Abstract
Deep hypothermia (cryoanesthesia) is often used as general anesthesia during surgery in neonatal rodents. Neonatal cryoanesthesia has been used recently to generate somatic brain transgenic (SBT) mouse models via intracerebral ventricular injection of rAAV vectors into both non-transgenic mice and numerous transgenic mouse models. Since, the evaluation of cognition is one of the main experimental endpoints in many of these studies, we examined the consequences of brief neonatal cryoanesthesia on the physical development and mnemonic function of adult mice. Two groups of 129FVBF1 pups from reciprocal breeding crosses underwent cryoanesthesia for 6 (Cryo6) or 12 (Cryo12) min, respectively, within the first hours (< 12hr) of postnatal life. A group of pups separated from the nest and kept in ambient temperature of 33 °C for 6 min served as a control. Our results revealed that lowering the temperature of pups to ~8 °C (Cryo6) or ~5 °C (Cryo12) did not affect their body weight at pre-weaning stage and in the adulthood. The evaluation of cognitive function in adult mice revealed strong and comparable to controls spatial reference, and context and tone fear memories of neonatally cryoanesthetized mice. Also, the experimental and control groups had comparable brain weight at the end of the study. Our results demonstrate that neonatal cryoanesthesia, lasting up to 12 min, has no adverse effects on the body weight of mice during development, and on their cognition in the adulthood.
Keywords: neonatal cryoanesthesia, mouse, reciprocal breeding, development, body weight, cognition
1. Introduction
Deep hypothermia, also referred to as cryoanesthesia [1], is used to induce anesthesia during surgical manipulations in neonatal rodent species [1–3]. Moderate hypothermia, which significantly reduces circulatory flow, cerebrospinal fluid pressure, and perception of pain [4–6], is also used as an accessory to other anesthesia procedures [7]. In neonatal rats cryoanesthesia abolished perception of pain and significantly decreased c-fos expression in neurons [8]. Lowering body temperature to ~20 °C significantly diminished synaptic transmission in rat pups, while further drop to ~10 °C completely blocked the transmission, an effect comparable to morphine analgesia [1]. Compared to methods that use injectable anesthetic agents [2] or saturated isoflurane gas [9], neonatal cryoanesthesia can be quickly and safely induced by placing pups directly on crushed ice in paper- or aluminum foil-lined tubes or grooves (to avoid freeze skin damage), or submerging them up to their neck in ice water (directly or placing them in a laboratory glove) (see [1] for detailed discussion of these methods). Since altricial newly born mouse and rat pups cannot maintain their body temperature and are functionally poikilothermic, with thermoregulatory ability developing only during the third week of life ([10, 11] cited in [1]), they are tolerant of low temperatures and can recover by re-warming, even from near 0 °C body temperature [12]. Such broad tolerance to low body temperatures of neonatal rodents likely represents an evolutionary adaptation to risk of bouts of hypothermia in natural environment of temperate climatic zones, when the parents leave their litters for periods of up to 4–9 hr during nocturnal foraging [13].
Cryoanesthesia is often used as a common anesthetic procedure for rat pups during neonatal gonadoctomy and other hormonal manipulations [2, 14], or neonatal cortical lesions [15, 16]. Recently cryoanesthesia has been extensively employed during neonatal gene delivery to the brain using viral vectors [17, 18] in mouse models of gene therapy or in the generation of somatic brain transgenic (SBT) mouse models of neurodegenerative diseases [19–24]. In our lab we have been generating somatic brain transgenic mouse models of Alzheimer’s disease like amyloidosis [19, 25] that present a new tool for elucidating mechanisms underlying amyloid β deposition in the brain [26–29]. Since many of these studies that manipulate genes expression in the brain include the evaluation of cognitive function in adult mice [30, 31], we investigated here whether brief neonatal cryoanesthesia by itself can exert long lasting effects on cognition when evaluated in adulthood. Most available evidence suggests that severe hypothermia affects memory formation in mice [32, 33], however, Mrosowsky reported no direct evidence for cold induced memory loss or impairments in learning reversal when hypothermia was initiated shortly after training [34]. These experiments used adult mice, longer bouts of hypothermia, and focused on relatively short-term effects of hypothermia on memory, thus were less directly related to long-term effects of neonatal cryoanesthesia on memory evaluated in adult mice. An interesting set of experiments performed in rats showed unequivocally that neonatal cryoanesthesia lasting for 60 min [35] or 9 min [36] resulted in deficits in the acquisition of the Morris water task when tested in adulthood (at 90 and 60 days postnatally, respectively). Rats and mice show relatively close developmental similarities [37], however, they are further apart with respect to behavioural similarities [38]. Therefore, in the present study we investigated whether neonatal cryoanesthesia might create a potential confounding variable affecting cognitive function in adult SBT mouse models.
Following the method adopted in our lab, which employs ~5-min long cryoanesthesia [26], we tested the effects of 6 (Cryo6) or 12 (Cryo12) min cryoanesthesia, administered to 129FVBF1 mouse pups within ~12 hr of postnatal life, on body weight during development and spatial reference and conditioned fear memories in 4–5-month old mice. Mice that underwent comparable experimental manipulation without neonatal cryoanesthesia served as a control group. Here we report that neonatal, 6- or 12-min long cryoanesthesia administered to 129FVBF1 mouse pups did not compromise their physical development or cognitive function evaluated in the adulthood.
