Abstract
While the chemotherapeutic effect of curcumin, one of three major curcuminoids derived from turmeric, has been reported, largely unexplored are the effects of complex turmeric extracts more analogous to traditional medicinal preparations, as well as the relative importance of the three curcuminoids and their metabolites as anti-cancer agents. These studies document the pharmacodynamic effects of chemically-complex turmeric extracts relative to curcuminoids on human breast cancer cell growth and tumor cell secretion of parathyroid hormone-related protein (PTHrP), an important driver of cancer bone metastasis. Finally, relative effects of structurally-related metabolites of curcuminoids were assessed on the same endpoints. We report that 3 curcuminoid-containing turmeric extracts differing with respect to the inclusion of additional naturally occurring chemicals (essential oils and/or polar compounds) were equipotent in inhibiting human breast cancer MDA-MB-231 cell growth (IC50=10–16μg/mL) and secretion of osteolytic PTHrP (IC50=2–3μg/mL) when concentrations were normalized to curcuminoid content. Moreover, these effects were curcuminoid-specific, as botanically-related gingerol containing extracts had no effect. While curcumin and bis-demethoxycurcumin were equipotent to each other and to the naturally occurring curcuminoid mixture (IC50=58 μM), demethoxycurcumin was without effect on cell growth. However, each of the individual curcuminoids inhibited PTHrP secretion (IC50=22–31μM) to the same degree as the curcuminoid mixture (IC50=16 μM). Degradative curcuminoid metabolites (vanillin and ferulic acid) did not inhibit cell growth or PTHrP, while reduced metabolites (tetrahydrocurcuminoids) had inhibitory effects on cell growth and PTHrP secretion but only at concentrations ≥10-fold higher than the curcuminoids. These studies emphasize the structural and biological importance of curcuminoids in the anti-breast cancer effects of turmeric and contradict recent assertions that certain of the curcuminoid metabolites studied here mediate these anti-cancer effects.
Keywords: curcumin, breast, cancer, curcuminoid, ginger, PTHrP, ferulic acid, tetrahydrocurcuminoids
Introduction
Breast cancer is the most common cancer among women worldwide [1]. However, the incidence of breast cancer in India is among the lowest in the world [2]. While the reasons for this are likely multifactorial, it is interesting to note that India also has the highest worldwide consumption of turmeric, a primary source of dietary curcumin. Curcumin has been well described in numerous in vitro studies to inhibit the growth of breast cancer cells [3]. Also, a separate beneficial effect of turmeric-derived curcuminoids in breast cancer, independent of effects on cell growth, has recently been identified in our laboratories; in an experimental model of breast cancer bone metastases, curcuminoids inhibited the development of osteolyic bone lesions, which occur in a majority of women with advanced metastatic disease [4], by inhibiting breast cancer cell secretion of osteolytic factors (e.g., parathyroid hormone-related hormone [PTHrP]) [5].
Turmeric has a rich history of medicinal use in Ayurveda, a Hindu system of traditional medicine native to India [6]. While chemically complex turmeric preparations are used ethnobotanically, purified curcumin(oid)-only products are the most common form of turmeric studied medicinally or sold as dietary supplements in western countries [7]. However, medicinal and/or health promoting effects of curcumin or turmeric’s naturally occurring mixture of curcuminoids (curcumin, demethoxycurcumin, and bis-demethoxycurcumin) have been called into question because serum levels are low when curcumin(oids) are administered orally in purified forms [8]. To resolve this apparent discrepancy between turmeric bioactivity and curcuminoid bioavailability, it has been proposed that curcuminoid metabolites, including degradative or reduced products, may be the bioactive moieties responsible for mediating curcumin(oid) effects [9]. Alternatively, or additionally, it is also possible that chemically complex (vs. curcuminoid-only) turmeric products may have differential pharmacodynamic and/or pharmacokinetic profiles that would explain/support their ethnobotanical use despite the low bioavailability documented for purified nontraditional, curcuminoid-only products. For example, emerging evidence from our laboratories and others have demonstrated independent bioactivity of another class of turmeric secondary metabolites, the essential oils [10], as well as enhanced oral curcuminoid bioavailability when administered in combination with the oils [11]. Thus, examination of possible roles of curcuminoid metabolites and/or curcuminoid interactions with other turmeric-derived compounds in mediating turmeric bioactivity is a viable research question with particular relevance to modern vs. traditional uses of turmeric for the purpose of limiting breast cancer occurrence or progression.
