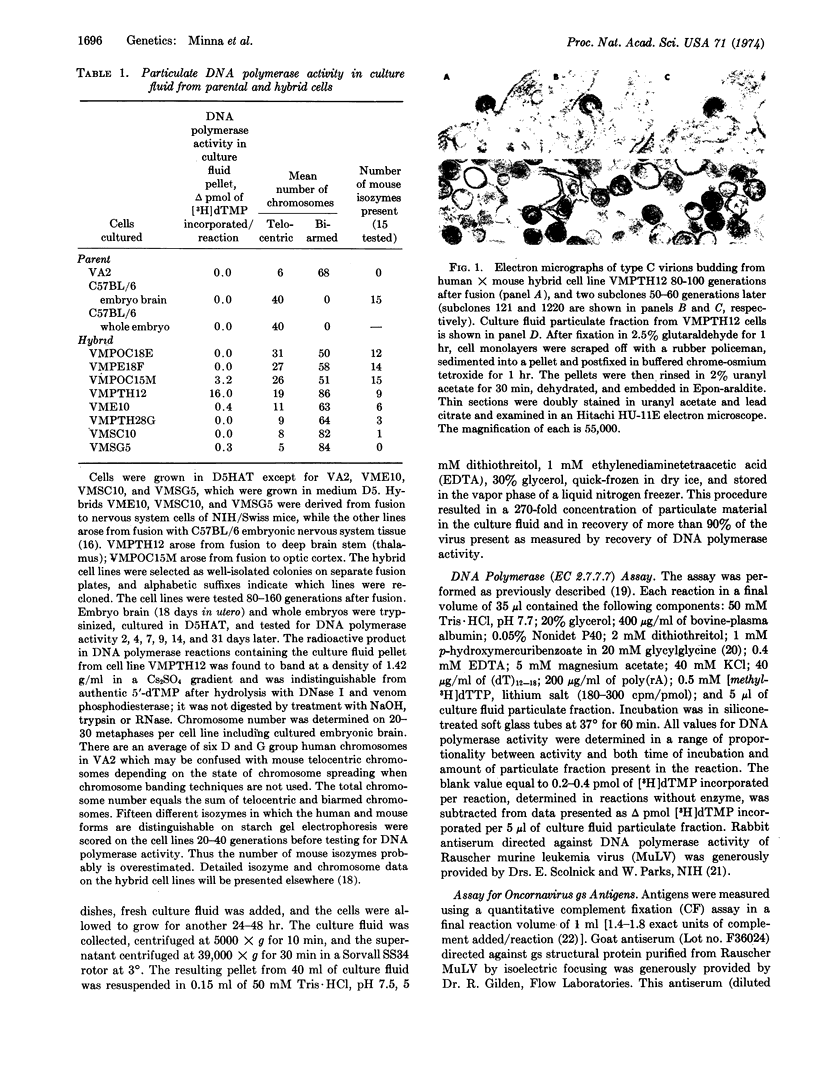
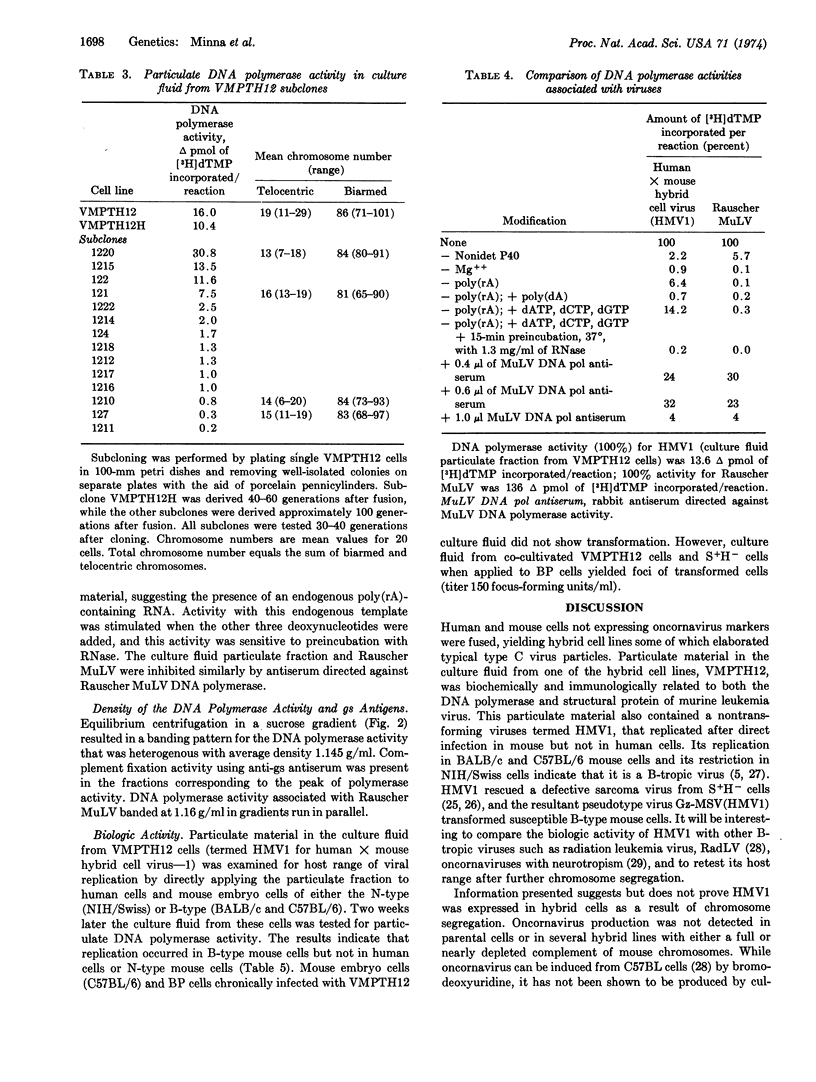
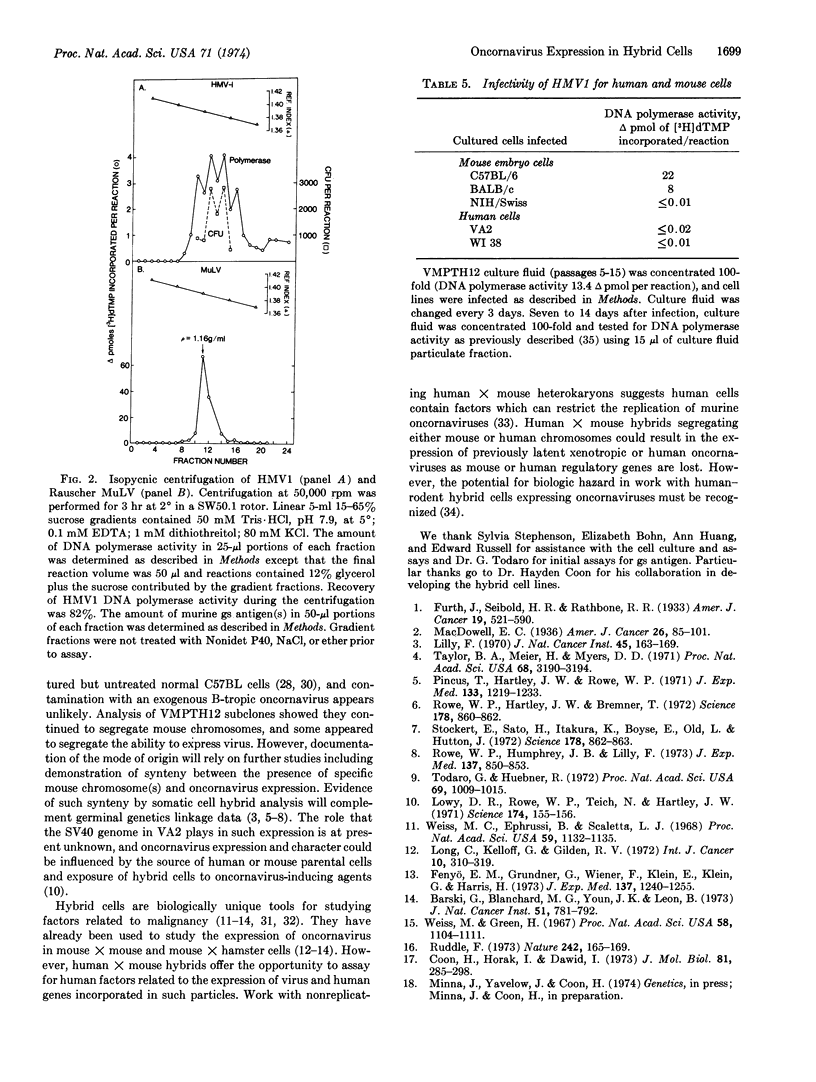
Abstract
Human × mouse hybrid clones obtained by fusing transformed human (VA2) cells with embryonic mouse brain cells were tested for their ability to spontaneously express type C virus particles. It had been previously shown that these hybrid cells preferentially retained human chromosomes while mouse chromosomes were lost. The culture fluid from one cell line was found to contain type C particle markers in abundance, and typical budding C particles were observed in the cells by electron microscopy. In contrast, no particle markers were detected in the culture fluid from parental cells and several other hybrid cell lines. Subclones of the virus-positive cell line continued to lose mouse chromosomes and were found to vary more than 100-fold in their culture fluid DNA polymerase activity. The hybrid cell viruses, termed HMV1, banded in a sucrose gradient between 1.14 and 1.16 g/ml, possessed viral group-specific antigens, and exhibited B-tropic host range for replication in mouse embryo cells, but did not replicate in human cells when directly applied. The virus did not transform mouse cells but was able to rescue the defective murine sarcoma virus from sarcoma-positive, helper-virus-negative cells. Activity of the DNA polymerase associated with HMV1 was similar to the activity of Rauscher murine leukemia virus (MuLV) DNA polymerase in its preference for poly(rA) over poly(dA) as a template, use of endogenous template, detergent requirement, and inhibition by antiserum directed against MuLV.DNA polymerase. The results suggest that human × mouse hybrid cells segregating mouse chromosomes can spontaneously express endogenous type C viruses and that such hybrid cell lines may be used for the isolation of latent mammalian oncornaviruses and analysis of viral gene regulation.
