Abstract
Introduction
Spinal cord injury (SCI) causes anorectal problems, whose pathophysiology remains poorly characterized. A comprehensive method of evaluating spino-anorectal function is lacking.
Aim
To investigate the neuropathophysiology of bowel dysfunction in SCI by evaluating motor evoked potentials (MEP) of anus and rectum following trans-spinal magnetic stimulation and anorectal physiology.
Methods
Translumbar and transsacral magnetic stimulations, anorectal manometry and pudendal nerve latency (PNTML) were performed in 39 subjects with SCI and anorectal problems and 14 healthy controls and data were compared. MEPs were recorded with an anorectal probe containing bipolar ring electrodes.
Results
The MEPs were significantly prolonged (p<0.05) bilaterally, and at lumbar and sacral levels, and at rectal and anal sites in SCI subjects compared to controls. 95% of SCI subjects had abnormal MEPs; 53% had abnormal PNTML. All subjects with abnormal PNTML also demonstrated abnormal MEP, but 16/17 subjects with normal PNTML had abnormal MEP. Overall SCI patients had weaker anal sphincters (p<0.05), higher prevalence of dyssynergia (85%) and altered rectal sensation (82%).
Conclusions
Translumbar and transsacral MEPs revealed significant and hitherto undetected lumbo-sacral neuropathy in 90% of SCI subjects. Test was safe and provided neuropathophysiological information that could explain bowel dysfunction in SCI subjects.
Keywords: Spinal cord injury, translumbar magnetic stimulation, transsacral magnetic stimulation, spino-anorectal pathway
Introduction
The annual incidence of spinal cord injury (SCI) is 40 per million, with approximately 11,000 new cases per year in the USA1. The most common causes are motor vehicle accidents, falls and spinal stenosis; less common causes include assaults, gun shot injuries and tumors2. In addition to the physical disability and psychological problems, both bowel and urinary dysfunction are common3, 4. Approximately, 39% of SCI subjects reported colorectal dysfunction and a significant impact on their quality of life5,6. Interestingly, disordered defecation was more common than fecal incontinence7, and 30% of SCI subjects regarded colorectal dysfunction as a greater problem than both bladder or sexual dysfunction.
The bowel dysfunction in SCI may be caused by motor and sensory deficits that affect the peripheral and autonomic nervous system8. The varied presentation of bowel symptoms could be a consequence of complex interactions between the neural injury, gut innervation and pelvic viscera. Currently, the evaluation of bowel dysfunction in SCI subjects may include MR imaging of the spinal cord, defecography, anorectal manometry as well as electrophysiological tests such as anal electromyography (EMG) and pudendal nerve terminal motor latency (PNTML)9. Although anal sphincter EMG (single fiber, concentric needle and surface plug electrodes) and PNTML can identify neuropathy, they are operator dependent, not standardized, invasive and poorly tolerated9–13.
Over the last decade, magnetic stimulation has been applied for studying nerve conduction. When applied topically, over specific sites, it can induce a varying magnetic field that stimulates underlying neural tissues with minimal discomfort. It is better tolerated than electrical or needle stimulation14. The stimulation can be performed over the motor cortex14,15,16 or over the lumbosacral region16,17,18,19. The test has been shown to be feasible and reproducible in healthy subjects20. However, whether it is useful in the evaluation of subjects with SCI is not known.
Magnetoelectrical stimulation is not focal21. Previous studies have shown that by placing a coil over the sacral region, the proximal pudendal nerve that originates from S2, S3 and S4 was stimulated17. Similarly, by placing a coil over the lumbar region, the portions of S2, S3, S4 nerves which courses intrathecally are stimulated. Thus, by placing a coil at both regions we were able to measure the conduction time of the intradural segments of the lumbosacral roots22 and as well as at the cauda equina17. We conducted tests on both sides since the injury may be unilateral or bilateral and/or may affect the nerves disproportionally23.
We tested the hypothesis that transspinal magnetic stimulation will provide comprehensive neuro-pathophysiologic information regarding the spino-anorectal dysfunction in patients with SCI. More specifically, it will reveal either unilateral or bilateral abnormal and prolonged motor evoked potentials (MEP) at the lumbar region and/or sacral region and at the rectal and/or anal sites in SCI subjects when compared to controls. Our aims were to develop a new methodology and determine the motor evoked potentials (MEP) of the anus and the rectum, on each side, and at both the lumbar and sacral levels, by performing translumbar and transsacral magnetic stimulation in subjects with SCI and to compare this with healthy controls. We also evaluated bowel symptoms and anorectal physiology in SCI subjects.