Studies reported here were undertaken to investigate two lines of inquiry related to turmeric use in breast cancer, examining (1) the relative pharmacodynamic effects of chemically complex vs. curcuminoid-only turmeric extracts on breast cancer cell growth and the secretion of osteolytic factors (PTHrP) important for breast cancer bone metastases progression, and (2) the relative pharmacodynamic effects of curcuminoids vs. their metabolites or other structurally- or botanically-related compounds on these same endpoints. These systematic studies were undertaken using human MDA-MB-231 cells, a frequently studied human breast cancer cell line that is triple negative for the estrogen, progesterone and EGF type 2 (HER2) receptors [12], thus representing an aggressive breast cancer phenotype [13], in order to query the importance of curcuminoids in mediating turmeric’s protective effects in breast cancer, as well as to assess structural features of the curcuminoids required for bioactivity in this common female malignancy.
Materials and Methods
Extract isolation and chemical analyses
Dried powdered rhizomes of turmeric (Curcuma longa L.) and ginger (Zingiber officinale Roscoe) were purchased from San Francisco Herb and Natural Food (San Francisco, CA) and experimental extracts were prepared and analyzed by HPLC for phenolic content as previously described [7,14,15]. Briefly, dried powdered turmeric rhizome was extracted with 1) methanol to prepare a crude turmeric extract containing curcuminoids, curcuminoid-related compounds, polar compounds, and essential oils (“crude turmeric,” 9.6% yield, 34% curcuminoids); 2) hexane to prepare an essential oil-only fraction (“turmeric essential oils”, 3.7% yield) or 3) the hexane marc was extracted with methanol to prepare an essential oil-free fraction containing curcuminoids, curcuminoid-related compounds and polar compounds (“curcuminoid fraction”, 3% yield; 52% curcuminoids, 28% polar compounds). A commercial curcuminoid-enriched essential oil-free product analogous in composition to currently available turmeric dietary supplements [16] was purchased from Fisher Scientific (#218580100, Lot A019754401; “curcuminoids” [76.9% curcumin, 17.6% demethoxycurcumin, 5.5% bisdemethoxycurcumin]). A crude ginger extract containing gingerols, gingerol-related compounds, polar compounds and essential oils (“crude ginger,” 6.4% yield) was prepared by extracting the dried ground ginger rhizome powder with dichloromethane (DCM). The resultant DCM extract was subjected to silica gel chromatography to yield 3 fractions: a fraction containing gingerols and their derivatives (“gingerol fraction,” 50% yield), a fraction containing only the essential oils (“ginger essential oils,” 22% yield) and a fraction containing polar compounds (30% yield, not tested here). Content of major phenolic compounds in extracts derived from dried ground rhizomes of turmeric (curcumin, demethoxycurcumin, and bis-demethoxycurcumin) and ginger (6-, 8-, and 10-gingerol) were determined by HPLC analysis as previously described [7,14]. Curcumin (#03926), demethoxycurcumin (#04230), bis-demethoxycurcumin (#04231), 6-gingerol (#07164), ferulic acid (#06005), and vanillin (#22305) were purchased from ChromaDex (≥ 92% pure). Tetrahydrocurcuminoids, manufactured by reduction of the naturally occurring mixture of curcuminoids present in turmeric (SBX0069; ≥96% pure), were a kind gift from Sabinsa Corporation. Stock solutions of experimental and commercial products were prepared using DMSO (100 – 300 mg/mL) and stored at 4 °C. Stability of curcuminoid containing stock solutions and/or extracts was verified at 12-month intervals by HPLC.
Cell cultures
Human breast cancer MDA-MB-231 cells (a kind gift from Dr. Theresa Guise, Indiana University), one of the most frequently studied estrogen-receptor negative human breast cancer cell lines, were used for these studies. To authenticate these cells, genomic DNA was genotyped for 9 Autosomal STR loci and Amelogenin (X/Y) using Promega Stem Elite PCR kit as per the manufacturer’s recommended cycling conditions with separation of PCR products by capillary electrophoresis on an AB 3730 DNA Analyzer. Positive and negative control samples and manufacturer’s allelic ladder were used to ensure allelic call calibrations. Electropherograms were analyzed and allelic values assigned using Soft Genetics, Gene Marker software version 1.85. Alleles were matched to STR Profile recorded with ATCC thus confirming the identity of the human breast cancer cell line MDA-MB-231 used here. For analysis of botanical effects on cell viability, cytotoxicity or PTHrP secretion, subconfluent MDA-MB-231 cells were plated at 5 × 104 cells/cm2 and incubated overnight in fresh DMEM medium containing 10% fetal bovine serum and antibiotics at 37°C and 5% CO2 in a humidified atmosphere. Cells were pre-incubated with indicated botanical treatments for 4 h and media was then refreshed with the addition of recombinant human TGF-β1 (5 ng/mL; R&D Systems) for 24 h to stimulate PTHrP secretion. Parathyroid hormone-related protein (PTHrP) was measured in conditioned media after 24 h by commercial radioimmunoassay (Diagnostic Systems Laboratories). Effects on cell viability and cytotoxicity were measured by assay of cell number assessed by mitochondrial reduction of MTT (ATCC) and conditioned media content of lactate dehydrogenase (LDH; Promega), respectively, as per manufacturer’s protocol. Treatment concentrations of phenol-containing turmeric and ginger extracts were normalized to, and expressed as, total content (μg/mL) of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) or gingerols (6-, 8- and 10-gingerol), respectively, while pure essential oils are expressed by weight (μg oil/mL).
