correlate with some of the prior cohorts is that a history of congestive heart failure was not predictive of stroke/ transient ischemic attack in multivariate analysis. This could be due to the low incidence of congestive heart failure in our cohort, possibly because women are more likely to have diastolic heart failure, thereby attenuating the impact of systolic heart failure in this cohort or because these women underreported diagnoses of heart failure. Nonetheless, at least two other studies have failed to support congestive heart failure as a risk factor for stroke in atrial fibrillation.26,29
In conclusion, both CHADS2, and CHA2DS2-VASc are predictive of stroke risk in ambulatory, post-menopausal female patients with atrial fibrillation. This study provides valuable information on the added value of using CHA2DS2-VASc to further risk-stratify women who have a CHADS2 score < 2, which, when combined with an assessment of bleeding risk, may have clinical implications for identifying patients who should be offered anticoagulation instead of aspirin therapy. Future studies are needed to clarify anticoagulation recommendations for CHADS2 < 2 individuals, particularly in the era of new oral anticoagulants.
Clinical Significance.
CHADS2, and CHA2DS2-VASc, the most widely-used stroke risk scores for patients with atrial fibrillation, guide decisions regarding anticoagulation.
CHADS2 classifies more than half of patients with atrial fibrillation as being at low or intermediate risk for stroke (score < 2).
CHA2DS2-VASc further risk-stratifies CHADS2 < 2 patients, which may help to guide clinical decisions regarding anticoagulation.
Acknowledgments
WHI Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, Nancy Geller
WHI Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg
WHI Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Georgetown-Howard Center for Clinical and Translational Sciences) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC); Sally Shumaker
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Abbreviations list
- AFI
Atrial Fibrillation Investigators
- SPAF
Stroke Prevention in Atrial Fibrillation
- WHI
Women's Health Initiative
- HR
hazard ratio
- CI
confidence interval
- NRI
net reclassification improvement
- US
United States
- ACC
American College of Cardiology
- AHA
American Heart Association
- ESC
European Society of Cardiology
- HRS
Heart Rhythm Society
Footnotes
Authorship Details: J Abraham, S Wassertheil-Smoller: Study concept and design
J Larson, J Abraham, S Wassertheil-Smoller, B Wilkoff: Data analysis and interpretation
J Abraham, J Larson: Drafting of the manuscript
M Chung, A Curtis, K Lakshminarayan, J Newman, M Perez, K Rerode, N Shara, A Solomon, M Stefanik, J Torner, S Wassertheil-Smoller, B Wilkoff: Critical revision of the manuscript for important intellectual content
Conflict of Interest: Dr. Curtis: Pfizer: honoraria (advisory board); Bristol Meyers Squibb: honoraria (advisory board)
None of the other authors have relevant relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. The American journal of cardiology. 2009 Dec 1;104(11):1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA : the journal of the American Medical Association. 2001 Jun 13;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 4.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. Journal of the American College of Cardiology. 2008 Feb 26;51(8):810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA : the journal of the American Medical Association. 2003 Aug 27;290(8):1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 6.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005 Sep 20;112(12):1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke; a journal of cerebral circulation. 1999 Jun;30(6):1223–1229. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Chao TF, Liu CJ, Chen SJ, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke; a journal of cerebral circulation. 2012 Oct;43(10):2551–2555. doi: 10.1161/STROKEAHA.112.667865. [DOI] [PubMed] [Google Scholar]
- 10.National Health Service NCCfCC, editor. NICE. NICE Clinical Guideline 36: The Management of Atrial Fibrillation. National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 11.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) Journal of the American College of Cardiology. 2006 Aug 15;48(4):854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003 Oct;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003 Oct;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 14.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA : the journal of the American Medical Association. 2003 May 28;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008 Jan 30;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 16.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Journal of the American College of Cardiology. 2011 Mar 15;57(11):e101–198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) European heart journal. 2010 Oct;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 18.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA : the journal of the American Medical Association. 2011 May 25;305(20):2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Archives of internal medicine. 1994 Jul 11;154(13):1449–1457. [PubMed] [Google Scholar]
- 20.The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. The Atrial Fibrillation Investigators. Archives of internal medicine. 1997 Jun 9;157(11):1237–1240. [PubMed] [Google Scholar]
- 21.Aguilar MI, H R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke of transient ischemic attack. The Cochrane Library. 2011;(4):1–34. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009 Sep 17;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 23.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007 Aug 11;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 24.Roy B, Desai RV, Mujib M, et al. Effect of warfarin on outcomes in septuagenarian patients with atrial fibrillation. The American journal of cardiology. 2012 Feb 1;109(3):370–377. doi: 10.1016/j.amjcard.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Bmj. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. European heart journal. 2012 Jan 13; doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 27.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: A nationwide cohort study. Thrombosis and haemostasis. 2012 May 31;107(6):1172–1179. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 28.Soliman EZ, Howard G, Meschia JF, et al. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke; a journal of cerebral circulation. 2011 Oct;42(10):2950–2953. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart RG, Pearce LA. Current status of stroke risk stratification in patients with atrial fibrillation. Stroke; a journal of cerebral circulation. 2009 Jul;40(7):2607–2610. doi: 10.1161/STROKEAHA.109.549428. [DOI] [PubMed] [Google Scholar]