Materials and Methods
Subjects
Consecutive subjects with a history of spinal cord injury (SCI) and symptoms of bowel dysfunction referred to a specialist unit at a tertiary care center were evaluated. Subjects with a history of bowel symptoms prior to spinal cord injury were not included in the present study. Bowel symptoms were assessed with a modified Rome II bowel symptom questionnaire. All subjects must have had a flexible sigmoidoscopy or colonoscopy to exclude mucosal disease within 12 months prior to enrollment. Subsequently, we performed translumbar and transsacral MEP studies using magnetic stimulation as described below. Anorectal manometry (ARM) and pudendal nerve terminal motor latency testing (PNTML) were also performed using standard protocols as described previously24. Healthy subjects with no symptoms or previous gastrointestinal or pelvic floor surgery and who had a normal physical examination were enrolled as controls. The study protocol was approved by the Institutional Review Board.
Translumbar and Transsacral MEP study Protocol
We used a specially designed anorectal probe for recording the motor evoked potentials (MEP), a recording device (Caldwell Sierra II wedge, SanDiego, CA) and a magnetic coil for stimulation. The anorectal probe (Koningsberg, CA) consisted of 2 pairs of bipolar steel ring electrodes; each pair was located 1 cm apart. The probe was placed in the anorectum such that the rectal recording electrodes were located at 10 cm and the anal recording electrodes were located at 2 cm from anal verge (Figure 1). The subject was then asked to lie in a prone position. The magnetic stimulations were performed by placing a Cadwell Focalpoint Coil™ (9-cm) (Cadwell, San Diego, CA) approximately 3–5 cm lateral to the midline and at the L3-L4 level to evoke the translumbar MEPs. These sites were chosen based on our pilot studies at multiple levels in the lumbar and sacral regions and in previous studies16,17.
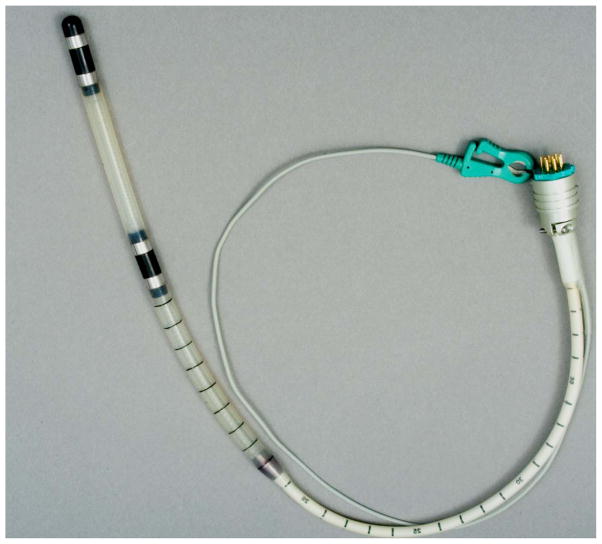
Figure 1.
Recording anorectal probe (Koningsberg, CA) containing 2 pairs of bipolar steel ring electrodes, located 10 cm apart.
Next, the coil was placed approximately 3–5 cm lateral to the midline at the S3–S4 level, and transsacral magnetic stimulation was performed on each side. Approximately 70–100% intensity (2 Tesla) of magnetic stimulation was used to evoke the MEPs, usually starting at 50% intensity. After magnetic stimulation, at each region and on both sides, the MEP responses were recorded simultaneously from the rectum and the anal canal, and were displayed on a monitor (Figure 2). At least 5 MEP responses were obtained from each stimulation site, and the three best responses were averaged to calculate the MEP response. The MEP responses obtained from the magnetic stimulation at the lumbar and sacral regions were designated as follows; translumbar-rectal MEP (TL-rMEP), translumbar-anal MEP (TL-aMEP), transsacral-rectal MEP (TS-rMEP) and transsacral-anal MEP (TS-aMEP), respectively. Because, we obtained responses from the left and right sides a total of eight MEP responses (4 from each side) were assessed in each subject.
Figure 2.