Statistical analyses
Data are expressed as mean ± SEM. Half maximal inhibitory concentrations (IC50) were obtained by analyzing concentration-response data using a four-parameter logistic equation, assigning a maximum value of 100% and a minimum value of > 0% (Prism 4.0 software, GraphPad, San Diego, CA). Statistically significant differences between IC50 values were determined by F test with p < 0.05 (Prism 4.0 software, GraphPad). Where indicated, differences between means were determined by one-way ANOVA with Student Newman Keuls post hoc test using Instat 3.0 software (Graphpad).
Results
Effects of multicomponent turmeric extracts on human breast cancer cell viability and PTHrP secretion
Chemically complex (crude extract or essential oil-free curcuminoid fraction) vs. purified curcuminoid-only turmeric extracts, when normalized to curcuminoid content, were statistically equipotent in decreasing human breast cancer MDA-MB-231 cell viability, with IC50 values of 10–16 μg curcuminoids/mL (Figure 1A). Isolated turmeric essential oils also decreased cell viability, but were less potent (Figure 1A). Chemically complex vs. curcuminoid-only extracts were equipotent inhibitors of osteolytic PTHrP secretion when normalized to curcuminoid content (IC50 = 2–3 μg/mL) (Figure 1B). Isolated essential oils actually increased PTHrP secretion, a potentially adverse biological effect (Figure 1B). However, the essential oil content of the crude turmeric extract did not appear to mitigate the beneficial PTHrP inhibitory effects of the curcuminoids since crude extract potency was statistically no different from that of curcuminoid extracts lacking essential oils (essential-oil free curcuminoid fraction or purified curcuminoids) (Figure 1B). To examine this further, PTHrP inhibitory effects of purified curcuminoids vs. turmeric essential oils were tested alone and in combination (Figure 1C). At the doses tested (5 μg curcuminoids or oil/mL), the PTHrP inhibitory effect of the curcuminoids was not blocked by addition of the oils; indeed, while similar in magnitude to the effects of curcuminoids, PTHrP inhibition by combined treatment was actually statistically greater than that of the curcuminoids alone (Figure 1C). Curcuminoid inhibition of cell viability, as determined by MTT assay, occurred in parallel with cytotoxicity, as determined by assay of LDH content of conditioned media (Figure 1D).
Figure 1.
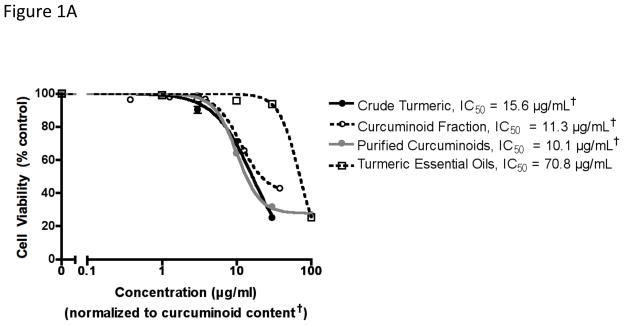
Effect of chemically complex turmeric extracts and fractions on human breast cancer MDA-MB-231 cell viability, cytotoxicity and secretion of osteolytic PTHrP. As indicated in the methods, MDA-MB-231 cells (n = 4–8/group) were treated with botanicals for 4 hours prior to stimulation with TGFβ for 24 hours, after which time (A) cell viability was assessed by MTT assay and (B) PTHrP secretion was determined by radioimmunometric assay of conditioned media. Concentrations of all curcuminoid-containing extracts were normalized to curcuminoid content (†). Half maximal inhibitory concentrations (IC50) were obtained using a four-parameter logistic equation with statistically significant differences between IC50 values determined by F test with p < 0.05. (C) Effects of purified curcuminoids (5 μg/ml) and turmeric essential oils (5 μg/ml) alone or in combination on PTHrP secretion (n = 4/group), and (D) effects of purified curcuminoids on cell viability (MTT assay) vs. cytotoxicity (LDH content of media) (n = 4/group) were compared with statistically significant effects determined by ANOVA with post-hoc testing. *p < 0.05 vs. control. ***p < 0.001 vs control.