2. Methods
2.1. Mice
FVB/NCrL (FVB) and 129S2/SvPasCrL (129) breeders were purchased from Charles River Laboratories (Wilmington, MA) at the age of 8 (females, ♀) and 9 (males, ♂) weeks. After two weeks of acclimation to the animal colony facility, reciprocal breeding trios (129♂/FVB♀♀, and FVB♂/129♀♀) were established. Apart from weekly handling, which included recording of body weight, and biweekly routine husbandry cage changes by the colony staff, the mice were not disturbed otherwise. Pregnant females, identified by substantial increase in body weight, were separated to cages supplied with nesting material (Nestlets, Code #NES3600, Ancare) and were monitored twice daily (~09:00 and ~16:00 h) for the birth of pups. Breeders and experimental mice were housed in same-sex groups (with the exception of active breeders and pre-weanling pups) of 2 – 4 in ventilated mouse 29 × 18 × 13 cm cages containing 1–2 nestlets, under standard laboratory conditions (12:12h light/dark cycle, lights on at 0600 h, ambient room temperature of 22°C), and water and food available ad libitum. The housing, husbandry and all experimental procedures were approved by the Institutional Animal Care and Use Committee of UF, and were in accordance with AAALAC and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Cryoanesthesia Procedure
Newly born pups underwent cryoanesthesia procedure within 3 to ~12 hr of their postnatal life. Since the biodistribution of the AAV2 pseudotype in the brain following intracerebroventricular injection during generation of SBT models is limited if injected beyond neonatal day 1 (P1) [39], we limited the application of cryoanesthesia to the first 12 hours of postnatal life. Litters were never disturbed immediately after birth, allowing the dam to lick the pups, gather them in the nest, and start nursing. Only half of a litter underwent the procedure at the time, regardless of the litter size, which minimized the disturbance and stress of the mother. The procedure took place in the adjacent to the colony room procedure suite under the ventilated hood with diffuse light. After opening the cage, the dam was encouraged to leave the nest by gentle touch on her back, if she already had not left the nest. The temperature of the nest was recorded by placing type T disc surface thermocouple probe (Cole Palmer, #K-08506-80) into a nest making sure that the surface probe was in the middle of pups huddle. Following, number of pups in the litter was counted, checked for runts, and the presence of milk in their stomach. Half of the pups were removed from the nest, and their weight, as a group, was recorded. The cage with the dam and the rest of pups was moved aside, allowing the dam continue to nurse remaining pups. Pups were cryoanesthetised on ice. An aluminum foil was placed on crushed ice and molded to form a narrow groove, wide enough to accommodate a single pup, thus maximizing its exposure to low temperature. Pups were placed in the groove in a row, preventing their body contact, the start time was recorded, and their surface body temperature was recorded every minute using the thermocouple probe. Since pups were not individually marked until the day of weaning, the body weight and the changes in surface temperature during cryoanesthesia procedure are reported as average per litter. Upon recovery from cryoanesthesia, pups were moved to a cage fitted with water thermopad lined with soft paper towels, and maintained at 33 °C, ~2 °C higher than the average nest temperature for 129 and FVB mothers recorded in our lab. During that time, pups were also warmed a few times in cupped hands, which stimulated their movement and ensured uniform recovery of each pup. Each batch of pups was returned to their home cage as a group when their movements were vigorous, the colour of their body was bright pink, indicating normal blood circulation, and their surface temperature reached ~28 °C. Pups were returned to their home cages within 8 – 10 min after placing them in a warming cage, and in total after ~18 ± 2 minutes from their initial removal from the home cage. At the same time, the second half of a litter underwent cryoanesthesia. Since all breeders were routinely handled after arriving to our animal facility, the dams were familiar with experimental manipulation and readily engaged in active nursing after the pups were returned to the nest.
2.3. Experimental design
Twelve litters, born to nulliparous mothers, comprising in total of 102 pups, were allocated to the experiment. Litters were pseudo randomly allocated to 3 experimental groups: two cryoanesthesia conditions and one handling-only control condition. Since the usual time of ice-induced cryoanesthesia applied to newly born mouse pups in our lab is ~5 min [26], we chose 6 and 12 min of cryoanesthesia in the study, with 12 min duration doubling the standard 6 min-long neonatal hypothermia. The pups in control conditions were removed from the dam for 6 min and kept in a holding cage with ambient temperature of 30 °C. Each group contained four litters, balanced across reciprocal breeding conditions, with sample size of 33 (19♂; 14♀) in the control, and 35 (18♀; 17♂) and 34 (20♀; 14♂) pups in 6- and 12-min cryoanesthesia groups, respectively. Pups were weaned at 21+1 day, and housed in same sex groups throughout the whole study. The body weight of pre-weanling pups was recorded on postnatal days 1, 7, 14, and 21, and is reported as average per litter. The body weight of adult mice was recoded at 28, 35, 50, 65, 85, and 95 days of life of the mice. During handling we used hand-cupping method. In this method mice were scooped up, allowed to stay on the experimenter’s cupped hand and were transferred to the holding cage or weighing container, without direct physical restraint [40]. Physical restraint associated with picking the mice up and restraining them by their tail increases anxiety responses in mice [40], which might affect their behaviour in subsequent cognitive tests. The spatial reference memory and cued response learning in Morris water maze (MWM) was followed by the evaluation of conditioned fear context and tone memories between 4 and 5.5 months of mice age. After the completion of the behavioural tests, the mice were sacrificed and their brain extracted and weighted.
2.4. Morris water maze (MWM) test
Mice were trained in the water maze test, 140 cm in diameter, as described [41]. The reference memory version of the test was run for 5 consecutive days with 4, 60-second, training trials per day. A mouse was released into water at semi-randomly chosen cardinal compass points (N, E, S, W [42]) and its swim path was recorded by image-tracking software (HVS Image). Dark, geometrical shapes (2–3 per wall), and two partitions separated experimenter, recording equipment, and a small cage rack from the testing area. The distance from the edge of the pool to the walls of the room or partitions was between 1 to 1.3 m. An escape platform, submerged 0.5 cm under water surface, was positioned in the center of the same NW quadrant of the pool (target quadrant, TQ) throughout training. The memory for platform location was evaluated in a probe trial (with escape platform removed) 24 h after the last day of training. During a visible platform test, run for 3 days with 4 trails per day during a week preceding spatial reference memory training, the platform was marked by a visible black cue and a curtain surrounded the pool.
2.5. Fear Conditioning test
The test was performed as previously described [43]. A 4-chamber conditioning apparatus (Coulbourn Inst.) was located in a dedicated room. A tone (80 dB, pulse (6 clicks per second), 30-s duration) was used as conditioned stimulus (CS) and a 0.45 mA, 2s foot shock, which co-terminated with a tone, as unconditioned stimulus (US). Mouse activity was recorded by FreezeFrame (Actimetrics) program, and freezing behaviour, defined as cessation of all movements other than respiratory activity [44], indicating fear memory of the association between CS and US was analyzed off-line. Each mouse received 2 CS-US pairings separated by a 60-s interval during one 5-min training session. After a day of recovery (D2), the contextual fear memory of mice was evaluated in the context of the original training chamber (D3), and a day later (D4), the fear memory elicited by tone only was evaluated in the modified context of the chamber (tone fear memory). Both tests were carried out in an extinction mode with no shock administered.
