Abstract
Central nervous system immune reconstitution inflammatory syndrome (CNS-IRIS) develops in 9 %–47 % of persons with HIV infection and a CNS opportunistic infection who start antiretroviral therapy and is associated with a mortality rate of 13 %–75 %. These rates vary according to the causative pathogen. Common CNS-IRIS events occur in relation to Cryptococcus, tuberculosis (TB), and JC virus, but several other mycobacteria, fungi, and viruses have been associated with IRIS. IRIS symptoms often mimic the original infection, and diagnosis necessitates consideration of treatment failure, microbial resistance, and an additional neurological infection. These diagnostic challenges often delay IRIS diagnosis and treatment. Corticosteroids have been used to treat CNS-IRIS, with variable responses; the best supportive evidence exists for the treatment of TB-IRIS. Pathogenic mechanisms vary: Cryptococcal IRIS is characterized by a paucity of cerebrospinal inflammation prior to antiretroviral therapy, whereas higher levels of inflammatory markers at baseline predispose to TB meningitis IRIS. This review focuses on advances in the understanding of CNS-IRIS over the past 2 years.
Keywords: Immune reconstitution inflammatory syndrome, HIV, AIDS, Central nervous system Infections, Antiretroviral therapy, Tuberculousmeningitis, Cryptococcal meningitis, Progressive multifocal leukoencephalopathy
Introduction
Central nervous system(CNS) opportunistic infections (OIs) are a significant cause of morbidity and mortality for people living with HIV [1]. CNS OIs mainly affect patients with advanced HIV and low CD4 counts. With the global scale-up of antiretroviral therapy (ART) over the last decade, the incidence of neurologic OIs has been decreasing as ART access expands [2, 3]. The World Health Organization (WHO) estimates that almost 10 million individuals are now receiving ART worldwide in low- and middle-income countries [4]. However, many persons still enter HIV care with advanced HIV and remain at risk for a CNS OI before they start ART or prior to immune restoration. Although ART has an enormous beneficial impact on survival, one important complication of ART is the immune reconstitution inflammatory syndrome (IRIS) that affects up to a quarter of patients starting ART [5•], mainly those with advanced HIV [6]. Most forms of IRIS cause symptom deterioration but are not life threatening. However, when the CNS is involved, death may result, making CNS-IRIS the most challenging form of IRIS to manage.
IRIS describes a constellation of symptoms and clinical features that may occur in previously immunosuppressed patients during rapid restoration of immune function in the presence of a pathogen or foreign antigen [7•]. The underlying immunodeficiency is most frequently secondary to HIV, but withdrawal of immunosuppressive medications (such as corticosteroids or immunosuppressant medication in transplant patients), as well as reversal of immunosuppressant states (e.g., pregnancy, malnutrition), can also result in IRIS events [8–10]. There are two common IRIS scenarios in HIV-infected persons that both occur in the first months after commencing ART. First, paradoxical IRIS, manifests with recurrence of symptoms of a previously recognized and treated OI, although the symptoms may be different from the initial presentation. Second, unmasking IRIS manifests with the inflammatory presentation of a newly diagnosed OI. In both situations, the immune system is rapidly transitioning from an inadequate response to a heightened inflammatory response toward the pathogen [8, 11]. Rare autoimmune forms of IRIS are also described where the target antigen is host.
Central Nervous Syndrome IRIS
Among patients with CNS OIs, the cumulative incidence of CNS-IRIS ranges from 9 % (in a Spanish cohort of patients with a variety of CNS OIs) to 47 % (in a South African study of patients with TB meningitis) [12, 13]. IRIS affecting the CNS has been associated with poor outcomes and mortality rates overall of approximately 20%–30 %; however, mortality rates vary widely depending on the underlying infection and individual circumstances [14•, 15•, 16••, 17, 18•]. The reasons for poor outcomes with CNS-IRIS vary by underlying infection, but a key factor is excessive inflammation and swelling occurring within the rigid anatomy that encases the CNS. A CNS inflammatory process can lead to increased intracranial pressure that may ultimately cause brain herniation and death [5•]. In addition, inflammation and neuronal dysfunction in vital brain structures, such as the brainstem’s respiratory center, may have fatal consequences. Survivors may suffer permanent neurologic disability [14•]. Treatment therefore centers on decreasing inflammation and reducing raised intracranial pressure, when present. A wide range of CNS-IRIS etiologies have been described (Table 1).
Table 1.
Forms of neurological IRIS reported in the literature
| Underlying Infection | Neurological Manifestation(s) |
|---|---|
| Cryptococcus neoformans | Meningitis |
| Intracerebral cryptococoma/abscess | |
| Cerebellitis | |
| Coccidiomycoses | Meningitis |
| Candida | Meningitis |
| Sporothrix schenckii | Meningitis |
| Mycobacterium tuberculosis | Meningitis |
| Intracerebral tuberculoma | |
| Radiculopathy | |
| Epidural abscess | |
| Mycobacterium avium complex | Mass lesion |
| Varicella zoster virus | Encephalitis |
| Transverse myelitis | |
| Vasculopathy | |
| Cytomegalovirus | Ventriculitis |
| Vasculitis | |
| Encephalitis | |
| Human immunodeficiency virus | Encephalitis/encephalopathy |
| Herpes simplex virus | Encephalitis |
| JC virus | Progressive multifocal leukoencephalopathy |
| Epstein–Barr virus | Cerebral lymphomatoid granulomatosis |
| Parvovirus B19 | Encephalitis |
| Toxoplasma | Encephalitis |
Forms of HIV-Related CNS-IRIS
Cryptococcal Meningitis IRIS
Both unmasking and paradoxical cryptococcal meningitis IRIS (CM-IRIS) have been well described with published consensus case definitions [7•]. Prospective studies, all published within the last 5 years, have reported an incidence of paradoxical CM-IRIS of 13 %–30 % among persons with CM surviving to receive ART [14•, 19–22, 23••, 24]. The median time of CM-IRIS onset ranges from 4 to 10 weeks [14•, 19–22].
In a 2012 review [1], the authors estimated that ~186,000 cases of CM-IRIS occur annually worldwide. This estimate was based on the estimate for CM incidence published by Park and colleagues [25] and multiplied by 20 %, that being the proportion of patients who develop CM-IRIS among those with CM who start ART [26]. All patients globally with CM were assumed to have started ART. While there are significant numbers of CM-IRIS worldwide, this estimate may be a substantial overestimation. The reason for this is that among the global burden of CM cases, only a proportion of them are actually diagnosed and receive treatment. Among those who are diagnosed, the in-hospital mortality is >50 % in routine care [27], often because of inadequate access to optimal care that includes amphotericin B and intracranial pressure control. Thus, only a minority of the global burden of CM cases survive to start ART; it is only these patients who are at the 20 % risk of developing CM-IRIS. Among those who do survive to start ART, CM-IRIS is a significant cause of early mortality on ART [14•].