Effects of turmeric’s phenolic and essential oil components on breast cancer cell viability and secretion of osteolytic PTHrP were compared to those of crude and similarly fractionated extracts prepared from rhizomes of ginger, a related plant of the Zingiberaceae family also known for its medicinal phenolics (gingerols) and essential oils [15]. The crude ginger extract (normalized to gingerol content) containing phenols, essential oils and polar compounds, as well as the essential oils alone, decreased cell viability with potencies greater than that of the crude turmeric extract (IC50 = 4 μg/mL) (Figure 2A). However, in contrast to the equipotent growth inhibitory effect of all curcuminoid-containing turmeric extracts, ginger’s phenolic fraction had no effect on breast cancer cell viability when administered in isolation (Figure 2A). While turmeric’s curcuminoids almost completely inhibited PTHrP secretion at doses as low as 10 μg/mL (Figure 1B), similar doses of ginger-derived phenolics were without effect (Figure 2B). Ginger essential oils, analogous to turmeric essential oils, increased PTHrP secretion (Figure 2B; p < 0.001 for 30 μg/mL).
Figure 2.

Effect of chemically complex ginger extracts and fractions on human breast cancer MDA-MB-231 cell viability and secretion of osteolytic PTHrP. As indicated in the methods, MDA-MB-231 cells (n = 4–8/group) were treated with botanicals for 4 hours prior to stimulation with TGFβ for 24 hours, after which time (A) cell viability was assessed by MTT assay and (B) PTHrP secretion was determined by radioimmunometric assay of conditioned media. Concentrations of all gingerol-containing extracts were normalized to gingerol content (†). Half maximal inhibitory concentrations (IC50) were obtained using a four-parameter logistic equation with statistically significant differences between IC50 values determined by F test with p < 0.05.
Effects of curcumin, demethoxycurcumin and bis-demethoxycurcumin vs. naturally occurring mixture of curcuminoids on breast cancer cell viability and secretion of osteolytic PTHrP
Because beneficial effects of chemically complex turmeric extracts on human MDA-MB-231 breast cancer cells, with respect to both cell viability and the secretion of osteolytic factors, appeared to be associated with curcuminoid content, the relative potency of purified curcumin, demethoxycurcumin or bis-demethoxycurcumin vs. the naturally occurring curcuminoid mixture on breast cancer cell viability and secretion of osteolytic PTHrP were determined. Curcumin and bis-demethoxycurcumin, typically the most and least abundant of turmeric’s curcuminoids, respectively [7], were statistically equipotent to the naturally occurring curcuminoid mixture in decreasing cell viability, while demethoxycurcumin was without any statistically significant effect (Figure 3A). In contrast to the differential effects of curcumin and bis-demethoxycurcumin vs. demethoxycurcumin on breast cancer cell viability, each of the individual curcuminoids inhibited PTHrP secretion (IC50 = 22–31 μM) and was statistically equipotent to the naturally occurring mixture of curcuminoids (Figure 3B).
Figure 3.
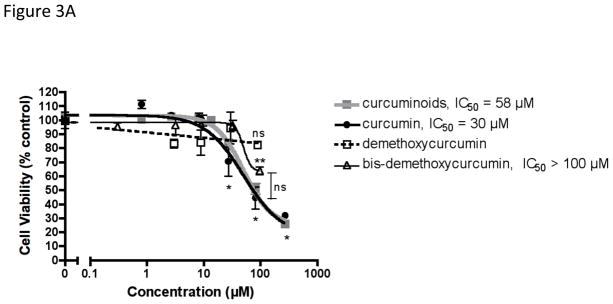

Comparative effects of curcuminoids, curcumin, demethoxycurcumin and bis-demethoxycurcumin on human breast cancer MDA-MB-231 cell viability and secretion of osteolytic PTHrP. MDA-MB-231 cells (n = 8–20/group) were treated with botanicals for 4 hours prior to stimulation with TGFβ for 24 hours, after which time (A) cell viability was assessed by MTT assay and (B) PTHrP secretion was determined by radioimmunometric assay of conditioned media. Half maximal inhibitory concentrations (IC50) were obtained using a four-parameter logistic equation with statistically significant differences between IC50 values determined by F test with p < 0.05. Statistically significant effects of each botanical (vs. controls or each other) were also determined by ANOVA with post hoc testing. * p < 0.001 (vs. control) for curcumin or curcuminoids. ** p < 0.001 (vs. control) for bis-demethoxycurcumin.