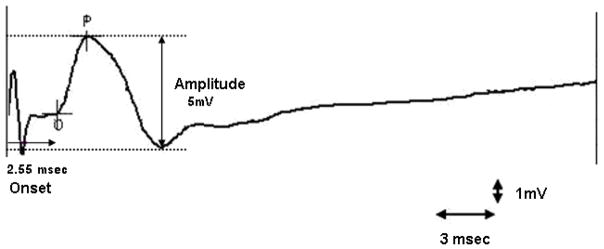
Typical motor evoked potential recording after magnetic stimulation
Anorectal physiology testing
Subjects with SCI also had anorectal manometry and rectal sensory testing. We placed a six-sensor solid state manometry probe with a balloon in the rectum and the test was performed by using previously published protocols25. We assessed the anal sphincter tone, anorectal pressure changes during squeeze and bearing down maneuvers and rectal sensory thresholds25. Additionally, we performed a balloon expulsion test by having the subject bear down and expel a 50 ml water-filled balloon25 and a saline continence test by asking the subject to retain 800 ml of saline that was infused at a constant rate of 60 ml/minute26.
Pudendal nerve terminal motor latency (PNTML)
The PNTML was measured by using the St. Marks disposable electrode (Dantec Electronic Bristol, UK). With the subject in the lithotomy position, the electrode mounted on a gloved finger was inserted into the rectum. The finger tip was positioned at the ischial spine and electrical stimuli (10–15 mA) were applied on each side. The compound muscle action potential response was recorded and the mean onset time of the three best responses was taken as the PNTML25.
Data and Statistical Analysis
SierraWin II XP software (Cadwell, WA) was used for recording the MEP data and for performing the measurements. The MEP latency was measured as the time interval (ms) between application of the stimulus and the onset of the first prominent action potential (Figure 2). The amplitude or the total height of the action potential was measured in microvolts. Magnetic stimulation of the lumbosacral region is usually associated with a stimulation artifact21. Thus, with our filter setting, minimum amplitude of response of 10 μV was considered a true physiological response and not an artifact.
Each group of responses consisted of 5 recordings that were analyzed individually, and the mean latency and mean amplitude of the three best responses were calculated. The responses were analyzed by two investigators initially (KT and AA) and were subsequently analyzed in a blinded manner by an independent investigator (SSCR) who was unaware of the subjects’ identity. If there was a discrepancy in the latency value (>5%), the data were reexamined and consensus was reached.
The results of MEPs and PNTML are expressed as mean (95% confidence interval) and manometric results as mean ± SD. The primary comparison was the MEP measurement at the same level (lumbar or sacral) and at the same site (rectal or anal) and on the same side (right or left) between the SCI subjects and controls. The mean differences for the MEP data between the healthy controls and SCI subjects were compared using nonparametric (Mann-Whitney U test). Holm’s sequential Bonferroni technique was used to control for multiple comparisons between the groups. The mean differences for the anal sphincter pressures, rectal sensation, saline continence tests and balloon expulsion tests between the two groups were compared using Student’s t test. The differences in the prevalence of dyssynergia between the two groups were compared with chi square test. SCI subjects were considered to have rectal hypersensitivity when two or more of the three rectal sensory threshold volumes (first sensation, desire to defecate, urgency to defecate) were lower than 2 standard deviations of the normal mean value and similarly they were considered to have rectal hyposensitivity when two or more of the three rectal sensory thresholds were higher than two standard deviations of the normal mean values27. Neuropathy was defined as a prolonged onset of the MEP latency time using the following cut offs that were greater than 2 SD of mean in healthy controls; > 3.5 msec for TL-rMEP, >4.15 for TL-aMEP, >4.05 for TS-rMEP and >4.5 for TS-aMEP, and for PNTML an onset time of >2.2 ms.
The diagnostic accuracy of MEP for identifying neuropathy in subjects with SCI was examined by constructing a receiver operating characteristic curve (ROC). The area under the curve (AUC) was taken as an overall measure of the diagnostic accuracy.
Results
Demographics
Forty four subjects with SCI were evaluated of whom 5 were excluded because their bowel symptoms preceded their injury. Thirty nine subjects with bowel symptoms and SCI (M/F 15/24, mean age 46.5 ± 14.0 years, range 21–78) were enrolled. Eighteen subjects (46%) presented with predominant symptoms of fecal incontinence, 13 (33%) with constipation and 8 (21%) with mixed symptoms of constipation and stool leakage. The causes of SCI were accidental back injury in 28 (72%) subjects; 8 had motor vehicle accident, 7 had falls, 9 had herniated disc and 4 had work place injury; 3 had gun shot wounds, 9 had degenerative disk disease and 3 subjects had resection of spinal tumor. The level of SCI was cervical (4), thoracolumbar (6), lumbar (14), lumbosacral (8), and pelvis (3). Eight subjects had injury at multiple levels. Twenty-six out of 39 subjects (67%) had a history of spinal surgery. Fourteen healthy subjects served as controls (M/F 5/9, mean age 42.9 ± 10.0, range 23–59 years).