The major risk factors for paradoxical CM-IRIS are related to high antigen burden and severity of pre-ART immune dysfunction. Antigen burden can be assessed either by cryptococcal antigen (CrAg) titer or by quantitative culture. Two studies in Thailand and Uganda reported higher baseline serum CrAg titer to be an independent predictor for IRIS [14•, 21]. In a study conducted in South Africa published in 2013, higher baseline cerebrospinal fluid (CSF) quantitative culture was an IRIS risk factor (median 111,000 in IRIS cases vs. 1,800 CFU/mL in non-IRIS patients, p =.004) [23••]. A second consistent risk factor for IRIS is an initial paucity of CSF inflammation, such as a lack of CSF leukocytes and/or normal CSF protein level, found in three independent cohorts in Kampala, Durban, and Cape Town [22, 23••, 28]. In the Ugandan study, a paucity of proinflammatory cytokines and chemokines (lower levels of IL-6, IL-8, TNF-α, and IFN-γ) at CM diagnosis characterized the CSF of persons that later developed cryptococcal IRIS [22]. Serum biomarkers of immune dysfunction may also predict IRIS: Future CM-IRIS patients had higher pre-ART levels of C-reactive protein (CRP), interleukin (IL)-4, and IL-17 and lower levels of VEGF, G-CSF, and TNF-α [14•]. These data demonstrate that risk stratification for development for CM-IRIS may be possible.
Cryptococcal IRIS most frequently presents as recurrent meningitis, usually with sterile CSF fungal cultures. However, cryptococcal IRIS manifestations may include intracranial space occupying lesions and extend beyond the CNS including lymphadenitis, pneumonitis, and ophthalmologic complications [14•, 20]. The diagnosis of cryptococcal IRIS is clinical, and factors considered are a temporal relationship to ART initiation, characteristic clinical presentation, and exclusion of failure of antifungal therapy that may be related to fungal resistance or poor adherence and exclusion of other infections [29]. Mortality for patients with CM-IRIS has been reported to be nearly twice that of patients with CM without IRIS [14•], although this finding is not uniform [19]. Recent prospective studies have reported mortality rates ranging from 7.7 % to 36 % [14•, 19, 21, 23••].
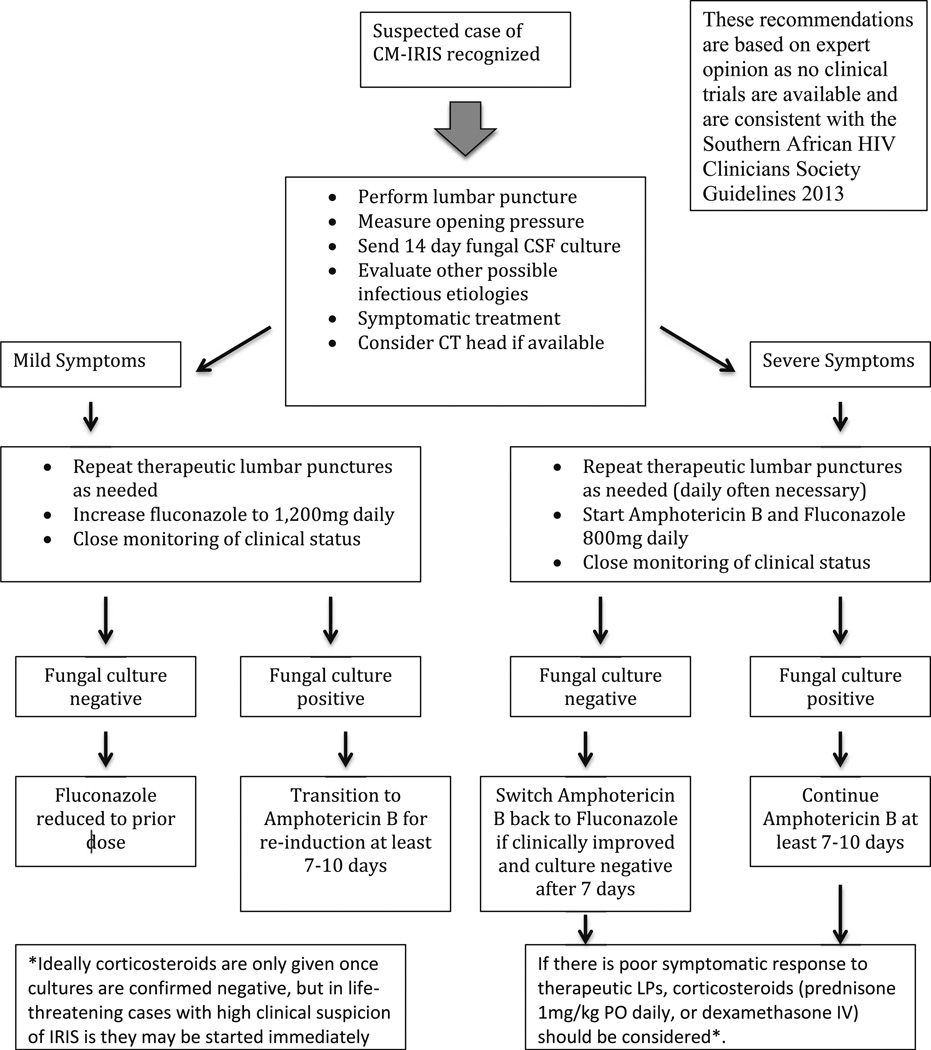
Elevated intracranial pressure (>25 cm H20) is found in approximately two thirds of CM-IRIS patients [19, 20, 30]. Control of elevated intracranial pressure via therapeutic lumbar puncture (LP) is essential [5•]. Pharmacologic treatment of CM-IRIS is anecdotal, without any data from clinical trials. Corticosteroids have been used in persistent and/or life-threatening CM-IRIS [14•, 31], and isolated cases have been treated with hydroxychloroquine, azathioprine, adalimumab, or thalidomide as alternatives to corticosteroids [9, 32–34]. In severe cases of CM-IRIS, intensification of antifungal therapy to amphotericin B should be considered, given that one cannot exclude antifungal treatment failure with relapse when the patient first presents [7•]. Interruption of ART is rarely necessary and is of uncertain benefit [35]. Our approach, consistent with the 2013 Southern African HIV Clinicians Society guidelines [36], is outlined in Fig. 1.
Fig. 1.
Recommended approach to the management of patients with suspected paradoxical CM-IRIS
The issue of the optimal timing of ART after CM was, until recently, unresolved. The counterbalancing risks are that of CM-IRIS with attendant mortality associated with earlier ART and the high mortality risk associated with deferring ART in patients with advanced HIV. Two small trials had conflicting results [37, 38]. The results of the Cryptococcal Optimal ART Timing (COAT) trial were presented at CROI in 2013 [39]. In the COAT trial of 177 ART-naïve persons with a first episode of CM, deferring ART for approximately 5 weeks was associated with lower mortality, as compared with initiation of ART in the first 1–2 weeks after CM diagnosis. The COAT trial was stopped early with 15 % better survival with deferred ART over early ART. In a small trial in Botswana, CM-IRIS was higher with early ART [40].