Effects of curcuminoid metabolites and related compounds on breast cancer cell viability and secretion of osteolytic PTHrP
The importance of structural elements of the curcuminoids, in addition to their differing phenolic methoxy-group substitutions, to their bioactivity in human breast cancer cells were further explored by comparison of curcuminoids with structurally related natural products (e.g., 6-gingerol), including reduced (tetrahydrocurcuminoids) and degradative (vanillin and ferulic acid) metabolites of the curcuminoids that have been reported to be produced in vitro and/or in vivo (Figure 4) [8,12,17–22]. None of the structurally related compounds altered breast cancer cell viability except for a minor inhibitory effect of the tetrahydrocurcuminoids at the highest concentration tested (300 μM) (Figure 5A); in contrast, this same concentration of curcuminoids (300 μM) caused the death of almost 80% of the breast cancer cells (Figure 5A and Figure 1D). With respect to inhibition of osteolytic PTHrP secretion, the degradative metabolites of the curcuminoids, vanillin and ferulic acid, were also without effect (Figure 5B); indeed, high dose ferulic acid actually stimulated PTHrP secretion, a potentially adverse biological effect. Tetrahydrocurcuminoids, a reductive metabolite of the curcuminoids, did significantly inhibit PTHrP secretion, but with a 10-fold lower potency (Figure 5B). Similarly, 6-gingerol, while inhibiting PTHrP secretion, was 10-fold less potent than the curcuminoids (IC50 = 133 μM vs. 16 μM); indeed, while a curcuminoid concentration of 100 μM completely inhibited PTHrP secretion, a similar dose of 6-gingerol was without any statistically significant effect (Figure 5B).
Figure 4.
Chemical structures of curcumin and related compounds tested.
Figure 5.
Comparative effects of curcuminoids, tetrahydrocurcuminoids, ferulic acid, vanillin, and 6-gingerol on human breast cancer MDA-MB-231 cell viability and secretion of osteolytic PTHrP. MDA-MB-231 cells (n = 8–16 per group) were treated with botanicals for 4 hours prior to stimulation with TGFβ for 24 hours, after which time (A) cell viability was assessed by MTT assay and (B) PTHrP secretion was determined by radioimmunometric assay of conditioned media. Half maximal inhibitory concentrations (IC50) were obtained using a four-parameter logistic equation with statistically significant differences between IC50 values determined by F test with p < 0.05. For each compound, statistically significant effects (vs. control) were determined by ANOVA with post-hoc testing. *p < 0.05. ** p < 0.01. ***p < 0.001.
Discussion
A large body of literature has documented beneficial effects of curcumin, one of three major curcuminoids derived from the medicinal turmeric rhizome, on cancer cell growth in vitro, including breast cancer [3,23]. Largely unexplored, however, have been the effects and bioactive components of chemically complex turmeric extracts that more closely model human exposure associated with culinary or traditional medicinal use of turmeric. Results from the studies presented here clearly demonstrate the unique ability of turmeric-derived phenolic compounds to inhibit breast cancer cell growth and the secretion of important osteolytic factors that drive breast cancer bone metastases in advanced disease [4]. These protective effects of turmeric’s phenolic compounds were specific to the curcuminoids as, for example, botanically and chemically related phenols isolated from ginger were without effect within a similar concentration range. The importance of studying the bioactivity of clinically-relevant, chemically-complex botanical extracts is underscored by the discovery that the secondary oils of turmeric (and of ginger) could have adverse effects in breast cancer, as the essential oils actually enhanced breast cancer cell secretion of osteolytic factors. Fortunately, however, in the case of chemically complex turmeric use, when turmeric’s essential oils were combined with the curcuminoids, the beneficial effect of the phenolic compounds was retained. Also of interest vis à vis the study of chemically complex botanicals, while the phenolics in turmeric were the most potent bioactive moieties with respect to breast cancer growth inhibition, the most potent secondary metabolites in ginger extracts were, instead, the essential oils.
The relative potency of the curcuminoids when used in combination or isolation to treat human breast cancer cells differed for each of the two investigated endpoints—cell growth vs. secretion of osteolytic PTHrP. Growth inhibitory effects of the curcuminoids in breast cancer have previously been attributed to their targeting of cell cycle and/or apoptotic cellular pathways [24–26]; our demonstration of equipotent effects of the curcuminoids on cell viability, as measured by MTT assay, and cytotoxicity, as measured by release of LDH into the media, is consistent with a pro-apoptotic, rather than anti-proliferative effect. Interestingly, demethoxycurcumin was less potent than the two other naturally occurring curcuminoids in blocking breast cancer cell viability. In contrast, all three curcuminoids were equipotent in blocking PTHrP secretion, having IC50 values that were ≥ 5-fold lower than those required to induce cell death. It has been reported that bisdemethoxycurcumin is the most stable of the curcuminoids [27] and that the stability of curcumin is enhanced in the presence of demethoxycurcumin [28]. However, because bisdemethoxycurcumin, curcumin and the naturally occurring mixture of curcuminoids were equipotent in inhibiting cell viability and blocking PTHrP secretion, this suggests that any potential differences in their relative stabilities did not alter bioactivity. Similarly, the lack of effect of demethoxycurcumin on cell viability was not likely due to a decrease in stability when administered in isolation since this compound remained equipotent to the other curcuminoids when blocking PTHrP secretion.
