Abstract
Humans typically consume “natural agents” that are believed to be chemoprotective and are known to decrease inflammation biomarker NF-κB in vitro; however, no intervention studies in humans have been done to date. This commentary documents the in vivo results as a powerful example for supporting the superiority of a complex mixture of natural agents. Human volunteers consumed two 500 mg capsules (BID) containing a mixture of natural agents for a period of 2 weeks, and blood samples were collected pre- and post-intervention. The purified lymphocytes were subjected to ex-vivo exposure to TNF-α or kept as untreated control. The mean NF-κB DNA binding activity was increased upon TNF-α treatment in pre-intervention samples; however, TNF-α was unable to induce NF-κB in post-intervention samples, suggesting that the mixture of four important natural agents could be useful to protect humans against oxidative stress.
Keywords: curcumin, epigallocatechin-3-gallate (EGCG), isoflavone, NF-κB, TNF-α, resveratrol
INTRODUCTION
Despite significant progress over the last 35 years to find a cure for cancer, this disease still remains a major public health problem, suggesting that prevention of cancer by employing multiple strategies could be the appropriate answer to solve this major public health issue. Moreover, because the incidence of cancer remains a major challenge for public health, chemoprevention by use of non-toxic dietary compounds is becoming a practical alternative for decreasing the prevalence of cancer. Many preclinical and clinical studies have demonstrated the beneficial effects of several powerful compounds, most of which function as pleiotropic agents, although one common target appears to be NF-κB, a transcription factor that plays important roles in the control of cell growth, differentiation, and apoptosis (1). Moreover, accumulating evidence from population-based and laboratory studies suggests that regular consumption of fruits and vegetables is inversely associated with the risk of certain malignancies (1,2). Previous studies from our laboratory have shown that soy isoflavone is a potent agent which can abrogate the TNF-α-induced activation of the DNA binding activity of NF-κB in lymphocytes of human volunteers (3) and that NF-κB is a legitimate target of natural agents as documented by many studies (1,4–6). Many studies have shown the beneficial effects of natural agents individually; however, to date, no such studies have attempted to show the biological activity of the combination of several biologically active natural agents. Since humans usually ingest a complex diet containing a mixture of several natural agents, such studies are warranted.
In our recent studies, we have shown that a specially formulated mixture of four active natural agents is biologically active, whereas the similar concentration of each independently had little or no activity in cell culture studies in vitro, suggesting that this combination could be superior compared to single agents for further studies (7). Therefore, a proof-of-concept pilot study was done to examine whether the consumption of our formulated 500-mg capsule containing curcumin (150 mg), resveratrol (75 mg), EGCG (150 mg), and soy isoflavone (125 mg) could protect human lymphocytes against oxidative stress induced by ex-vivo exposure of lymphocytes to TNF-α, which could be similar to those observed in our previous human intervention studies using soy isoflavone as a single agent (3). This commentary shows that in vivo effects of a complex mixture of natural agents can be demonstrated, and such human studies should be the future goal to advance the field in support of the use of natural agents chemoprevention-chemoprotection and other human applications.
STUDY DESIGN AND MAJOR FINDINGS IN SUPPORT OF SIMILAR FUTURE STUDIES IN HUMANS
Since human interventions studies using natural agents are lacking, we have done a pilot proof-of-concept study in 11 normal healthy adults: five males and six females who volunteered to participate. The median age of the 11 participants was 42 years (range 22–58). Their racial distribution was: six Caucasian, one Middle Eastern, and one Asian. The capsule used in this study was formulated by Dr. Fazlul Sarkar and was manufactured and supplied by Vitacost (Vitacost.com, Lexington, NC, USA). The capsule contained 150 mg curcumin (30%), 75 mg resveratrol (15%), 150 mg EGCG (30%) and 125 mg soybean extract (25%). Subjects were considered compliant if at least 80% of intended pills were consumed. After the study was completed, upon questioning, the volunteers experienced no side effects during the study period.