2.6. Data analysis
General linear model of factorial ANOVA (Statistical Package for Social Sciences, SPSS v.20, Inc. Chicago), with cryoanesthesia conditions, genetic background of a dam (129 or FVB), sex, and brain weight as between subject, and body weight, or learning and memory scores as within subject factors, was used to analyze the data. Simple effects were evaluated using one-way ANOVA. When necessary, degrees of freedom were adjusted by Greenhouse-Geisser epsilon correction for the heterogeneity of variance. Bonferroni adjustment of α level (MODLSD Bonferroni t-tests, SPSS v21) was applied in multiple planned comparisons. Comparisons between two independent groups were done using Student t-test. Comparisons against chance performance were done using Kolmogorov-Smirnov one sample t-test. The critical α level was set to 0.05, and all values in the text and figures represent means ± SEM. Due to limitation of space only significant results pertaining to the main experimental factors and interactions are reported.
3. Results
3.1. Litter size and nest quality
Based on 6 litters in each reciprocal breeding, the average litter size of FVB dams tended to be larger, though not significantly, than the litter size of 129 dams (9.3 ± .42 and 7.7 ± 0.71, respectively, t(10) = −2.0, p = 0.07), with the median of 9 and the range 8 to 11 for FVB dams, and 7 and the range of 6 to 11 for 129 dams. No runts were found in any of the experimental and control litters. Although not quantified, routine observations indicated that the nests of 129 dams were larger, with all nestlets shredded and shaped into closed ball with pups and the mother inside. In contrast, the nests of FVB dams were more open, with larger pieces of nestlets at the base and the pups almost always visible in the nest. The difference in the nest quality was reflected in the temperature of the nest, which was significantly higher in nests of 129 dams than in nests of FVB dams (31.3 °C ± 0.4 and 29.9 ± 0.5, for 129 and FVB respectively, t(12) = 2.4, p < 0.05). The difference in the nest temperatures did not affect the average weight of a pup in a litter recorded at the time of the cryoanesthesia procedure (1.3g ± 0.02 and 1.3g ± 0.03, for 129 and FVB litters, respectively, t(10) = 0.4, p = 0.7).
3.2. Change in body temperature during cryoanesthesia
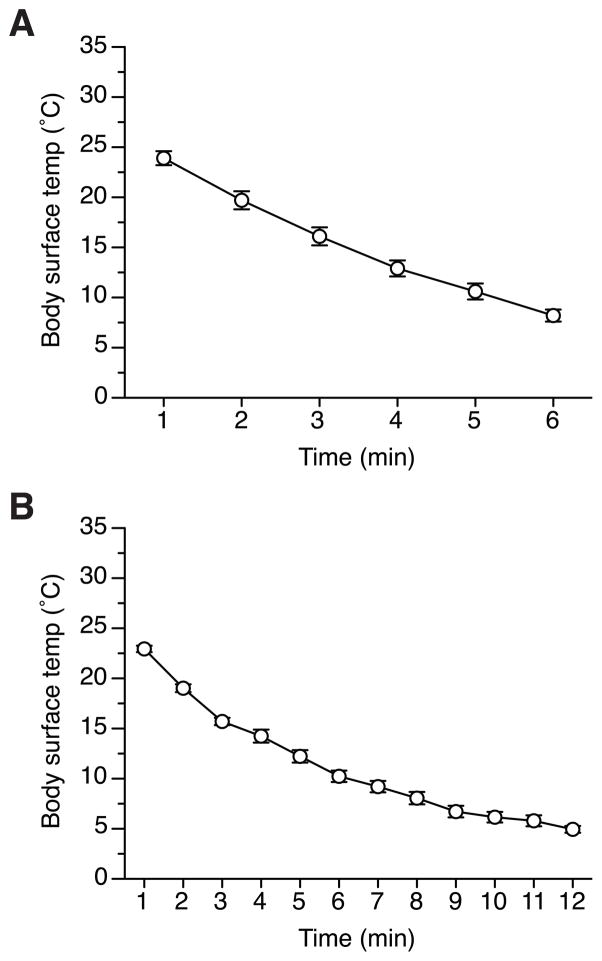
The changes in the body surface temperature of pups during 6- or 12-min cryoanesthesia are presented in Figure 1. Since there was no significant maternal effects in each conditions (F(1,5) = 2.1, p = 0.21, and F(1,6) = 1.1, p = 0.34, for Cryo6- and Cryo12 groups, respectively), or significant interactions involving dam’s strain, the results were pooled across reciprocal breeding within each condition for the presentation and further analyses. Overall, the decrease in the body surface temperature was significant during both 6-min (F(5,30) = 491.6, p < 0.001, Fig. 1A) and 12-min (F(11,77) = 200.7, p < 0.001, Fig. 1B) cryoansthesia. After 6 min of cryoanesthesia the body temperature decreased to 8–10 °C, while at the end of 12 min, it dropped to ~5 °C. At ~10 °C no body movements of pups were observed. There was no difference in changes in body temperature between the first and the second batch of pups undergoing cryoanesthesia in each litter (data not shown).
Figure 1.
The change in the body surface temperature (averaged per litter) of 3 to 10-hr-old 129FVBF1 pups during (A) 6 min and (B) 12 min cryoanesthesia procedure. Vertical bars represent SEM.