Physical and rectal examination
All subjects were ambulant with a normal sensory and motor testing during clinical neurological evaluation, except two subjects who had mild paraparesis but without any major motor deficit of the lower limbs. On digital rectal examination, perianal sensation was abnormal in 9 subjects (absent in 4, decreased in 5) and the anocutaneous reflex was abnormal in 14 subjects (absent 5, decreased 9).
Translumbar Motor Evoked Potentials (TL-MEP)
A typical example of TL-MEP response in a healthy subject and in a subject with SCI is shown in Figure 3. The mean rectal MEP following translumbar stimulations on the left side and on the right side were significantly prolonged in SCI subjects (p<0.01) when compared to healthy controls (Table 1). The differences remained statistically significant after the Holm’s sequential Bonferroni adjustment for multiple comparisons. All subjects tolerated the procedure without any adverse events. The rectal MEP could not be recorded in 2 subjects and the anal MEP could not be recorded in 1 subject.
Figure 3.
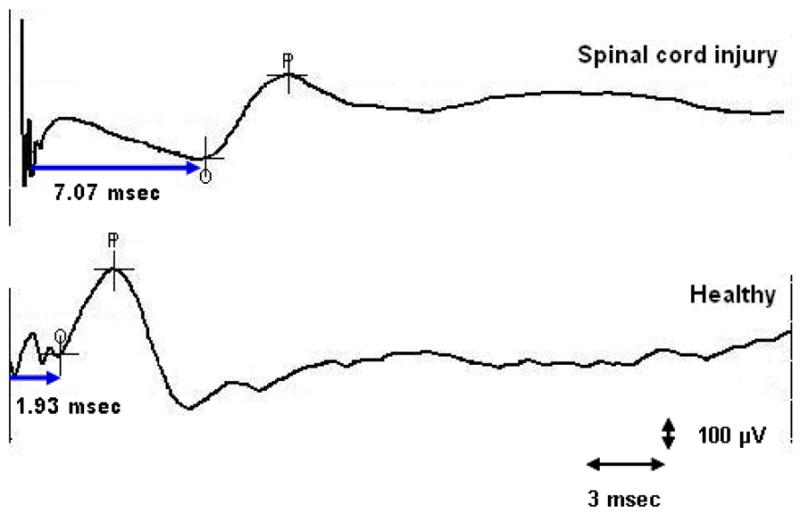
Translumbar motor evoked potential response in a subject with spinal cord injury and in a healthy subject.
Table 1.
| Left | p | Right | p | |||
|---|---|---|---|---|---|---|
| SCI, n = | Control, n = | SCI, n = | Control, n = | |||
| TL-rMEP ms | 5.3±4.2 | 2.7±0.6 | 0.01 | 5.5±5.1 | 2.6 ±0.9 | 0.002 |
| TL-aMEP ms | 6.7±3.4 | 3.2±1.0 | 0.002 | 7.1±4.3 | 2.9±1.1 | 0.0001 |
| TS-rMEP ms | 5.8±5.6 | 3.0±1.1 | 0.047 | 4.9±3.3 | 3.0±1.0 | 0.078 |
| TS-aMEP ms | 6.0±3.0 | 3.0±1.0 | 0.0001 | 5.5±3.5 | 3.0±1.0 | 0.006 |
| PNTML ms | 3.3± 3.4 | 1.7± 0.2 | 0.006 | 2.9 ±1.9 | 1.7 ± 0.6 | 0.009 |
Transsacral Motor Evoked Potentials (TS-MEP)
A typical example of a TS-MEP response in a healthy subject and a SCI subject is shown in Figure 4. The MEP responses following transsacral magnetic stimulation on the left and on the right sides and at the anal and rectal sites were significantly prolonged in subjects with SCI when compared to healthy controls (p < 0.01), except the right sacro-rectal MEP (table 1). The differences remained statistically significant after adjustment for multiple comparisons. The rectal MEPs could not be obtained in 2 subjects and the anal MEPs could not be obtained in 2 subjects.
Figure 4.