While not investigated here, because our previous studies have identified Smad-mediated transforming growth factor (TGF)-β signaling as the cellular pathway targeted by curcuminoids when inhibiting PTHrP secretion in human breast cancer MDA-MB-231 cells [5], the demonstration in these studies of equipotent effects of each of the curcuminoids in blocking PTHrP secretion suggests that each of these three compounds may have similar efficacy in blocking Smad-mediated TGF-β signaling. TGF-β signaling induces PTHrP secretion independent of any stimulatory effects on cell growth rate; indeed, prolonged treatment with TGF-β at high concentrations decreases MDA-MB-231 cell number (29). Therefore, the inhibitory effects of curcuminoids on cell viability cannot be attributed to blockade of Smad-mediated TGF-β, but rather is likely due to targeting of a second, non- TGF-β pathway regulating cell death. The less potent effects of the curcuminoids in blocking cell viability (via PTHrP secretion) as well as the differential effects of the individual curcuminoids in limiting cancer cell viability (vs. their equipotent effects in blocking PTHrP secretion) suggests that: 1) the structural elements of the curcuminoids required for their bioactivity vary with the biological target, and that 2) curcuminoids have multiple biological targets within a given cell [23]. Evidence from other studies supports this conclusion, as, for example, curcumin has been reported to be the least effective of the curcuminoids in blocking MMP-3 secretion from the same cell line used here [30]. With regard to anti-oxidative properties, curcumin has typically been the most potent of the three curcuminoids when tested head-to-head [22,31,32], a phenomenon that has been attributed to the functional importance of its two methoxy groups (compared to one for demethoxycurcumin and none for bis-demethoxycurcumin). Cyclooxygenase-2 (COX-2) inhibition, another much studied function of curcumin, has also been attributed to the 2-methoxy phenolic structure [33]. Consistent with our studies, however, many anti-cancer studies have described demethoxycurcumin or bis-demethoxycurcumin as equally and sometimes more potent than curcumin [27,34,35], indicating that the methoxy moieties are not conferring bioactivity in these cases. From a practical standpoint, given that a majority of turmeric dietary supplements available for clinical use or research are comprised of the naturally occurring mixture of curcuminoids [7], our demonstration of equal or greater potency of the mixture vs. single compounds justifies continued testing of curcuminoids as a mixture for their chemotherapeutic potential in breast cancer.
Given the low circulating levels of curcuminoids in vivo, as well as the diverse documented effects of curcuminoids in biological systems, a potential role for known curcuminoid metabolites in mediating their bioactivity has been postulated [9,36]. Among these discussions, a postulate has been put forth that ferulic acid and vanillin, both degradative products of the curcuminoids, are responsible for the biological effects of the curcuminoids, including their anti-tumor effects [9]. While it may be possible that other metabolites of curcuminoids are bioactive, evidence presented here clearly demonstrates that the degradative metabolites, ferulic acid and vanillin, are not able to inhibit breast cancer cell growth or the secretion of osteolytic PTHrP. Indeed, ferulic acid at high doses acted in opposite fashion as compared to curcuminoids and stimulated, rather than suppressed, breast cancer cell PTHrP secretion. Consistent with our findings, vanillin and ferulic acid, even at high concentrations, have been demonstrated by others to have minimal effect on other known curcuminoid targets, including cyclooxygenase enzyme and NF-κB [37–40], suggesting that these metabolites are not mediating curcuminoid biological effects. What is more, vanillin and ferulic acid have not been identified as abundant degradation products of curcuminoids in vitro [19,41,42] and are not reported to be major metabolites in vivo [21,22], casting further doubt on the postulate that vanillin and ferulic acid are responsible for the bioactivity of curcuminoids. Thus, results reported here using MDA-MB-231 cells as a breast cancer model system suggest that turmeric-derived curcuminoids possess potent anti-cancer activity, while certain other structurally related compounds, such as ferulic acid, a major phenolic compound that is also found in the cell walls of important dietary monocotyledons (e.g., rice, wheat, maize) [43,44], do not.