3.3. Body weight
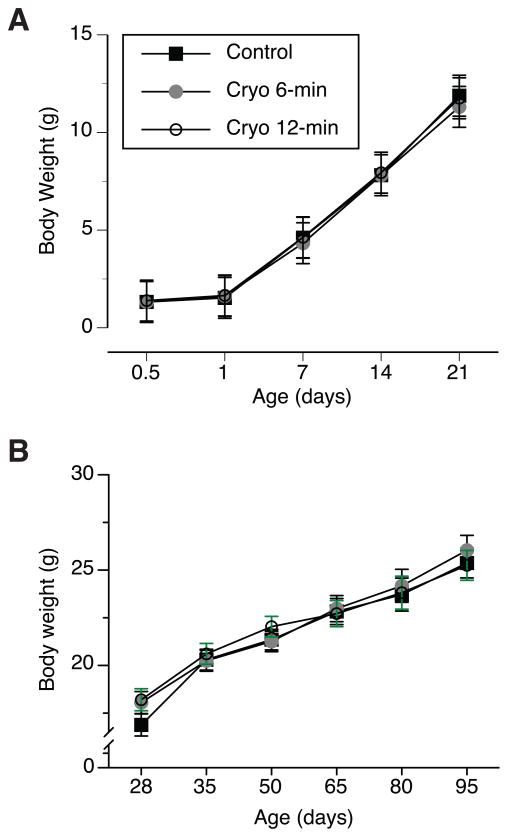
The body weight of pups significantly increased within the first 21 days of their life (F(3,18) = 1400.5, p < 0.001, Fig 2A), with no significant main effects of treatment, dam strain, or interactions between the experimental factors. The body weight continued to increase after weaning within the studied age, between 28 and 95 days (F(5,30) = 400.5, p < 0.001, Fig 2B). The analysis also revealed a significant effect of maternal strain on the change in the body weight of adult mice (F(1,6) = 7.9, p < 0.05). The 129FVBF1 mice born to FVB mothers were on average heavier (23.1g ± 0.52) than the adult progeny of 129 dams (21.0g ± 0.05). Since there were more males than females in the cohort of mice born to FVB mothers than in the cohort of 129 mothers (29♂; 27♀ and 16♂; 30♀, for FVB and 129 dams, respectively), we included sex and maternal strain background as factors in the analysis of the body weight of adult mice. The analysis revealed significant increase in the body weight (F(2,196) = 233.0, p < 0.001), sex effect (F(1,98) = 340.9, p < 0.001), with males being heavier than females, but also confirmed significant effect of maternal genetic background (F(1,98) = 24.2, p < 0.001). The comparison of the body weight of mice between reciprocal breeding within each sex revealed that both males and females born to FVB mothers were significantly heavier from their counterparts born to 129 mothers. The analysis of the body weight of mice at the end of the study revealed no significant effects of neonatal crypanesthesia (30.8g ± 0.8; 31.3g ± 0.8; 30.6g ± 0.6 for the control, Cryo6 and Cryo12 groups, respectively), but significant effects of sex and maternal genetic background (F(1,90) = 160.9, p < 0.001, and F(1,90) = 11.9, p = 0.001, respectively). In summary, 6- and 12- min cryoanesthesia did not affect the physical development of mice, evaluated by the changes in body weight at pre- and post-weaning age; however, the body weight of the adult mice was significantly affected by sex and maternal strain background.
Figure 2.
Body weight (mean ± SEM) of pups that underwent 6- and 12-min long neonatal cryoanesthesia (Cryo 6-min, and Cryo 12-min, respectively), and control pups, which were only removed from the nest and handled, during (A) pre-weaning and (B) post-weaning and stage of development.
3.4. Brain weight
The post mortem analysis of the brain weight revealed no differences between control and Cryo6, and Cryo12 groups (0.495g ± 0.004; 0.5g ± 0.004; 0.497g ± 0.004, respectively), but significant sex differences (F(1,90) = 4.8, p < 0.05), with females having larger brains than males (0.501g ± 0.003 and 0.492g ± 0.003, respectively). No other factors or interactions were significant.
3.5. Morris water maze test
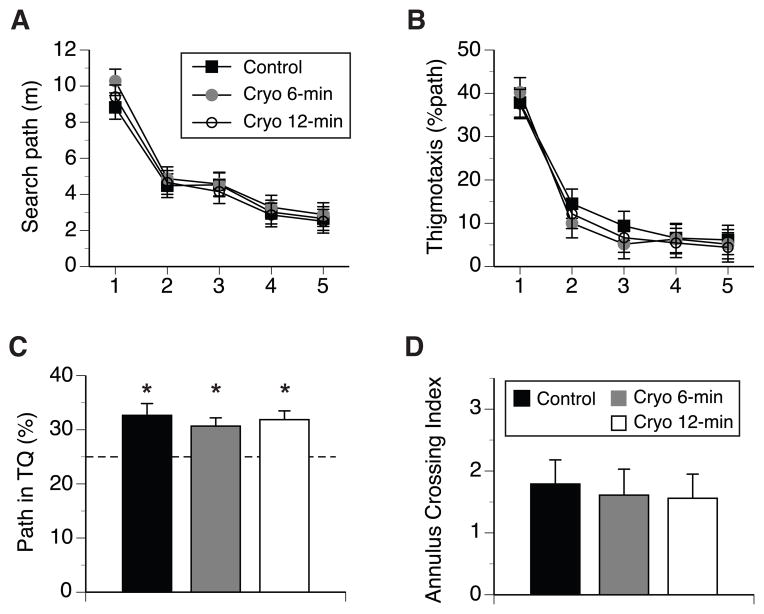
Mice in Cryo6, Cryo12 and control groups did not differ in their swim path length and swim speed to the platform marked by a black cue, with no other factors and interactions significant at α = 0.05 (data not shown). Learning acquisition phase. All mice significantly decreased their swim paths while searching for the submerged escape platform during 5-day training (F(3,256) = 132.1, p < 0.001, Greenhouse-Geisser epsilon correction of df, Fig. 3A). There was a significant main effect of treatment (F(2,90) = 3.3, p < 0.05), with other factors and interactions not significant. Mice in Cry6 group tended to have longer search paths than the mice in the control and Cry12 groups (5.2m ± 0.14 versus 4.8m ±0.18 and 4.7m ± 0.14, respectively), the differences only bordered significance level (p = 0.052 for control vs Cryo6, and p = 0.059 for Cryo6 vs Cryo12 groups, MODLSD Bonferroni t-tests). The inspection of Fig. 3A indicated that Cryo6 mice showed slightly longer search paths during the first day of training, however the analysis of path length for that day did not reveal a significant treatment effect (F(2,101) = 2.1, p = 0.12). Excluding day 1 from the analysis revealed no differences due to treatment in search paths (F(2,90) = 1.5, p = 0.24), suggesting that the effect of treatment on the length of search paths observed in the global analysis was weak and caused by the variability in paths lengths during day 1 of training. Since it has been demonstrated that wall hugging or thigmotaxic swim behaviour significantly affects the efficiency of search in the water maze [41, 45], we investigated whether cryoanesthesia affected thigmotaxic behavior during WM training in the present study. All mice spent ~40% of their search path swimming along the wall of the pool during the first day, which significantly decreased as training progressed (F(3,289) = 504.8, p < 0.001, Fig. 3B). None of the other main factors were significant, but there was a significant days by treatment interaction (F(6,289) = 2.6, p < 0.02). Post-hoc analysis revealed that control mice showed significantly longer thigmotaxic swims compared to Cryo6, but not Cryo12 groups on days 2 and 3 (p < 0.03 and p < 0.01, respectively, MODLSD Bonferroni t-tests). Cryoanesthesia did not affect the swim speed or the rate of floating of the mice, but there was a significant maternal effect on swim speed (F(1,90) = 5.3, p < 0.05), and mice born to 129 dams swam significantly faster than the mice of FVB mothers (0.24m/s ± 0.003 and 0.23m/s ± 0.003, respectively). The analysis revealed no significant main effect of sex or significant interactions involving sex. Spatial reference memory. All mice showed a positive bias in the search for the location of the escape platform during the probe trial administered 24hr (Day 6) following the 5-day training. Cryoanesthesia did not affect the spatial memory of the mice, and none of other factors or interactions was significant. The proportion of search paths spent by mice in the TQ was significantly higher than chance in each treatment group (t(32) = 3.5, t(34) = 3.7, and t(33) = 4.2, all ps < 0.001, for the control, Cryo6, and Cryo12 mice; Kolmogorov-Smirnov one-sample t-test, against 25% chance test value, Fig. 3C). Similarly, an annulus crossing memory index, which expresses the swims over the platform annulus in TQ, adjusting for crosses of platform annuli in other quadrants of the pool [42], was positive and significantly higher from zero chance level (crossing of all 4 annuli with comparable frequency) within each treatment group (t(32) = 4.6, t(34) = 3.8, and t(33) = 4.0, all ps < 0.001, for the control, Cryo6, and Cryo12 mice; Kolmogorov-Smirnov one-sample t-test, against 0 chance test value, Fig. 3D). The swim speed between treatment groups during the probe trial was comparable, with females swimming faster than males (F(1,90) = 7.3, p < 0.01; 0.29m/s ± 0.002 and 0.27m/s ± 0.003, respectively), and mice of 129 dams swimming faster than their counterparts born to FVB mothers (F(1,90) = 7.8, p < 0.01; 0.29m/s ± 0.003 and 0.27m/s ± 0.002, respectively).