Unmasking of Cryptococcosis on ART
Prospective studies on unmasking cryptococcosis during early ART have reported incidences of 0.4 %–1.7 % (2/490, 5/295, 3/423) in broad cohorts of HIV-infected patients in Uganda and South Africa [24, 41, 42]. The incidence is higher as the CD4+ count decreases [41, 43], and mortality rates of 75 % were reported in one Uganda cohort study [41]. Symptom onset can range from within days of starting ART to 3–4 months [24, 41, 42].
Cryptococcus provides a useful model for understanding unmasking of opportunistic infections on ART. This is not a latent infection being reactivated, but a subclinical active infection that becomes apparent with ART. Detectable cryptococcal antigenemia represents dissemination. If ART is not received, these persons will progress to symptomatic meningitis in weeks to months [41]. Conversely, if ART is initiated, persons may or may not develop unmasking cryptococcosis. The two most important risk factors appear to be the inverse interplay between CD4+ count and antigen burden. Once ART is initiated, the immune system must rapidly recover to control asymptomatic cryptococcal infection. If the CD4+ count is <100 cells/µL and the serum CrAg titer is >1:8, unmasking of cryptococcosis is frequent [43–47]. However, preemptive fluconazole therapy may abort this trajectory. Pre-ART CrAg screening with preemptive fluconazole therapy for those positive has recently been recommended for patients with CD4+ counts <100 cells/µL [41]. Implementing screening and preemptive programs could decrease the numbers of unmasking cryptococcal cases, and this public health strategy is being tested in an ongoing randomized controlled trial (RCT) in Uganda, the ORCAS trial (personal communication, D. R. Boulware).
Neurological TB-IRIS
Tuberculosis-associated-IRIS (TB-IRIS) is the commonest form of IRIS in high HIV/TB coinfection settings, with both unmasking and paradoxical forms described [11]. Paradoxical TB-IRIS, which is the best studied of the two forms, occurs in 8 %–54 % of TB patients who typically experience symptomatic improvement on appropriate TB drugs and then develop new, recurrent, or worsening TB symptoms or signs after subsequently starting ART [11, 48]. Neurological involvement occurs in a substantial proportion of these patients, accounting for 12 %–31 % of cases in two series of paradoxical TB-IRIS [17, 18•]. In a very high TB endemic setting, paradoxical neurological TB-IRIS (neuro-TB-IRIS) was also the most common cause for CNS deterioration in patients within 1 year of starting ART, accounting for 21 % of cases, and occurred in 47 % (16/34) of tuberculous meningitis (TBM) patients after ART initiation [13]. Paradoxical neuro-TB-IRIS may occur both in patients who present with neurological TB and in those who present with extra-neural TB, at initial TB diagnosis. Manifestations include meningitis, brain tuberculomas, brain abscesses, radiculomyelitis, and spinal epidural abscesses [16••, 17, 18•, 49]. The median time to developing paradoxical neuro-TB-IRIS is 14 days after starting ART, but IRIS may occur more than 3 months after ART initiation [16••, 18•].
Risk factors for paradoxical TBM-IRIS have recently been described in a prospective study of HIV-associated TBM [16••]. In this study, ART-naïve patients were enrolled at TBM diagnosis. ART was started 14 days after TB treatment initiation. Patients were followed up for 9 months, and findings were compared between those who developed TBM-IRIS and controls who did not. At TBM diagnosis, patients who developed TBM-IRIS had features suggesting a higher degree of TB dissemination, as compared with patients who did not develop TBM-IRIS, such as chest radiograph abnormalities (81 % vs. 50 %, p =.08). Raised CSF neutrophil counts (median, 50 vs. 3 cells/µL, p =.02) at baseline predicted future TBM-IRIS and CSF concentrations of inflammatory cytokines such as TNF-α, IFN-γ, and IL-6, which have been implicated in TB-IRIS pathogenesis in two studies published in 2011–2012 [50, 51], were also significantly higher in TBM-IRIS patients at baseline. These findings suggest a potential role for immunomodulatory therapies to prevent TBM-IRIS, which should be investigated in RCTs. TBM-IRIS was further associated with high baseline CNS bacillary load as evidenced by CSF Mycobacterium tuberculosis culture positivity; the organism was cultured at TBM diagnosis in 15/16 TBM-IRIS patients (94 %), as compared with 6/18 controls (33 %). As has been suggested previously for TB-IRIS [11] as well as for CM-IRIS [14•, 21, 23••], a high baseline microbial load seems to drive a CNS inflammatory response that ultimately manifests as IRIS. This emphasizes the need for optimal antimicrobial therapy prior to ART initiation to reduce the risk for paradoxical IRIS.
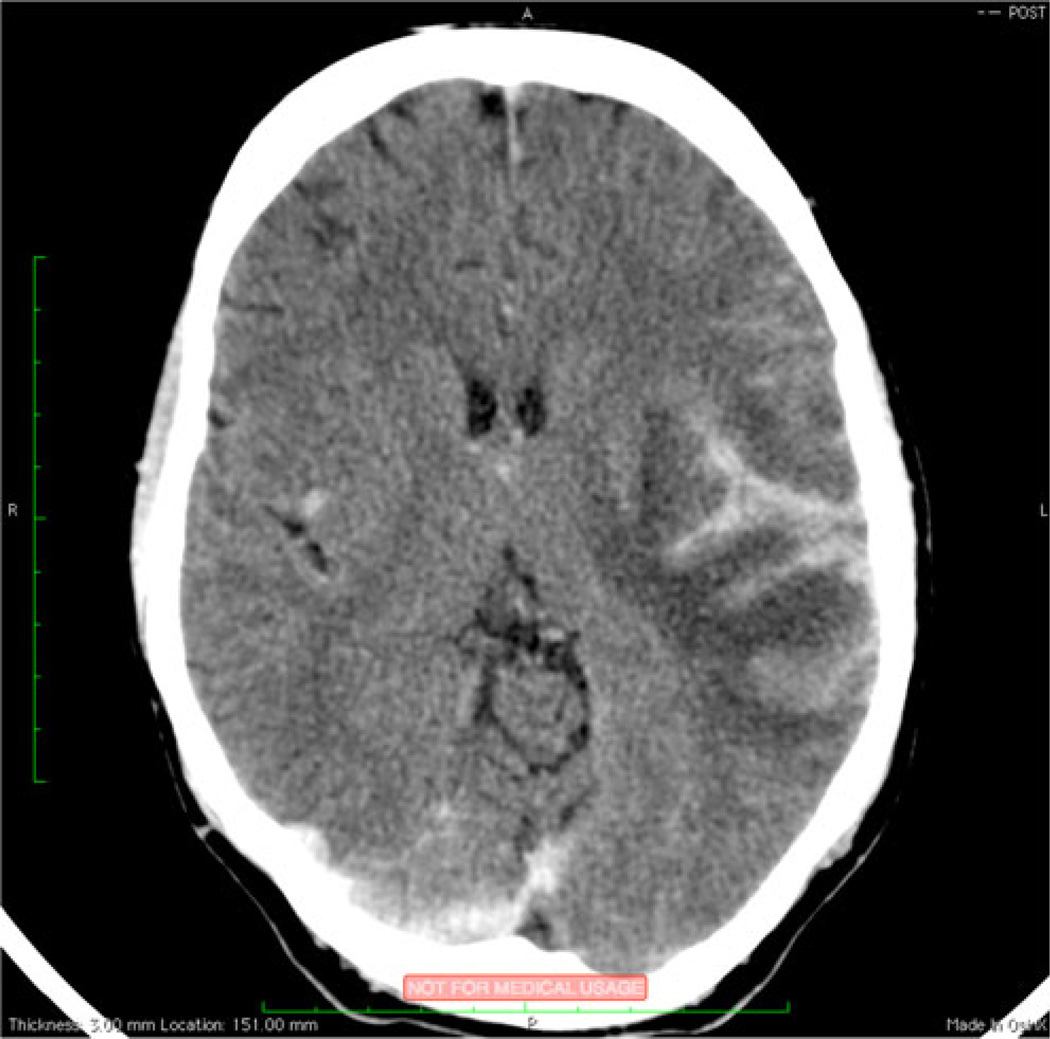