Blood samples were collected (two 8 mL cell preparation tubes (BD, Franklin Lakes, NJ, USA)) and processed immediately on day 1 (pre-intervention) and day 15 (post-intervention) of the study. Lymphocytes from the blood were isolated and cultured in RPMI 1640 medium supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin/streptomycin (Invitrogen) for 3 h to repair any damage and then divided into two parts. One was kept at 37°C without any treatment, and the other was treated with 50 ng/mL TNF-α for 20 min, followed by the preparation of nuclear protein extracts following standard procedures and was then subjected to DNA binding activity of NF-κB following our standard procedure (4). Simple spaghetti plots were used to graphically illustrate the individual subject’s NF-κB values under various experimental conditions. Descriptive statistics were used to summarize the NF-κB levels. These statistics included the mean and its 80% confidence interval (CI). The 80% confidence level is appropriate for a small pilot study. The statistic of primary interest in an intervention trial is the mean difference over time and pre-/post- intervention. Under each of three different experimental conditions, we computed the mean difference. Each distribution of those differences was highly statistically significantly non-normal, which would invalidate the paired t-test. No transformation could be found that would normalize the difference distribution for all three experimental conditions. Accordingly, the nonparametric Wilcoxon signed-rank test was used to test each of the three mean differences vs. zero. No correction for multiple comparisons was applied to the results, since this is a small pilot study aimed at generating preliminary data of potential value for designing a subsequent confirmatory trial.
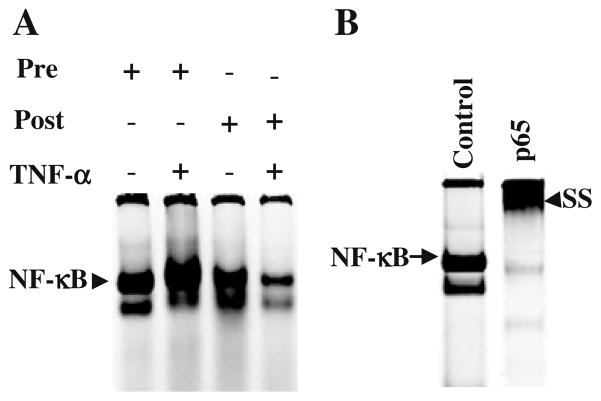
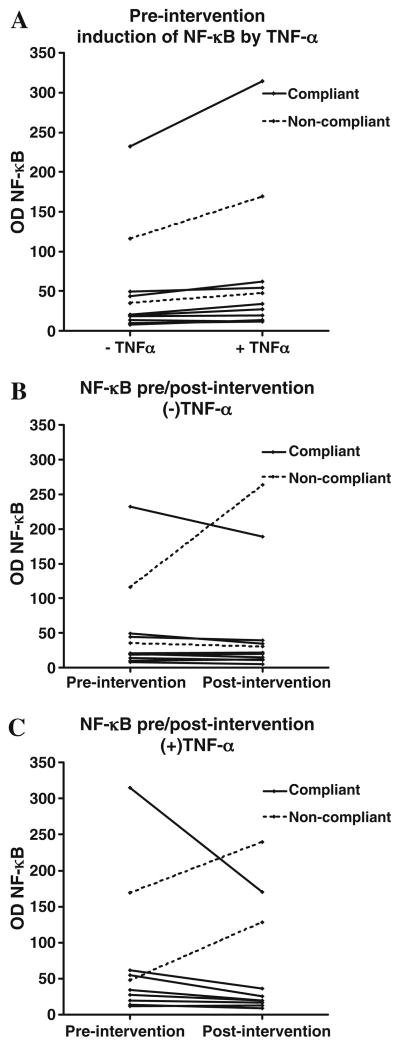
Our findings are shown in Fig. 1, where Fig. 1A represents a typical EMSA result of a compliant subject (samples were taken from subject 11). In this subject, similar to other compliant subjects, the DNA binding activity of NF-κB was increased by TNF-α treatment of pre-intervention lymphocytes relative to pre-intervention samples without TNF-α treatment. TNF-α failed to induce the DNA binding activity of NF-κB in post-intervention sample compared to the pre-intervention samples treated with TNF-α. Supershift (ss) assay was performed (Fig. 1B) using p65 antibody to verify the specificity of the DNA binding activity of NF-κB. As shown in Fig. 2A (where intervention compliance does not yet matter), TNF-α-induced activation of NF-κB among the two non-compliant subjects was comparable to that of the nine compliant subjects. Fig. 2B indicates that without TNF-α, NF-κB, levels stayed about the same over the two-week intervention period or decreased somewhat. One of the two non-compliant subjects showed an aberrant increase. However, with TNF-α present, NF-κB levels typically decreased among the compliant subjects, and both of the two non-compliant subjects showed an aberrant increase (Fig. 2C). Two of the compliant subjects did have a small increase in NF-κB levels.