Figure 3.
Spatial reference memory was evaluated in 4-month-old neonatally cryoanesthetized and control mice. (A) Search path for the location of the escape platform during training stage. (B) Thigmotaxic, wall-hugging swim within a distance of 10 cm from the wall. (C) Spatial memory of platform location was evaluated during 60s probe trial with the escape platform removed. Percent of path spent searching a quadrant containing the escape platform during training (target quadrant, TQ). (D) Annulus crossing memory index (#crosses of platform site minus an average of crosses of virtual platform locations in the other three quadrants), represents memory index that controls for alternative search strategies relying on equidistant swims along the wall like chaining [42]. Vertical bars represent SEM. * p < 0.001 indicates the comparison of each group against 25% chance level performance.
3.6. Conditioned Fear Memory
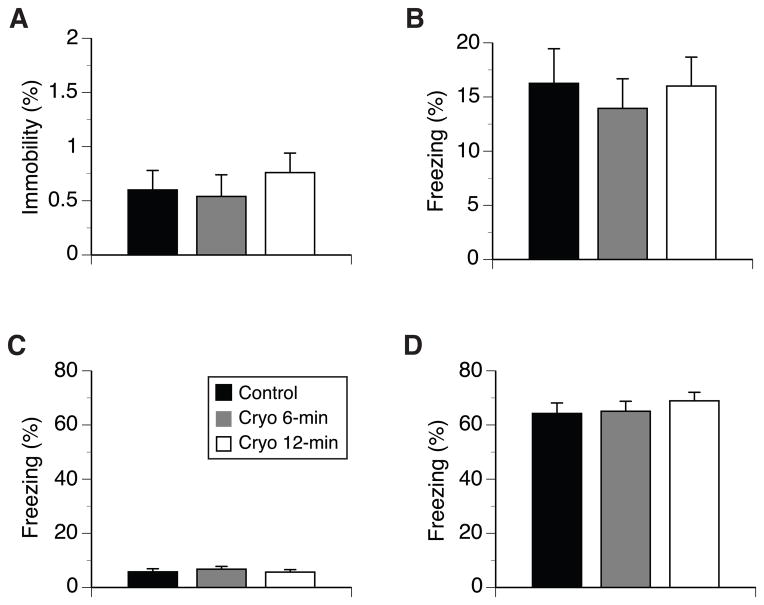
All mice actively explored the training chamber during the 2-min period preceding the fist CS-US pairing, spending about 1.5% of time or less on pauses (Fig. 4A). Overall, males paused longer than females (F(1,90) = 16.4, p <0.001; 1.1% ± 0.2 and 0.21% ± 0.1, respectively), otherwise there were no differences in exploration with respect of other experimental factors. Also, cryoanesthesia did not affect the immediate response to foot shock (averaged over the two post CS-US periods) of the mice (12.9% ±3.6, 12.9% ± 2.7, and 11.4% ± 2.8 for control, Cryo6, and Cryo12 groups, respectively). The fear memory for the context of the training chamber is shown in Fig. 4B. There were no differences between the groups in freezing during the context test, and none of the other factors or interactions were significant. Since in delay FC paradigm, the contextual cues are in the background in relations to tone CS, freezing in the context test was lower than freezing observed in the tone test (below). The response of the mice to the altered context of the training chamber and to tone CS during its presentation is shown in Fig. 4C and D, respectively. Mice of all experimental groups spent low, about 6%, and comparable percent of time freezing during pre-CS phase (Fig. 4C), but they showed strong and significantly higher freezing during the tone presentation than during pre-CS phase (F(1,90) = 897.4, p < 0.001, Fig. 4D). None of the experimental factors differentiated the freezing of mice during pre-CS phase; however, during tone presentation females froze longer than males (F(1,90) = 18.7, p < 0.001; 73.3% ± 2.6 and 54.7% ± 3.4, respectively), and mice of FVB mothers froze longer than mice of 129 mothers (F(1,90) = 5.8, p < 0.02; 69.2% ± 2.6 and 58.8% ± 3.4, respectively).
Figure 4.
Behaviour of 5-month-old neonatally cryoanesthetized and control mice in fear conditioning test. (A) Pauses during the exploration of the novel environment of the training FC chamber during the first 120 sec of the test. (B) Mean percent of freezing exhibited by mice during context test carried out on day 3 after training. (C) Freezing behavior of mice during 3-min exploration of the modified training chamber, which preceded (D) 3-min phase of the test during which a tone (CS) was presented.