The same study reported at time of TBM-IRIS presentation that patients had a significant recurrence of CSF inflammation with high neutrophil and lymphocyte counts and increased protein levels [16••]. Inflammation was also evident on brain CT and spinal magnetic resonance imaging (MRI), which revealed features consistent with pachymeningitis and radiculomyelitis, respectively, in some TBM-IRIS patients (Fig. 2). However, normal brain imaging (including CT and MRI) does not exclude the diagnosis of TBM-IRIS [16••, 18•, 52]. CSF culture for TB is typically, but not invariably, negative at time of neuro-TB-IRIS diagnosis [16••]. As with IRIS associated with other etiologies, no confirmatory test for TB-IRIS exists. The diagnostic approach to neuro-TB-IRIS suspects should focus on the exclusion of other causes for deterioration, such as poor adherence to TB drugs, drug reactions or toxicities, infection with a TB drug-resistant strain, and an alternative or additional opportunistic etiology [11].
Fig. 2.
TB-IRIS pachymeningitis. Postcontrast brain-computed tomography shows focal leptomeningeal enhancement involving the left sylvian fissure (black arrow) with adjacent cerebral edema in a patient with tuberculous meningitis immune reconstitution inflammatory syndrome
In general, paradoxical TB-IRIS is associated with low mortality (3.2 % in a meta-analysis published in 2010) [26]. However, paradoxical neuro-TB-IRIS is more frequently associated with death; mortality at 6–9 months reaches 30 % [16••, 17]. In a recent case series, 3 of 4 patients (75 %) who presented with TBM-IRIS died [18•].
The optimal management of neuro-TB-IRIS is uncertain, since no treatment is of proven benefit [5•]. In an RCT of prednisone versus placebo for the treatment of paradoxical TB-IRIS that excluded patients with neuro-TB-IRIS, prednisone was associated with more rapid symptom relief and reduced the duration of hospitalization and need for therapeutic procedures [53]. No treatment trials in neuro-TB-IRIS have been conducted. Corticosteroid treatment is known to reduce mortality in severe forms of TB outside the context of IRIS, such as meningitis [54], and has anecdotally been associated with good outcome in neuro-TB-IRIS [17]. However, corticosteroids do not prevent neuro-TB-IRIS; 13/16 TBM-IRIS cases were already receiving prednisone (0.75–1.5 mg/kg/day) when they developed TBM-IRIS [16••]. Our practice is to treat all TB-IRIS patients with neurological involvement with prednisone (starting dose, 1.5 mg/kg/day or equivalent) tapering the dose after 2–4 weeks according to the individual patient’s response [5•]. Patients usually require 2–4 months of treatment; however, some patients may experience relapses when treatment is reduced or stopped, necessitating steroid treatment for longer periods. An attempt should be made to continue ART during the IRIS episode, since interrupting ART may increase susceptibility to other OIs and predispose to drug resistance. IRIS may also recur when ART is restarted after interruption. We suggest that temporary discontinuation of ART be considered only in cases with a persistent or severely depressed level of consciousness and in cases with severe neurological disability unresponsive to corticosteroid treatment.
The optimal time to start ART in patients with HIV-associated neuro-TB remains uncertain. A shorter interval between TB treatment and ART initiation increases the risk of developing TB-IRIS. Regardless of this risk, three RCTs published in 2011 showed a mortality benefit for starting ART early, as compared with later (2–4 weeks vs. 8 weeks after TB treatment initiation), in severely immune suppressed (CD4+ count <50 cells/µL) patients [55–57]. These three trials enrolled predominantly patients with extra-neural TB, and thus the findings may not be generalizable to patients with neurological forms of TB who are at high risk of developing potentially fatal neuro-TB-IRIS [58]. Another RCT published in 2011 involving TBM patients compared early (within 7 days of TB treatment initiation) with delayed (8 weeks after TB treatment initiation) ART [59••]. This TBM-specific study, which included severely immunosuppressed patients (median CD4 count=41 cells/µL), found no difference in mortality between the two treatment arms but an increased frequency of severe adverse events with early ART initiation. On the basis of these findings, some guidelines recommend deferring ART to 4–6 weeks after TB treatment initiation in patients who present with TBM [60], and our practice is now to start ART at 4 weeks in TBM patients. Table 2 compares CM-IRIS and TBM-IRIS.
Table 2.
Comparison of paradoxical TB meningitis IRIS and paradoxical cryptococcal meningitis IRIS
| TBM-IRIS | CM-IRIS | |
|---|---|---|
| Time between ART initiation and onset | Median 14 days | Median 30–60 days |
| Incidence | 8 %–54 % (applies to all forms of TB-IRIS) |
13 %–30 % |
| Risk Factors, prior to ART initiation* | At initial TBM diagnosis:
|
At initial CM diagnosis:
High serum CrAg pre-ART |
| CSF cellular picture of IRIS (compared with baseline) | Increased CSF Protein | Increased CSF cell count Presence of neutrophils CSF Protein is elevated |
| Serum/plasma biomarkers associated with IRIS | CRP, IFN-γ IL-6, IL-8, TNF-α, IL-10, IL-12p40** | CRP, IFN-γ, IL-6, IL-8, GCSF, GMCSF, VEGF, IL-1RA, IL-2, IL-7, IL-17, D-dimer |
| Management | Exclude drug resistance Corticosteroids |
Exclude culture-positive relapse Therapeutic LP(s) Possible corticosteroids |
| Outcome | Mortality 13 %–75 % Disability 25 % |
Mortality 27 %–36 % |
In both TB and CM, higher organism/antigen load is a risk factor for IRIS, but in general, CM is characterized by much higher organism load than is TBM and, prior to ART, a poorer immune response in the CSF, unlike TBM.