The absence of an inhibitory effect of vanillin and ferulic acid, both of which lack the curcuminoids’ heptadienone chain, on MDA-MB-231 cell viability and PTHrP secretion suggest that this structural element may be important for curcuminoid bioactivity in breast cancer. Consistent with this hypothesis, loss of this structural element when curcuminoids are reduced to form tetrahydrocurcuminoids, a biotransformation that occurs in vitro and in vivo [21,22], was associated with a 10-fold decrease in potency with respect to inhibition of PTHrP secretion and a > 10- fold loss of potency with respect to inhibitory effects on cell growth. Tetrahydrocurcuminoids have similarly been demonstrated by others to be less potent than the curcuminoids as an anti-tumor agents [45–47], while retaining anti-oxidative properties relative to their parent compounds [32,47,48], likely due to the common presence of hydroxyl moieties [31,46]. While the exact functional significance of the curcuminoid heptadienone chain in breast cancer was unstudied here, previous studies have reported anti-cancer bioactivity of curcumin analogues that retain this Michael acceptor dienone moiety [49]. The heptadienone chain has recently been reported by Schneider et al [41,42] to be a required element for the autooxidation of curcumin, an alternative metabolic pathway not evaluated here [41]. Thus future studies are needed to assess a potential role of oxidative metabolites in mediating the anticancer effects of the curcuminoids reported here.
Interestingly, while gingerols present in complex ginger extracts were without effect at clinically relevant concentrations in targeting breast cancer cells, higher doses of purified 6-gingerol were equipotent to the tetrahydrocurcuminoids in blocking PTHrP secretion (and similarly ineffective in inhibiting cell growth). This suggests that the complete lack of activity of the mono-phenolic degradative metabolites of the curcuminoids (ferulic acid and vanillin) cannot simply be attributed to the absence of the second phenolic group. Clearly, however, these findings again point to turmeric-derived curcuminoids as the most potent of a series of botanically-and structurally-related naturally-occurring phenolics with respect to anti-breast cancer activity.
Conclusion
Turmeric has a rich history of medicinal use. Our findings demonstrated that polyphenolic curcuminoids were responsible for the anti-breast cancer activity of chemically complex turmeric extracts, and that a naturally-occurring mixture of the three curcuminoids was as potent as the individual curcuminoids in inhibiting cancer cell growth and expression of the osteolytic factor PTHrP. We also demonstrated that curcuminoid metabolites, vanillin, ferulic acid and tetrahydrocurcuminoids were not potent inhibitors of breast cancer cell growth or secretion of osteolytic PTHrP, despite recent assertions that they are responsible for the bioactivity of curcuminoids. These studies emphasize the structural and biological importance of curcuminoids in the anti-cancer effect of turmeric.
Acknowledgments
This work was supported by the National Institutes of Health grant number F31AT004875 to L.E.W. and R21AT003614 to J.L.F. from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NCCAM, ODS or the National Institutes of Health.
List of Abbreviations
- PTHrP
parathyroid hormone-related protein
- MTT
3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide
- LDH
lactate dehydrogenase
- TGF-β
transforming growth factor- β
Contributor Information
Laura E. Wright, Email: Lauraw1@u.arizona.edu.
Jen B. Frye, Email: jabeisch@u.arizona.edu.
Bhavana Gorti, Email: bgorti@u.arizona.edu.
Barbara N. Timmermann, Email: jfunk@u.arizona.edu, btimmer@ku.edu.
Janet L. Funk, Email: jfunk@u.arizona.edu.