4. Discussion
The present study investigated the effects of neonatal deep hypothermia (cryoanesthesia) administered to mouse pups within the first 12 hr of postnatal life on their adult cognitive function. Cyoanesthesia is the method of choice for reliable and safe anesthesia of neonatal rodents [46], however, evidence from rat studies suggests that the procedure might have some delayed detrimental effects on brain morphology and behavior [35, 36, 47]. Since neonatal cryoanesthesia is routinely used during recently developed technologies of somatic gene expression in the mouse brain [18] to study the development of neural systems or generation of somatic brain transgenic models of neurodegeneration [19, 26], it is important to identify whether similar long-term detrimental effects of the procedure are observed in a mouse.
Our results clearly demonstrate that the exposure of newly born 129FVBF1 mouse pups to 6 or 12 min of deep hypothermia resulted in the drop of body temperature to ~10 °C or ~5 °C respectively, which did not affect the cognitive function and the body and brain weights of mice in adulthood. Similarly to published studies on rats [35, 36], we evaluated the mice in the spatial reference memory version of the Morris water maze test, but we also extended our cognitive evaluation to Pavlovian associative learning of conditioned fear. Our results showed that neither learning acquisition nor spatial reference memory were compromised in 4-month old adult mice that experienced cryoanesthesia lasting up to 12 min within the first 12 hours of postnatal life. Locomotor behaviour, like active swim, rate of floating, as well as thigmotaxis, with the latter implicated in the plasticity of behavioral search strategies in water maze [48, 49] or increased fearfulness [50], were also not compromised in neonatally cryoanesthetized. Supporting these results, experimentally cryoanesthetized and control mice also developed strong contextual and tone conditioned fear memories. Mice in all groups did not differ in their initial exploration of the novel training chamber, and exhibited only few pauses or immobility bouts during their initial exploration of the training environment. They also showed comparable and active exploration of the modified chamber in the 3-min period preceding the delivery of tone CS, and showed a strong freezing response to tone CS. Since the performance in the spatial reference memory of WM and FC paradigms is not correlated [51, 52], with the former implicating hippocampus-dependent [53, 54] and the latter both hippocampus- and amygdala-dependent [55] memory systems, the lack of cryoanesthesia effects in both paradigms provides strong validation of our conclusion that neonatal deep hypothermia does not have any significant delayed detrimental effects on cognitive function in mice.
Neonatal cryoanesthesia also did not significantly affect the physical development of mice in terms of pre- and post-weaning changes in the body weight. Mice in all groups showed comparable increase in their body weight at the pre-weaning age, suggesting comparable maternal care and pups’ growth across experimental conditions. Also, the experimental end-point analysis of the brain weight also revealed no differences between neonatally cryoanesthetized and control mice. The average brain weights of 0.500g ± 0.004; 0.497g ± 0.004 for Cryo6 and Cryo12 mice, respectively, were comparable to the brain weight of the control mice (0.495g ± 0.004), and were within a range of the brain weights reported in the literature [56, 57]. Although we cannot exclude subtle changes in the dendritic arborization reported by Kolb and Cioe [36], the lack of differences in several behavioural measures and in a general physical development suggests that such differences, if exist, might have a minimal effect on adult cognitive function in a mouse, and in most cases might not be detected in cognitive tests. The reported effects of neonatal cryoanesthesia on the performance in water maze and brain morphology of adult Long-Evans rats [35, 36, 47] are interesting and should be investigated further in both species to elucidate possible differences between the species, the timing and duration of the procedure, and the possible modifications of the effects by environmental enrichments or socialization. The reported significant decreases in the volume of visual cortex [47], in the weight of hippocampus [35], as well as in cortical thickness and dendritic arborization [36] are likely not benign and will be reflected in compromised behavioural and specifically, cognitive propensities of the animals. These observed effects might be idiosyncratic for rats or even limited only to Long-Evans strain, or they can be the result of relatively impoverished housing conditions in animals facilities or unusually severe, non-biological hypothermia conditions employed in experimental studies. Since both neonatal rats and mice are poikilothermic and unable to maintain their body temperature, they should well tolerate periodic drops in their body temperature when left in the nest by their foraging parents [13]. The of lack of adverse effect of severe (~5 °C) but brief neonatal hypothermia on cognitive function in adult mice demonstrated in our study seems to be in agreement with the studies investigating this issue in white-footed mouse (Peromyscus leucopus), which demonstrated no adverse effects of longer, up to 2.5 hr, neonatal hypothermia (2 – 4 °C), administered between 4 – 10 postnatal days on adult cognitive function evaluated in conditioned taste aversion and water maze tests [58].
An interesting, but somewhat unexpected, result of our study indicates that maternal strain exerts a long-term effect on body weight of 129FVBF1 offspring. While there is extensive evidence of maternal genotype effect on pups body weight [59, 60], retrieval [61, 62], postnatal care and pups ultrasonic vocalization [62, 63], as well as adult behaviour, including response to stress and anxiety levels [59, 64], most of these results used cross-fostering pups between genetically different mothers. In our case, we observed the differences in the body weight of adult, but not pre-weanling, mice between reciprocal breeding paradigms of FVB/NCrL and 129S2/SvPasCrL mice. Our results also demonstrated that with the exception of motor differences in swim speed, where the mice born to 129 mothers were swimming faster than their counterparts born to FVB dams, we did not observe any maternal effect on cognitive function or brain weight in adult 129FVBF1 progeny. Similar maternal effects on the body weights in reciprocal crosses of other strains were reported previously [56]. In conclusion, the employment of reciprocal crosses in our studies revealed maternal effects on body weight in adult mice, however, the genotype of the mother did not affect the response to cryoanesthesia, adult cognitive function, or brain weight of F1 progeny.
A practical conclusion of our study that is directly applicable to SBT research is that brief, up to 12 min, neonatal cryoanesthesia administered within the first 12 hours of postnatal life presents safe and efficient anesthesia method allowing surgical manipulation of mouse pups, free from long-term deleterious developmental and cognitive consequences. Additionally, we did not observe any mortality of experimental mice or rejection of the pups by their mothers caused by the procedure. We also confirmed in a mouse that deep hypothermia lasting for ~5 min results in the drop of pups’ body temperature to ~10 °C, with no observable body movements, that is in agreement with published results of deep anesthesia in rat pups [1]. The extension of neonatal cryoanesthesia to 12 min also did not affect the development or cognitive function of the adult mice, providing evidence for broader safe experimental window that might be required during SBT surgical manipulations. Staging the procedure in two batches of pups minimizes the risk of increased agitation of the mothers caused by pups’ removal, and reduces possible rejection of the pups after the procedure. In our experiments, the mothers were actively nursing the pups within 3 to 5 min after returning them to the nest. The frequent handling of the breeders is important in this respect and has been recommended as the way to minimize mortality of pups [3]. In our study, all breeders were well habituated to routine handling through weekly handling. However, the expecting mothers were not disturbed within at least 4 – 6 days before the estimated day of delivery of a litter, a procedure also observed in other labs [30]. Daily checks for the presence of new litter were successfully performed without opening the cage and disturbance of the expecting females.