Biomarkers elevated in serum of TB-IRIS patients in studies not limited to patients with CNS TB-IRIS
Progressive Multifocal Leukoencephalopathy IRIS
Progressive multifocal leukoencephalopathy (PML), a demyelinating disease of the brain caused by the JC polyomavirus, occurs in 3 %–5 % of persons with AIDS worldwide [61]. Both paradoxical (also termed PML-delayed IRIS) and unmasking (also termed PML-simultaneous IRIS) PML-IRIS have been described [15•]. PML-IRIS is also the most frequent form of CNS-IRIS reported in HIV-negative patients recovering from iatrogenic immunosuppression (discussed below). One study found PML-IRIS to occur in 18 % of persons with PML diagnosed pre-ART [62], while a study published in 2012 reported IRIS in 40%of patients diagnosed with PML in a mixed cohort of patients with HIV and other causes of immunosuppression [63•]. In two studies (one prospective and another retrospective), the median time to PML-IRIS was 4 weeks from starting ART with paradoxical IRIS and 7–8 weeks with unmasking IRIS [15•, 63•]. In both studies, only a minority of cases occurred more than 6 months after starting ART [15, 63]. Neurological deficits correspond to the brain area involved and include findings such as cognitive dysfunction, focal weakness, sensory changes, and visual disturbances. In the most severe cases, cerebral edema and herniation occur, and this may be rapidly fatal [15•, 64]. In one study, the mortality associated with paradoxical PML-IRIS was 53 % and with unmasking IRIS was 31 %. The authors hypothesized that delayed recognition of IRIS in paradoxical cases contributed to poor outcome [15•]. Few studies have addressed risk factors for PML-IRIS. One retrospective study of 87 patients with PML noted that the baseline CD4 count was lower in patients who developed PML-IRIS, as compared with PML patients who did not develop IRIS (mean 35 vs. 72) [65].
The diagnosis of PML-IRIS may be difficult, since the diagnostic methods used are expensive and not available in most resource-limited settings. In addition, CSF PCR for JC virus in patients not on ART is >95 % sensitive [66], but the PCR is less sensitive in patients on ART (sensitivity 58 %) [67]. It is also difficult to distinguish between a patient deteriorating due to IRIS or from progression of JC virus infection. Brain MRI shows enhancement more often with PML-IRIS than with PML [64]. In a retrospective PML-IRIS series of 54 HIV infected persons, 57 % had MRI enhancement [15]. A prospective cohort study published in 2012 including patients with mixed HIV status (19/42 PML cases and 12/17 IRIS cases were HIV-infected) reported enhancement in 88 % of MRI scans in cases of PML-IRIS versus 15 % of non-IRIS PML cases [63]. In the same study, a ratio lipid/lactate to creatine >1.5 combined with contrast enhancement yielded a 79 % probability of IRIS [63], providing potential new methods of PML-IRIS diagnosis. Less gadolinium enhancement may be seen in HIV-infected persons than in HIV-uninfected persons with PML-IRIS due to lower CSF leukocyte counts in the former group [64].
There is no specific therapy for PML’s causative agent, JC virus [64]. This is a major determinant of the high mortality associated with PML-IRIS. In the same retrospective study of 54 HIV infected persons with PML-IRIS, 12 received corticosteroid treatment. There was no difference in mortality associated with receiving corticosteroids [15•]. Restricting analysis to only those 12 patients and comparing survivors and nonsurvivors, there was a nonsignificant trend toward earlier initiation and longer duration of corticosteroids among survivors [15•]. These data are not compelling evidence for the benefit of corticosteroids, and there is no clinical trial evidence supporting corticosteroid use in PML-IRIS. Steroid use remains controversial because there is concern that immunosuppression may be deleterious for controlling JC virus replication; however, many experts advocate use of corticosteroids in life-threatening cases such as patients with cerebral edema [68].
HIV Associated Neurocognitive Disorder IRIS
HIV itself causes neurocognitive impairment with severity varying from mild impairment to dementia, due to direct viral invasion of the CNS [69]. In one cohort study spanning 1996–2002, 55 % of HIV patients had some degree of HIV-associated neurocognitive disorder (HAND), while 10.4 % went on to develop dementia, as compared with approximately 20 % of persons with AIDS in the pre-ART era [70]. A Danish cohort study published in 2011 reported an incidence of dementia in HIV patients similar to that of the general population (<1 %) [71]. Although the incidence and prevalence of dementia has decreased in HIV infected populations in the ART era, the prevalence of HAND overall has been fairly constant, since milder forms remain common [72].
IRIS has been reported to complicate HAND as an encephalitic process in patients initiating ART [73]. HAND-IRIS has been described as worsening of preexisting HAND, as well as the cause of new onset HAND [74]. Cases with fulminant disease complicated by cerebral edema with thorough exclusion of other neuro-infections have been described, and these may represent the most severe forms of HAND-IRIS [75]. Although imaging findings vary, white matter lesions are often seen on MRI in HAND-IRIS cases and may be concentrated in the periventricular area [74–76].
While several groups are investigating HAND pathologic mechanisms [72, 77, 78], little investigation of HAND-IRIS has been undertaken. Johnson and Nath have postulated that CNS IRIS may be an important component of the development of HIV-related neurocognitive disorders on ART [79]. More recently, their group published a study suggesting that direct activation of T-cells by HIV Tat protein that persists in the CSF of patients despite virological suppression on ART may play a role in the development of HAND-IRIS [80]. Case reports have described variable pathologic features, including a CD8+ lymphocytic infiltrate (either perivascular or diffuse) and demyelination [74–76]. An important question that should be addressed in future studies is whether HIV itself or a component of HIV is the antigenic target [74, 81] or whether the pathology reflects an autoimmune CNS process in the context of ART [74] occurring on the background of chronic HIV damage and immune dysregulation in the CNS.
Of reported HAND-IRIS cases, some degree of symptomatic improvement has been reported both with and without corticosteroid use [73, 76]. However, no controlled trials have examined treatment modalities for HAND-IRIS.
Other Forms of CNS IRIS
Many other infections have been reported as causes of CNS-IRIS, although the numbers of cases reported for each of these infections is few. CNS toxoplasmosis is a common opportunistic infection in patients with AIDS [82], but toxoplasmosis-IRIS is rarely described; only case reports have been published [83, 84]. Symptomatic improvement was reported with corticosteroid therapy in one of these cases [83]; corticosteroids were not used in others. In persons receiving trimethoprimsulfamethoxazole (cotrimoxazole) prior to ART, unmasking of toxoplasmosis is exceptionally rare. Other causes of CNS IRIS, many of which have been reported as single-case reports, are listed in Table 1.
Non-HIV-Related Forms of CNS-IRIS
IRIS is now recognized in non-HIV-infected patients recovering from immunosuppression, such as refeeding syndrome in severely malnourished refugee populations, flares of rheumatologic conditions post-partum, and bone marrow transplant recipients during recovery from neutropenia. An underlying theme in all forms of IRIS is exposing large amounts of foreign antigen to an immune system with improving ability to respond and cause inflammation. Barber and colleagues suggest that both ART-related and non-HIV IRIS is due to hyper-responsiveness of the innate immune system as T-cell function recovers [85••].