Fig. 1.
(A) Typical example of EMSA showing induction of NF-κB by TNF-α and decreased DNA binding activity of NF-κB in post-intervention sample in both +/− TNF-α treatment group. (B) Supershift (ss) analysis with p65 Ab, which was performed using lymphocyte nuclear protein extracts for demonstrating the specificity of the band. Nuclear extract was pre-incubated with anti-phospho-p65 NF-κB-specific Ab for 30 min then ran on 8% polyacrylamide gel.
Fig. 2.
Line graphs showing change in DNA binding activity of NF-κB under various conditions.
The pre-intervention median and mean levels of NF-κB among the nine compliant subjects were higher after TNF-α was applied (Table I). The mean change was an increase of +14.76 arbitrary OD units which was statistically significantly different from zero (p =0.0195). From the CI, we can be 80% confident that the true mean change in NF-κB was at least +2.59 OD units and at most +26.94 OD units. Our findings revealed that without TNF-α, NF-κB levels were only slightly reduced among the nine compliant subjects and that the mean change of −7.68 OD units was not quite significantly different from zero (p=0.0547). Most importantly, in the presence of TNF-α, NF-κB levels decreased significantly (p=0.0195). The mean change was a decrease of −25.36 arbitrary OD units. We can be 80% confident that the true mean change in NF-κB was a reduction of at least −4.06 OD units and at most −46.65 OD units.
Table 1.
NF-κB Descriptive Statistics for the 9 Intervention Compliant Subjects
| Time point | Median | Mean | 80% CI lower limit for the mean | 80% CI upper limit for the mean | SD | Min. | Max. | |
|---|---|---|---|---|---|---|---|---|
| Pre-intevention | (−) TNF-α | 19.39 | 46.32 | 13.21 | 79.43 | 71.21 | 8.14 | 232.04 |
| (+) TNF-α | 27.49 | 61.08 | 16.03 | 106.14 | 96.76 | 11.80 | 314.51 | |
| Differencea | 5.82 | 14.76* | 2.59 | 26.94 | 26.15 | −1.89 | 82.47 | |
| (−) TNF-α | Pre | 19.39 | 46.32 | 13.21 | 79.43 | 71.12 | 8.14 | 232.04 |
| Post | 19.41 | 38.64 | 11.86 | 65.42 | 57.52 | 5.23 | 189.08 | |
| Differenceb | −3.46 | −7.68** | −14.26 | −1.09 | 14.15 | −42.96 | 1.74 | |
| (+)TNF-α | Pre | 27.49 | 61.08 | 16.03 | 106.14 | 96.76 | 11.80 | 314.51 |
| Post | 19.32 | 35.73 | 11.87 | 59.59 | 51.25 | 8.49 | 170.57 | |
| Differencec | −7.60 | −25.36* | −46.65 | −4.06 | 45.74 | −143.94 | 0.76 |
P =.0195;
P =.0547. Units for all statistics in table are arbitrary units of optical density (OD).
CI = confidence interval (for the true mean).
Difference* = (After TNF-α - Before TNF-α);
Difference** = (Post-intervention - Pre-intervention);
Difference* = (Postintervention - Pre-intervention).
GENERAL DISCUSSION AND THE IMPLICATIONS OF THE CURRENT FINDINGS
This pilot study clearly provides support in favor of a complex mixture of natural agents as a supplement to protect humans against oxidative stress. Since inflammation and oxidative stress are believed to be intimately associated with the etiology of cancer (8), anti-oxidants may be useful for the prevention of cancer. However, this field is highly controversial, and there is no direct evidence showing that anti-oxidant use for a long period of time could prevent the development of cancer in humans. There are many cellular markers that are associated with inflammation and oxidative stress, among which the activation of NF-κB transcription factor is considered the most important. It is involved in the regulation of many downstream genes that are known to control inflammation, cell growth, apoptosis, and cytokine response (9). Although the exact mechanism by which chemoprotection takes place remains unclear, it has been suggested that inhibiting the expression of critical pro-inflammatory cytokines, such as TNF-α, could be a potent means by which one could inhibit the activation of NF-κB. Thus, decreasing inflammation in human lymphocytes and other normal organs could provide a protective role against the development of cancer and other chronic diseases. Our previous studies have shown that supplementation with soy isoflavone abrogates NF-κB activation by TNF-α in human lymphocytes (3). Similar studies have also shown that curcumin, resveratrol, and EGCG, all could individually decrease the activation of NF-κB induced by TNF-α (9), suggesting that the combination of isoflavones, curcumin, resveratrol, and EGCG could be useful for protecting the normal functioning of individuals against inflammation and oxidative stress.