In summary, cryoanesthesia within the first 12 hr of postnatal life does not affect the immediate physical development of mouse pups and later cognitive function in adulthood. Therefore, brief, up to 12 min in duration, neonatal cryoanesthesia should not likely present confounding deleterious effects in experimental paradigms focusing on behavioural endophenotypes evaluated later in life.
Highlights.
We studied the effects of deep hypothermia (cryoanesthesia) on cognition in mice.
Cryoanesthesia within 10 hrs after birth did not affect the body weight of pups.
The body weight of neonatally cryoanesthetized adult mice normal.
Up to 12 min cryoanesthesia did not affect spatial or fear memories in adult mice.
Neonatal cryoanesthesia also did not affect the brain weight of adult mice.
Acknowledgments
We would like to thank Ms. Amelia March and Mr. Ryan Keith for help during data collection, Dr. Mary Reinhart for helpful comments, and the staff of Animal Care Services of UF for husbandry care and help during the project. This work was supported by NIA R21AG035054 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher Janus, Email: cjanus@ufl.edu.
Todd Golde, Email: tgolde@ufl.edu.
References
- 1.Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiology & behavior. 1986;38:887–90. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- 2.Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Laboratory Animal Science. 1997;47:386–95. [PubMed] [Google Scholar]
- 3.Cunningham MG, McKay RD. A hypothermic miniaturized stereotaxic instrument for surgery in newborn rats. Journal of neuroscience methods. 1993;47:105–14. doi: 10.1016/0165-0270(93)90026-n. [DOI] [PubMed] [Google Scholar]
- 4.Yoon WY, Chung SP, Lee HS, Park YS. Analgesic pretreatment for antibiotic skin test: vapocoolant spray vs ice cube. Am J Emerg Med. 2008;26:59–61. doi: 10.1016/j.ajem.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Hughes PS. Cold air anesthesia in dermasurgery: comparative study. Dermatol Surg. 2006;32:165–6. doi: 10.1111/1524-4725.2006.32027. [DOI] [PubMed] [Google Scholar]
- 6.Ring ME. The history of local anesthesia. J Calif Dent Assoc. 2007;35:275–82. [PubMed] [Google Scholar]
- 7.Serra-Guillen C, Hueso L, Nagore E, Vila M, Llombart B, Requena Caballero C, et al. Comparative study between cold air analgesia and supraorbital and supratrochlear nerve block for the management of pain during photodynamic therapy for actinic keratoses of the frontotemporal zone. The British journal of dermatology. 2009;161:353–6. doi: 10.1111/j.1365-2133.2009.09184.x. [DOI] [PubMed] [Google Scholar]
- 8.Yi DK, Barr GA. The suppression of formalin-induced fos expression by different anesthetic agents in the infant rat. Dev Psychobiol. 1996;29:497–506. doi: 10.1002/(SICI)1098-2302(199609)29:6<497::AID-DEV2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Drobac E, Durand E, Laudenbach V, Mantz J, Gallego J. A simple method for short-term controlled anesthesia in newborn mice. Physiology & behavior. 2004;82:279–83. doi: 10.1016/j.physbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald LR, Bacon RL. Metabolic Rate and Thermal Regulation in Neonatal Mice. Anatomical Record. 1952;113:532. [Google Scholar]
- 11.Lagerspetz KYH. Development of thermal regulationin laboratory mice. Helg Wiss Meeresunters. 1066;14:559–71. [Google Scholar]
- 12.Adolf EF. How do infant mammals tolerate deep hypothermia? In: Hardy JD, editor. Temperature, its measurment and control in science and industry: Part 3 Biology and medicine. New York: Reinhold; 1963. pp. 511–5. [Google Scholar]
- 13.Hill RW. The amount of maternal care in Peromyscus leucopus and its thermal significance for the young. Journal of mammalogy. 1972;53:774–90. [PubMed] [Google Scholar]
- 14.Booth JE. Sexual behaviour of neonatally castrated rats injected during infancy with oestrogen and dihydrotestosterone. J Endocrinol. 1977;72:135–41. doi: 10.1677/joe.0.0720135. [DOI] [PubMed] [Google Scholar]
- 15.Kolb B. Recovery from early cortical damage in rats. I. Differential behavioral and anatomical effects of frontal lesions at different ages of neural maturation. Behavioural brain research. 1987;25:205–20. doi: 10.1016/0166-4328(87)90069-6. [DOI] [PubMed] [Google Scholar]
- 16.Kolb B, Holmes C, Whishaw IQ. Recovery from early cortical lesions in rats. III. Neonatal removal of posterior parietal cortex has greater behavioral and anatomical effects than similar removals in adulthood. Behavioural brain research. 1987;26:119–37. doi: 10.1016/0166-4328(87)90161-6. [DOI] [PubMed] [Google Scholar]
- 17.Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–40. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passini MA, Wolfe JH. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol. 2001;75:12382–92. doi: 10.1128/JVI.75.24.12382-12392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–8. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–10. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 21.Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–8. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadalla KK, Bailey ME, Spike RC, Ross PD, Woodard KT, Kalburgi SN, et al. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol Ther. 2013;21:18–30. doi: 10.1038/mt.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein RL, Dayton RD, Tatom JB, Diaczynsky CG, Salvatore MF. Tau expression levels from various adeno-associated virus vector serotypes produce graded neurodegenerative disease states. Eur J Neurosci. 2008;27:1615–25. doi: 10.1111/j.1460-9568.2008.06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCown TJ, Xiao X, Li J, Breese GR, Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain research. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 25.Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, et al. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. The Journal of clinical investigation. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Miller VM, Levites Y, West KJ, Zwizinski CW, Moore BD, et al. BRI2 (ITM2b) inhibits Abeta deposition in vivo. J Neurosci. 2008;28:6030–6. doi: 10.1523/JNEUROSCI.0891-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–59. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarty P, Tianbai L, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol Neurodegener. 2012;7:36. doi: 10.1186/1750-1326-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarty P, Ceballos-Diaz C, Beccard A, Janus C, Dickson D, Golde TE, et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010;184:5333–43. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naert A, Gantois I, Laeremans A, Vreysen S, Van den Bergh G, Arckens L, et al. Behavioural alterations relevant to developmental brain disorders in mice with neonatally induced ventral hippocampal lesions. Brain Res Bull. 2013;94:71–81. doi: 10.1016/j.brainresbull.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen B, Levites Y, Chakrabarty P, Rosario A, March A, Felsenstein KM, et al. Are shorter Aβ peptides protective? Society for Neuroscience; New Orleans: 2012. [Google Scholar]