An interesting new development has been the reports of PML-IRIS occurring in HIV-negative persons after discontinuation of immunosuppressant medication—most notably, natalizumab [86–88], but also fumaric acid and efalizumab [89, 90]. Natalizumab, used in the treatment of multiple sclerosis, is a recombinant humanized monoclonal antibody directed against α4 integrin, an adhesion molecule expressed on circulating lymphocytes involved in recruitment to sites of inflammation. Iatrogenic immunosuppression allows for reactivation of latent JC virus with viral replication. When nataluzimab is discontinued, the immune system responds to the foreign antigen causing pathologic IRIS with unmasking of PML or rapid worsening of diagnosed PML. JC-virus-specific CD4+ lymphocytic infiltrate into brain tissue is associated with PML-IRIS response, as well as JC virus clearance [88]. An insight that might be drawn from such cases is that if natalizumab withdrawal precipitates IRIS, natalizumab may be considered as a treatment for severe life-threatening cases of HIV-related CNS-IRIS associated with infections for which effective antimicrobial therapy exists. Other treatments that block specific immune effector pathways (such as TNF-alpha blockers) may also have a role in such cases [91]. The role of such experimental therapies needs to be addressed in controlled research settings.
Other sporadic cases of CNS-IRIS have been reported in relation to withdrawal of immunosuppressants: A case of TB-IRIS occurred after discontinuation of infliximab [92]; CM-IRIS has occurred after withdrawal of immunosuppressive medications for solid organ transplant [93, 94]; and candida encephalitis IRIS was reported after withdrawal of immunosuppressive therapy related to hematopoietic stem cell transplant [95].
Conclusion
CNS-IRIS is a significant cause of morbidity and mortality worldwide in persons with HIV and is being increasingly recognized in persons without HIV, given the increasing use of immunomodulatory medications. While CNS-IRIS related to TB, Cryptococcus, and PML are most frequent, a number of other, more rare causes of CNS-IRIS have been noted. Advances in diagnosis and prediction have been made in the past few years; however, significant work remains, particularly in regard to treatment. Research priorities should include further characterization of IRIS pathogenesis, role and timing of amphotericin B and corticosteroid use in CM-IRIS, more rapid diagnostic tools for CNS-IRIS risk factors for the development of PML-IRIS, and further characterization of HAND-IRIS and the role of IRIS in the development of HAND on ART.
Acknowledgment
Suzaan Marais has received grant support for her institution from Wellcome Trust.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Nathan Bahr, David R Boulware, James Scriven, Robert J Wilkinson, and Graeme Meintjes declare no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Nathan Bahr, Email: Bahrx026@umn.edu, Division of Infectious Diseases & International Medicine, Department of Medicine, University of Minnesota, MTRF 3-222, 2001 6th Street SE, Minneapolis, MN 55455, USA.
David R. Boulware, Email: Boulw001@umn.edu, Division of Infectious Diseases & International Medicine, Department of Medicine, University of Minnesota, MTRF 3-222, 2001 6th Street SE, Minneapolis, MN 55455, USA.
Suzaan Marais, Email: marais.suzaan@gmail.com, Clinical Infectious Diseases Research Initiative, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town 7925, South Africa; Division of Neurology, Department of Medicine, University of Cape Town, Cape Town, South Africa.
James Scriven, Email: James.Scriven@liverpool.ac.uk, Clinical Infectious Diseases Research Initiative, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town 7925, South Africa; Liverpool School of Tropical Medicine, Liverpool University, Liverpool, UK.
Robert J. Wilkinson, Email: Robert.Wilkinson@uct.ac.za, Clinical Infectious Diseases Research Initiative, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town 7925, South Africa; Department of Medicine, Imperial College London, London W2 1PG, UK; Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, Cape Town, South Africa; National Institute of Medical Research, London, UK.
Graeme Meintjes, Email: graemein@mweb.co.za, Clinical Infectious Diseases Research Initiative, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town 7925, South Africa; Department of Medicine, Imperial College London, London W2 1PG, UK; Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, Cape Town, South Africa.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mateen FJ, Nath A. Central nervous system – immune reconstitution inflammatory syndrome in resource-limited settings: current burden and future needs. AIDS. 2012;26:1851–1855. doi: 10.1097/QAD.0b013e3283574e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirza SA, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis. 2003;36(6):789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 3.d’Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in EuroSIDA cohort. Annals of Neurol. 2004;55(3):320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global update on HIV treatment 2013: results, impact and opportunities. [Last accessed 13, July, 2013]; Available online at http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf.
- 5. Meintjes G, Scriven J, Marais S. Management of immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2012;9:138–150. doi: 10.1007/s11904-012-0129-5. This is a comprehensive update of management strategies for various common forms of IRIS.
- 6.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16–21. doi: 10.1007/s11904-007-0003-z. [DOI] [PubMed] [Google Scholar]
- 7. Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution syndrome in HIV-1 infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. This paper provides case definitions of CM-IRIS based on current literature. Variation in case definitions of CM-IRIS used across studies has handicapped a broad understanding of CM-IRIS and the ability to compare studies.
- 8.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of Natalizumab therapy. Arch Neurol. 2011;68(2):186–191. doi: 10.1001/archneurol.2010.257. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan S, Banerjee C, Holt PA. Cryptococcal immune reconstitution syndrome during steroid withdrawal with hydroxychloroquine. Internat J Infect Dis. 2011;15:e70–e73. doi: 10.1016/j.ijid.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Katusiime C, Ocama P, Kambugu A. A case report: Herpes zoster IRIS in pregnancy. Afric Health Sci. 2009;9(4):294–295. [PMC free article] [PubMed] [Google Scholar]
- 11.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riveiro-Barciela M, Falco V, Burgos J, et al. Neurological opportunistic infections and neurological immune reconstitution syndrome: impact of one decade of highly active antiretroviral treatment in a tertiary hospital. HIV Medicine. 2013;14:21–30. doi: 10.1111/j.1468-1293.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 13.Asselman V, Thienemann F, Pepper DJ, et al. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS. 2010;24(18):2871–2876. doi: 10.1097/QAD.0b013e328340fe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after Cryptococcus meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. This prospective study of 101 patients provides possible serum biomarkers for predicting CM-IRIS, in addition to adding to understanding of CM-IRIS pathogenesis.
- 15. Tan K, Roda R, Ostrow L, et al. PML-IRIS in patients with HIV infection: Clinical manifestations and treatment with steroids. Neurology. 2009;72:1458–1464. doi: 10.1212/01.wnl.0000343510.08643.74. This retrospective study provides the best understanding of HIV-related PML-IRIS risk factors that is currently available.
- 16. Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculosis meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2013;56(3):450–460. doi: 10.1093/cid/cis899. This prospective study provides insights regarding the pathogenesis of neuro-TB-IRIS through biomarkers characterization and identifies clinical risk factors for the development of neuro-TB-IRIS.