Moreover, we have also demonstrated by in vitro studies that combining chemotherapeutic agents with genistein, a major isoflavone from soybeans, could reduce the required dose of chemotherapeutic agents while still achieving better anticancer outcomes in different types of cancers, such as breast, prostate, pancreatic, and lung cancers (5). These results collectively suggest that supplementation with mixtures of natural agents could improve anti-cancer therapies as well as offer chemoprotection to healthy human lymphocytes in vivo.
Taken together, oxidants can trigger inflammatory cell signaling pathways, such as the activation of NF-κB, which may have adverse general health effects if such conditions are not resolved and may lead to the development of chronic diseases, including cancer. Our present study suggests that regular (albeit short-term) dietary consumption of capsules containing curcumin, isoflavones, resveratrol, and EGCG may exert chemoprotective effects on human lymphocytes or even in other normal tissues by inhibiting pro-oxidant-induced activation of NF-κB (Fig. 2C). Although further in-depth clinical trials are needed, we speculate that by consuming these capsules, one may experience a decrease in free-radical-induced DNA damage, hydrogen peroxide, nitric oxide, and oxidative stress. Thus, these compounds may be able to increase the activity and/or expression of anti-oxidative enzymes, such as glutathione S-transferase (GST), glutathione perioxidase (GPx), NADPH:quinone oxidoreductase (NQO), heme-oxygenase-1 (HO-1), and UDP glucuronosyl transferase (UGT-1A), and thus confer protection against oxidative stress, and may also protect against inflammation, which awaits further investigations, either with this approach or with mixtures of many other natural agents. Overall, our results are consistent with published studies of individual natural agents, suggesting that these polyphenols could function as potential chemoprotective agents by protecting cells from inflammation-induced activation of NF-κB. However, further clinical studies are warranted in order to fully elucidate the mechanisms of chemoprotection of these compounds, especially our formulated capsule, for the maintenance of normal health status.
CONCLUSION
Based on our results, we conclude that our formulated capsule containing a mixture of four important natural agents was well tolerated and could be useful to protect human lymphocytes from oxidative stress, as demonstrated by the disabling of TNF-α-induced activation of the DNA binding activity of NF-κB. Our previous in vitro studies have shown that the combination treatment with these agents was more potent than single agent alone (7); however, no such studies have been documented in humans in vivo. Therefore, our current findings represent the first in vivo evidence in humans showing the beneficial effects of this capsule containing four natural agents that indeed represent a human situation in which humans consume complex foods containing a complex mixture of compounds. This proof-of-concept study represents an example which should guide future studies using complex mixtures of natural agents for prevention and/or treatment of human malignancies.
Footnotes
DISCLOSURE STATEMENT
There is no potential conflict of interest.
Contributor Information
Kristin Dominiak, Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan, USA.
Jaclyn McKinney, Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan, USA.
Lance K. Heilbrun, Department of Internal Medicine, Wayne State University School of Medicine, Detroit, Michigan, USA
Fazlul H. Sarkar, Email: fsarkar@med.wayne.edu, Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan, USA. Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, 740 Hudson Webber Cancer Research Center, 4100 John R, Detroit, Michigan 48201, USA
References
- 1.Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–9. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 2.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 3.Davis JN, Kucuk O, Djuric Z, Sarkar FH. Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radic Biol Med. 2001;30:1293–302. doi: 10.1016/s0891-5849(01)00535-4. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Zhang Y, Wang Z, Che M, Chiao PJ, Abbruzzese JL, et al. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int J Cancer. 2007;120:906–17. doi: 10.1002/ijc.22332. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–42. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 6.El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res. 2006;66:10553–9. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Desmoulin S, Banerjee S, Kong D, Li Y, Deraniyagala RL, et al. Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci. 2008;83:293–300. doi: 10.1016/j.lfs.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]