Keywords: B-tropic virus, DNA polymerase, group-specific antigens, type C virus
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Parks W. P., Scolnick E. M., Todaro G. J. Antibody to the RNA-dependent DNA polymerase of mammalian C-type RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 May;68(5):920–924. doi: 10.1073/pnas.68.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski G., Blanchard M. G., Youn J. K., Leon B. Expression of malignancy in interspecies Chinese hamster X mouse cell hybrids. J Natl Cancer Inst. 1973 Sep;51(3):781–792. doi: 10.1093/jnci/51.3.781. [DOI] [PubMed] [Google Scholar]
- Coon H. G., Horak I., Dawid I. B. Propagation of both parental mitochondrial DNAs in rat-human and mouse-human hybrid cells. J Mol Biol. 1973 Dec 15;81(3):285–298. doi: 10.1016/0022-2836(73)90142-3. [DOI] [PubMed] [Google Scholar]
- Engel E., McGee B. J., Harris H. Cytogenetic and nuclear studies on A9 and B82 cells fused together by sendai virus: the early phase. J Cell Sci. 1969 Jul;5(1):93–119. doi: 10.1242/jcs.5.1.93. [DOI] [PubMed] [Google Scholar]
- Ephrussi B., Davidson R. L., Weiss M. C., Harris H., Klein G. Malignancy of somatic cell hybrids. Nature. 1969 Dec 27;224(5226):1314–1316. doi: 10.1038/2241314a0. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Grundner G., Wiener F., Klein E., Klein G., Harris H. The influence of the partner cell on the production of L virus and the expression of viral surface antigen in hybrid cells. J Exp Med. 1973 May 1;137(5):1240–1255. doi: 10.1084/jem.137.5.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Phillips L. A., Sarma P. S., Peebles P. T., Chopra H. C. Presence of sarcoma genome in a "non-infectious" mammalian virus. Nat New Biol. 1971 Nov 17;234(46):69–72. doi: 10.1038/newbio234069a0. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Russell E., Sarma P. S., Sarin P. S., Hall W., Chopra H. C. Properties of noninfectious and transforming viruses released by Murine sarcoma virus-induced hamster tumor cells. J Virol. 1973 Oct;12(4):931–936. doi: 10.1128/jvi.12.4.931-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Sarma P. S., Bassin R. H. Properties of a murine sarcoma virus isolated from a tumor arising in an NZW-NZB F 1 hybrid mouse. II. Physical and biological characteristics. Int J Cancer. 1972 Jan 15;9(1):234–241. doi: 10.1002/ijc.2910090125. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S. Group-specific antigens of RNA tumor viruses as markers for subinfectious expression of the RNA virus genome. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1021–1025. doi: 10.1073/pnas.69.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. T., Andresen W. F., Sanford K. K., Evans V. J., Hartley J. W. Virus particles and murine leukemia virus complement-fixing antigen in neoplastic and nonneoplastic cell lines. Science. 1967 Apr 7;156(3771):85–88. doi: 10.1126/science.156.3771.85. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Long C., Kelloff G., Gilden R. V. Variations in sarcoma and leukemia virus activity in somatic cell hybrids. Int J Cancer. 1972 Sep 15;10(2):310–319. doi: 10.1002/ijc.2910100211. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Bremner T. Genetic mapping of a murine leukemia virus-inducing locus of AKR mice. Science. 1972 Nov 24;178(4063):860–862. doi: 10.1126/science.178.4063.860. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Humphrey J. B., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. 3. Assignment of the Fv-1 locus to linkage group 8 of the mouse. J Exp Med. 1973 Mar 1;137(3):850–853. doi: 10.1084/jem.137.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle F. H. Linkage analysis in man by somatic cell genetics. Nature. 1973 Mar 16;242(5394):165–169. doi: 10.1038/242165a0. [DOI] [PubMed] [Google Scholar]
- Stockert E., Sato H., Itakura K., Boyse E. A., Old L. J., Hutton J. J. Location of the second gene required for expression of the leukemia-associated mouse antigens G IX . Science. 1972 Nov 24;178(4063):862–863. doi: 10.1126/science.178.4063.862. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Meier H., Myers D. D. Host-gene control of C-type RNA tumor virus: inheritance of the group-specific antigen of murine leukemia virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3190–3194. doi: 10.1073/pnas.68.12.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Richter C. B. Murine leukemia virus: restriction in fused permissive and nonpermissive cells. Science. 1972 Nov 3;178(4060):516–518. doi: 10.1126/science.178.4060.516. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Ephrussi B., Scaletta L. J. Loss of T-antigen from somatic hybrids between mouse cells and SV40-transformed human cells. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1132–1135. doi: 10.1073/pnas.59.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Green H. Human-mouse hybrid cell lines containing partial complements of human chromosomes and functioning human genes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1104–1111. doi: 10.1073/pnas.58.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]