- 17.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–e107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 18. Agarwal U, Kumar A, Behera D, et al. Tuberculosis associated immune reconstitution inflammatory syndrome in patients infected with HIV: meningitis a potentially life threatening manifestation. AIDS Res Ther. 2012;9(1):17. doi: 10.1186/1742-6405-9-17. A small descriptive study highlighting the significant mortality associated with neurological TB-IRIS.
- 19.Bicanic T, Meintjes G, Rebe K, et al. Immune Reconstitution Inflammatory Syndrome in HIV-associated Cryptococcal meningitis: a prospective study. J Acquir Immune Defic Sydnr. 2009;51:130–134. doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 20.da Cunha Colombo ER, Mora DJ, Silva-Vergara ML. Immune reconstitution inflammatory syndrome (IRIS) associated with Cryptococcus neoformans infection in AIDS patients. Mycoses. 2010;54:e178–e182. doi: 10.1111/j.1439-0507.2010.01870.x. [DOI] [PubMed] [Google Scholar]
- 21.Sungkanuparph S, Giller SG, Chetchotisakd P, et al. Cryptococcal immune reconstitution syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49:931–934. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 22.Boulware DR, Bonham SC, Meya DB, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202(6):962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27:000–000. doi: 10.1097/QAD.0b013e3283614a8d. This carefully performed prospective study describes risk factors for CM-IRIS. A particularly important finding is that CSF culture positivity prior to ART initiation predicts IRIS.
- 24.Haddow LJ, Easterbrook PJ, Mosam A, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49:1424–1432. doi: 10.1086/630208. [DOI] [PubMed] [Google Scholar]
- 25.Park BJ, Wannemuehler KA, Marston BJ, et al. Estimation of the current burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 26.Muller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhein J, Boulware DB. Prognosis and management of cryptococcal meningitis in patients with human immunodeficiency virus infection. Neurobehavioral HIV Medicine. 2012;4:45–61. [Google Scholar]
- 28.Jarvis J. CSF cytokine profiles in patients with HIV-associated cryptococcal meningitis: correlates with clinical outcome. Presented at the 8th International Conference on Cryptococcus and Cryptococcosis; May 4, 2011; Charleston, SC. [Google Scholar]
- 29.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21(16):2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 30.Sungkanuparph S, Jongwutiwes U, Kiertiburanakul S. Timing of cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in patients with AIDS and cryptococcal meningitis. J Acquir Immune Defic Syndr. 2007;45(5):595–596. doi: 10.1097/QAI.0b013e318061b5eb. [DOI] [PubMed] [Google Scholar]
- 31.Lortholary O, Fontanet A, Memain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 32.Scourfield A, Waters L, Tittle V, et al. Azathioprine use as a novel treatment for cryptococcal immune reconstitution and inflammatory syndrome in HIV. 17th Annual Conference of the British HIV Association; Bournemouth. 2011. abstract P92. [Google Scholar]
- 33.Brunel AS, Reynes J, Tuaillon E, et al. Thalidomide for steroid-dependent immune reconstitution inflammatory syndromes during AIDS. AIDS. 2012;26(16):2110–2111. doi: 10.1097/QAD.0b013e328358daea. [DOI] [PubMed] [Google Scholar]
- 34.Sitapati AM, Kao CL, Cachay ER, et al. Treatment of HIV-related inflammatory cerebral cryptococcoma with adalimumab. Clin Infect Dis. 2010;50:e7–e10. doi: 10.1086/649553. [DOI] [PubMed] [Google Scholar]
- 35.Musubire AK, Meya DB, Mayanja-Kizza H, et al. Challenges in diagnosis and management of cryptococcal immune reconstitution inflammatory syndrome (IRIS) in resource limited settings. African Health Sciences. 2012;12(2):226–230. doi: 10.4314/ahs.v12i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govender NP, Meintjes, Bicanic T, et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. [Last accessed: 21, June 2013]; Available at: http://www.sahivsoc.org/upload/documents/Guidelines%20for%20the%20Prevention,%20Diagnosis%20and%20Management%20of%20Cryptococcal%20Meningitis%20in%20HIV-infected%20persons%202013%20update.pdf. [Google Scholar]
- 37.Makadznage AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-Saharan Africa. Clin Infect Dis. 2010;50:1532–1538. doi: 10.1086/652652. [DOI] [PubMed] [Google Scholar]
- 38.Zolopa A, Anderson J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulware DB, Meya DB, Muzoora C, et al. ART initiation within the first 2 weeks of cryptococcal meningitis is associated with higher mortality: a multisite randomized trial. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta. 2013. abstract #144. [Google Scholar]
- 40.Bisson GP, Nthobatsong R, Thakur R, et al. The use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in Botswana. J Acquir Immune Defic Syndr. 2008;49(2):227–229. doi: 10.1097/QAI.0b013e318183181e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 43.French N, Gray K, Watera C, Nakiyingi J, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 44.Jarvis JN, Lawn SD, Vogt M, et al. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenaemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 46.Micol R, Lortholary O, Sar B, et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr. 2007;45:555–559. doi: 10.1097/QAI.0b013e31811ed32c. [DOI] [PubMed] [Google Scholar]
- 47.Govender NP, Chetty V, Roy M, et al. Phased implementation of screening for cryptococcal disease in South Africa. S Afr Med J. 2012;102:914–917. doi: 10.7196/samj.6228. [DOI] [PubMed] [Google Scholar]
- 48.Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS ONE. 2013;8(5):e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal JE, Cimerman S, Schiavon Nogueira R, et al. Paradoxical reaction during treatment of tuberculous brain abscess in a patient with AIDS. Rev Inst Med Trop Sao Paulo. 2003;45(3):177–178. doi: 10.1590/s0036-46652003000300012. [DOI] [PubMed] [Google Scholar]
- 50.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37(5):1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meintjes G, Skolimowska KH, Wilkinson KA, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2012;186(4):369–377. doi: 10.1164/rccm.201201-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marais S, Scholtz P, Pepper DJ, et al. Neuroradiological features of the tuberculosis-associated immune reconstitution inflammatory syndrome. Int J Tuberc Lung Dis. 2010;14(2):188–196. [PMC free article] [PubMed] [Google Scholar]
- 53.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):141–151. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 55.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanc FX, Sok T, Laureillard Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torok ME, Farrar JJ. When to start antiretroviral therapy in HIV-associated tuberculosis. N Engl J Med. 2011;365(16):1538–1540. doi: 10.1056/NEJMe1109546. [DOI] [PubMed] [Google Scholar]
- 59. Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–1383. doi: 10.1093/cid/cir230. This is the only randomized controlled trial to specifically focus on timing of ART initiation in patients with tuberculous meningitis. Although this trial did not assess the occurrence of IRIS, findings suggest that early ART has no mortality benefit in severely immunosuppressed patients with tuberculous meningitis.