References
- 1.Centers for Disease Control and Prevention. 1999–2008 Incidence and Mortality Web-Based Report. Atlanta GA: Department of Health and Human Services, Center for Disease Control and Prevention, and National Cancer Institute; [Accessed 26 Oct 2012]. homepage on the internet. Available from: http://www.cdc.gov/uscs. [Google Scholar]
- 2.World Health Organization, International Agency for Research on Cancer. GLOBOCAN. Lyon, France: 2008. [Accessed 26 Oct 2012]. homepage on the internet. Available from: http://globocan.iarc.fr. [Google Scholar]
- 3.Ji JL, Huang XF, Zhu HL. Curcumin and its formulations: potential anti-cancer agents. Anticancer Agents Med Chem. 2012;12:210–8. doi: 10.2174/187152012800228733. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;8:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Wright LE, Frye JB, Lukefahr AL, et al. Curcuminoids block TGF-β signaling in human breast cancer cells and limit osteolysis in a murine model of breast cancer bone metastasis. J Nat Prod. 2012 doi: 10.1021/np300663v. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnaswamy K. Traditional Indian spices and their health significance. Asia Pac J Clin Nutr. 2008;17 (Suppl 1):265–8. [PubMed] [Google Scholar]
- 7.Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arth Rheum. 2006;54:3452–64. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 8.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;4:453–70. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 9.Liang S, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends Mol Med. 2012;18:138–43. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.) J Agric Food Chem. 2010;58:842–9. doi: 10.1021/jf9027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benny M, Antony B. Bioavailability of Biocurcumax (BCM-095™) Spice India. 2006;19:1–51. [Google Scholar]
- 12.Subik K, Lee JF, Baxter L, et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl) 2010;20:35–41. [PMC free article] [PubMed] [Google Scholar]
- 13.Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast Dis. 2010;32:99–122. doi: 10.3233/BD-2010-0304. [DOI] [PubMed] [Google Scholar]
- 14.Funk JL, Frye JB, Oyarzo JN, Timmermann BN. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J Nat Prod. 2009;72:403–7. doi: 10.1021/np8006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.) J Agric Food Chem. 2010;58:842–9. doi: 10.1021/jf9027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk JL, Oyarzo JN, Frye JB, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–5. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucoronidation in mice. Drug Metabolism Disposition. 1999;27:486–94. [PubMed] [Google Scholar]
- 18.Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–76. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 19.Pfeiffer E, Hohle S, Solyom AM, Metzler M. Studies on the stability of turmeric constituents. J Food Eng. 2003;56:257–59. [Google Scholar]
- 20.Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008;52:1074–81. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 21.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 22.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;12:761–8. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 23.Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–55. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16:916–22. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Sun SH, Huang HC, Huang C, Lin JK. Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur J Pharmacol. 2012;690:22–30. doi: 10.1016/j.ejphar.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoll F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;15:1305–15. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 28.Han G, Cui JJ, Bi R, Zhao LL, Zhang WG. Study on stability of curcumine, demethoxycurcumin and bisdemethoxycurcumin. Zhongguo Zhong Yao Za Zhi. 2008;33:2611–4. [PubMed] [Google Scholar]
- 29.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonrao M, Yodkeeree S, Ampasavate C, Anuchapreeda S, Limtrakul P. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch Pharm Res. 2010;33:989–98. doi: 10.1007/s12272-010-0703-6. [DOI] [PubMed] [Google Scholar]
- 31.Jeong GS, Oh GS, Pae HO, et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp Mol Med. 2006;38:393–400. doi: 10.1038/emm.2006.46. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Nakamura T, Iyoki S, et al. Elucidation of the anti-allergic activities of curcumin- related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;8:1438–43. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- 33.Fujisawa S, Ishihara M, Murakami Y, Atsumi T, Kadoma Y, Yokoe I. Predicting the biological activities of 2-methoxyphenol antioxidants: effects of dimers. In Vivo. 2007;2:181–8. [PubMed] [Google Scholar]
- 34.Thapliyal R, Maru GB. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem Toxicol. 2001;39:541–7. doi: 10.1016/s0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 35.Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J Nutr Biochem. 2007;20:87–95. doi: 10.1016/j.jnutbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2012 Sep 20; doi: 10.1002/biof.1042. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Dhillon N, Sung B, Kurzrock R, Aggarwal BB. Could antitumor activity of curcumin in patients be due to its metabolites? A response. Clin Cancer Res. 2009;15:7108–9. [Google Scholar]
- 38.Murakami Y, Hirata A, Ito S, et al. Re-evaluation of cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in macrophages stimulated with lipopolysaccharide. Anticancer Res. 2007;27:801–7. [PubMed] [Google Scholar]
- 39.Jung KJ, Go EK, Kim JY, Yu BP, Chung HY. Suppression of age-related renal changes in NF-κB and its target gene expression by dietary ferulate. J Nutr Biochem. 2009;20:378–88. doi: 10.1016/j.jnutbio.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Jayaprakasam B, Vanisree M, Zhang Y, Dewitt DL, Nair MG. Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. J Agric Food Chem. 2006;54:5375–81. doi: 10.1021/jf060899p. [DOI] [PubMed] [Google Scholar]
- 41.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventative agent curcumin. J Bio Chem. 2011;286:1114–24. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol Med. 2012;18:361–3. doi: 10.1016/j.molmed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10:97–108. doi: 10.1007/s10522-008-9160-8. [DOI] [PubMed] [Google Scholar]
- 44.Klepacka J, Fornal L. Ferulic acid and its position among the phenolic compounds of wheat. Crit Rev Food Sci Nutr. 2006;46:639–47. doi: 10.1080/10408390500511821. [DOI] [PubMed] [Google Scholar]
- 45.Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinomas in nude mice. World J Gastroenterol. 2008;14:2003–9. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res. 2004;27:683–92. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 47.Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55:1646–54. doi: 10.1002/mnfr.201100454. [DOI] [PubMed] [Google Scholar]