- 32.Beitel RE, Porter PB. Deficits in retention and impairments in learning induced by severe hypothermia in mice. J Comp Physiol Psychol. 1968;66:53–9. doi: 10.1037/h0020593. [DOI] [PubMed] [Google Scholar]
- 33.Boyd SC, Caul WF. Evidence of state dependent learning of brightness discrimination in hypothermic mice. Physiology & behavior. 1979;23:147–53. doi: 10.1016/0031-9384(79)90135-5. [DOI] [PubMed] [Google Scholar]
- 34.Mrosovsky N. Retention and Reversal of Conditioned Avoidance Following Severe Hypothermia. J Comp Physiol Psychol. 1963;56:811–3. doi: 10.1037/h0039412. [DOI] [PubMed] [Google Scholar]
- 35.Nunez JL, Koss WA, Juraska JM. Hippocampal anatomy and water maze performance are affected by neonatal cryoanesthesia in rats of both sexes. Horm Behav. 2000;37:169–78. doi: 10.1006/hbeh.2000.1572. [DOI] [PubMed] [Google Scholar]
- 36.Kolb B, Cioe J. Cryoanethesia on postnatal day 1, but not day 10, affects adult behavior and cortical morphology in rats. Brain Res Dev Brain Res. 2001;130:9–14. doi: 10.1016/s0165-3806(01)00182-1. [DOI] [PubMed] [Google Scholar]
- 37.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 38.Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiology & behavior. 1995;58:687–93. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, et al. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One. 2013;8:e67680. doi: 10.1371/journal.pone.0067680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010:1–2. [Google Scholar]
- 41.Yue M, Hanna A, Wilson J, Roder H, Janus C. Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiology of aging. 2011;32:590–603. doi: 10.1016/j.neurobiolaging.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learning & memory (Cold Spring Harbor, NY. 2004;11:337–46. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna A, Iremonger K, Das P, Dickson D, Golde T, Janus C. Age-related increase in amyloid plaque burden is associated with impairment in conditioned fear memory in CRND8 mouse model of amyloidosis. Alzheimers Res Ther. 2012;4:21. doi: 10.1186/alzrt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 45.Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627–34. [PubMed] [Google Scholar]
- 46.Flecknell PA. Laboratory Aminal Anesthesia. San Diego: Academic Press; 1987. [Google Scholar]
- 47.Nunez JL, Kim BY, Juraska JM. Neonatal cryoanesthesia affects the morphology of the visual cortex in the adult rat. Brain Res Dev Brain Res. 1998;111:89–98. doi: 10.1016/s0165-3806(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 48.Tremml P, Lipp HP, Muller U, Wolfer DP. Enriched early experiences of mice underexpressing the beta-amyloid precursor protein restore spatial learning capabilities but not normal openfield behavior of adult animals. Genes Brain Behav. 2002;1:230–41. doi: 10.1034/j.1601-183x.2002.10405.x. [DOI] [PubMed] [Google Scholar]
- 49.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol Sci. 1998;13:118–23. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- 50.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 51.Ferbinteanu J, Holsinger RM, McDonald RJ. Lesions of the medial or lateral perforant path have different effects on hippocampal contributions to place learning and on fear conditioning to context. Behavioural brain research. 1999;101:65–84. doi: 10.1016/s0166-4328(98)00144-2. [DOI] [PubMed] [Google Scholar]
- 52.Wrenn CC, Marriott LK, Kinney JW, Holmes A, Wenk GL, Crawley JN. Galanin peptide levels in hippocampus and cortex of galanin-overexpressing transgenic mice evaluated for cognitive performance. Neuropeptides. 2002;36:413–26. doi: 10.1016/s0143-4179(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 53.Morris RG. Further studies of the role of hippocampal synaptic plasticity in spatial learning: is hippocampal LTP a mechanism for automatically recording attended experience? J Physiol Paris. 1996;90:333–4. doi: 10.1016/s0928-4257(97)87912-0. [DOI] [PubMed] [Google Scholar]
- 54.Morris RG, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci. 1997;352:1489–503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahlsten D. Maternal effects on mouse brain weight. Brain research. 1983;285:215–21. doi: 10.1016/0165-3806(83)90054-8. [DOI] [PubMed] [Google Scholar]
- 57.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16364–9. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill RW, Eshuis RK. Learning in mature mice (Peromyscus leucopus) subjected to deep hypothermia as neonates. J Comp Psychol. 1988;102:44–8. doi: 10.1037/0735-7036.102.1.44. [DOI] [PubMed] [Google Scholar]
- 59.Blaney CE, Gunn RK, Stover KR, Brown RE. Maternal genotype influences behavioral development of 3xTg-AD mouse pups. Behavioural brain research. 2013;252C:40–8. doi: 10.1016/j.bbr.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 60.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–78. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlier M, Roubertoux P, Cohen-Salmon C. Differences in patterns of pup care in Mus musculus domesticus l-Comparisons between eleven inbred strains. Behav Neural Biol. 1982;35:205–10. doi: 10.1016/s0163-1047(82)91213-4. [DOI] [PubMed] [Google Scholar]
- 62.Carlier M, Roubertoux P, Cohen-Salmon C. Early development in mice: I. Genotype and post-natal maternal effects. Physiology & behavior. 1983;30:837–44. doi: 10.1016/0031-9384(83)90245-7. [DOI] [PubMed] [Google Scholar]
- 63.Cohen-Salmon C, Carlier M, Roubertoux P, Jouhaneau J, Semal C, Paillette M. Differences in patterns of pup care in mice. V--Pup ultrasonic emissions and pup care behavior. Physiology & behavior. 1985;35:167–74. doi: 10.1016/0031-9384(85)90331-2. [DOI] [PubMed] [Google Scholar]
- 64.Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, et al. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev Psychobiol. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]