- 60.The South African Antiretroviral Treatment Guidelines, 2013. [Last accessed: 21, June 2013]; Available at: http://www.sahivsoc.org/upload/documents/2013%20ART%20GuidelinesShort%20Combined%20FINAL%20draft%20guidelines%2014%20March%202013.pdf. [Google Scholar]
- 61.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 62.Cinque P, Pierotti C, Vigano MG, et al. The good and evil of HAART in HIV-related progressive multifocal leukoencephalopathy. J Neurovirol. 2001;7:358–363. doi: 10.1080/13550280152537247. [DOI] [PubMed] [Google Scholar]
- 63. Gheuens S, Ngo L, Wang X, et al. Metabolic profile of PML lesions in patients with and without IRIS. Neurol. 2012;79:1041–1048. doi: 10.1212/WNL.0b013e318268465b. This study included both HIV-positive and -negative patients. It had a prospective design and characterized the metabolic markers of PML-IRIS.
- 64.Ferenczy MW, Marshall LJ, Nelson CD, et al. Molecular biology, epidemiology, and pathogenesis of Progressive Multifocal Leukoencephalopathy, the JC Virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):4871–4506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison DM, Newsome SD, Skolasky RL, et al. Immune reconstitution is not a prognostic factor in progressive multifocal leukoencephaopathy. J Neuroimmuno. 2011;238:81–86. doi: 10.1016/j.jneuroim.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology. 2013;80(15):1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marzocchetti A, Di Giambenedetto S, Cingolani A, et al. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43(8):4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lima MA, Koralnik IJ. New features of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy and natalizumab. J Neurovirol. 2005;11(Supp 3):52–57. doi: 10.1080/13550280500513325. [DOI] [PubMed] [Google Scholar]
- 69.Bachis A, Avdoshina V, Zecca L, et al. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neuroscience. 2012;32(28):9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus – associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005;11:265–273. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- 71.Lescure FX, Omland LH, Engsig FN, et al. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin Infect Dis. 2011;52(2):235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- 72.Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmes MV, Desai M, Dosekun O, et al. Is the diagnosis at HAND? Lancet. 2010;375:1134. doi: 10.1016/S0140-6736(09)62101-2. [DOI] [PubMed] [Google Scholar]
- 74.Miller RF, Isaacson PG, Hall-Craggs M, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol. 2004;108:17–23. doi: 10.1007/s00401-004-0852-0. [DOI] [PubMed] [Google Scholar]
- 75.Oelschlage C, Dziewas R, Reichelt D, et al. Severe leukoencephalopathy with fulminant cerebral edema reflecting immune reconstitution inflammatory syndrome during HIV infection: a case report. J Med Case Reports. 2010;4:214. doi: 10.1186/1752-1947-4-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venkataramana A, Pardo CA, McArthur JC, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurol. 2006;67:383–388. doi: 10.1212/01.wnl.0000227922.22293.93. [DOI] [PubMed] [Google Scholar]
- 77.Pacifici M, Delbue S, Ferrante P, et al. Cerebrospinal fluid miRNA profile in HIV-encephalitis. J Cellul Physiol. 2013;228:1070–1075. doi: 10.1002/jcp.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gelman BB, Chen T, Lisinicchia JG, et al. The national neuroAIDS tissue consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24:284–290. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- 80.Johnson TP, Patel K, Johnson KR, et al. Induction of IL-17 and nonclassical T-cell activation by HIV_Tat protein. [Last accessed 3, August 2013];Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1308673110. Epub ahead of print available at http://www.pnas.org/content/early/2013/07/26/1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lescure FX, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57:101–108. doi: 10.1093/cid/cit175. [DOI] [PubMed] [Google Scholar]
- 82.Mayor AM, Fernandez Santos DM, Dworkin MS, et al. Toxoplasmic encephalitis in an AIDS cohort at Puerto Rico before and after highly active antiretroviral therapy. Am J Trop Med Hyg. 2011;84(5):838–841. doi: 10.4269/ajtmh.2011.10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tremont-Lukat IW, Garciarena P, Juarbe R, El-Abassi RN. The immune inflammatory reconstitution syndrome and central nervous system toxoplasmosis. Ann Intern Med. 2009;150(9):656–657. doi: 10.7326/0003-4819-150-9-200905050-00025. [DOI] [PubMed] [Google Scholar]
- 84.Tsambiras PE, Larkin JA, Houston SH. Case report. Toxoplasma encephalitis after initiation of HAART. AIDS Read. 2001;11(12):608–610. 615–616. [PubMed] [Google Scholar]
- 85. Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nature Reviews Microbiol. 2012;10:150–156. doi: 10.1038/nrmicro2712. This paper makes a significant conceptual contribution to the IRIS field by proposing a mechanism by which HIV-related and non-HIV-related IRIS might share similar pathogenic features.
- 86.Gheuens S, Smith DR, Wang X, et al. Simultaneous PML-IRIS after discontinuation of natalizumab in a patient with MS. Neurology. 2012;78:1390–1393. doi: 10.1212/WNL.0b013e318253d61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwab N, Hohn KG, Schneider-Hohendorf T, et al. Immunological and clinical consequences of treating a patient with natalizumab. Multiple Sclerosis Journal. 2012;18(3):335–344. doi: 10.1177/1352458511421919. [DOI] [PubMed] [Google Scholar]
- 88.Aly L, Yousef S, Schippling S, et al. Central role of JC virus-specific CD4+ lymphocytes in a progressive multifocal leukoencephalopathyimmune reconstitution inflammatory syndrome. Brain. 2011;134:2687–2702. doi: 10.1093/brain/awr206. [DOI] [PubMed] [Google Scholar]
- 89.Ermis U, Weis J, Schulz JB. PML in a patient treated with Fumaric Acid. New Engl J Med. 2013;368(17):1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- 90.Schwab N, Ulzheimer JC, Fox RJ, et al. Fatal PMJ associated with efalizumab therapy: Insights into integrin αLβ2 in JC virus control. Neurology. 2012;78:4258–4267. doi: 10.1212/WNL.0b013e3182478d4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47:e83–e85. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 92.Jorge JH, Graciela C, Pablo AP, Luis SH. A life-threatening central nervous system-tuberculosis inflammatory reaction nonresponsive to corticosteroids and successfully controlled by infliximab in a young patient with a variant of juvenile idiopathic arthritis. J Clin Rheumatol. 2012;18:189–191. doi: 10.1097/RHU.0b013e318258b725. [DOI] [PubMed] [Google Scholar]
- 93.Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756–1761. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 94.Legris T, Massad M, Purgus R, et al. Immune reconstitution inflammatory syndrome mimicking relapsing cryptococcal meningitis in a renal transplant recipient. Transpl Infect Dis. 2011;13:303–308. doi: 10.1111/j.1399-3062.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 95.Airas L, Paivarinta M, Roytta M, et al. Central nervous system immune reconstitution inflammatory syndrome (IRIS) after hematopoietic SCT. Bone Marrow Transplantation. 2010;45:593–596. doi: 10.1038/bmt.2009.186. [DOI] [PubMed] [Google Scholar]