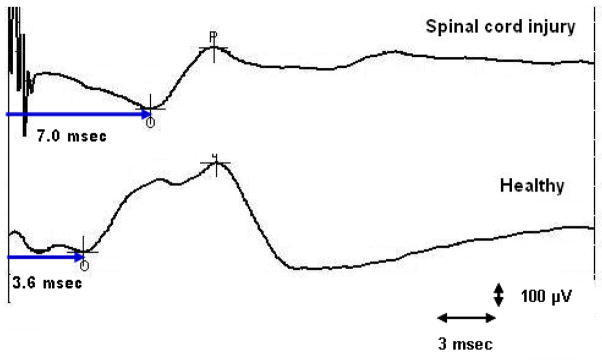
Transsacral motor evoked potential response in a subject with spinal cord injury and in a healthy subject.
Anorectal manometry (ARM)
The manometric findings are summarized in table 2. Both the internal and the external anal sphincter pressures were significantly weaker in SCI subjects compared to controls. The cough reflex was impaired in 4 subjects (10%). The rectoanal inhibitory reflex was impaired in 7 subjects (18%), i.e. the volume required to elicit this reflex was significantly higher than controls (>100 cc). The sensory thresholds were quite variable. Seventeen subjects (44%) showed features of rectal hyposensitivity, 15 had rectal hypersensitivity (38%) and 11(28%) had normal rectal sensation. Thirty-three subjects (85%) showed a dyssynergic pattern during attempted defecation and 11 subjects (28%) were unable to expel the balloon. All 13 subjects who complained of constipation exhibited dyssynergia and 6/8 who complained of mixed symptoms also had dyssynergia.
Table 2.
| Spinal cord injury [mean ± SD] | Controls [mean ± SD] | p-value | |
|---|---|---|---|
|
| |||
| Resting sphincter pressure (mm Hg) | 53 ± 22 | 69 ± 15 | 0.0024 |
| Maximal squeeze pressure (mm Hg) | 117 ± 85* | 168 ± 36 | 0.037 |
| Sustained squeeze pressure (mm Hg) | 77 ± 46* | 148 ± 34 | 0.00002 |
| No. with dyssynergic pattern of defecation | n = 33 (85%) | - | |
| Threshold volumes for rectal sensation | |||
| - First sensation (cc) | 44 ± 43.3 | 20±7.6 | 0.058 |
| - Desire to defecate (cc) | 65 ± 54 | 106±44 | 0.354 |
| - Urge to defecate (cc) | 151 ± 71 | 179±53 | 0.785 |
| - Maximal tolerable volume (cc) | 206 ± 73 | 240±52 | 0.279 |
| Rectal Sensation | |||
| -Hyposensitivity | n=17(44%) | ||
| -Hypersensitivity | n=15(38%) | ||
| -Normal sensation | n=11 (28%) | ||
| Saline continence test | |||
| - Volume infused at first leak (ml) | 161 ± 202* | 652±333 | 0.0002 |
| - Total volume retained (ml) | 253 ± 305* | 738±82 | 0.0002 |
| Balloon expulsion test (s) | 90 ± 122 | 59±75 | |
| - unable to expel balloon | n = 11 (26%) | n=0 | |
In subjects with symptoms of fecal incontinence, the saline continence test was abnormal in 15/18 (83%) subjects, all of whom had significant leakage. The test was normal in 3/18 subjects.
Pudendal nerve terminal motor latency (PNTML)
The PNTML could not be obtained in 3 subjects with SCI due to technical problems. Thus, the PNTML responses were analyzed in 36 subjects. The PNTML was abnormal in 19/36 patients (53%). Twelve subjects had bilateral pudendal neuropathy, 4 had unilateral right side pudendal neuropathy and 3 had unilateral left side pudendal neuropathy. Overall, subjects with SCI had significantly prolonged (p <0.01) pudendal nerve latencies when compared to healthy subjects (table 1).
Comparison of MEP and PNTML
An abnormal neural conduction, defined as a prolonged MEP (values outside the upper limit of normal range) in at least one spino-anorectal pathway was detected in 37/39 subjects (95%) whereas an abnormal PNTML defined as prolonged latency on any one side was detected in 19/36 subjects (53%). Twelve subjects with bilateral pudendal neuropathy showed abnormal MEPs on both sides. Seven subjects with unilateral pudendal neuropathy (4 right, 3 left) also had corresponding and abnormal MEPs. In 17/36 subjects with bilateral and normal PNTML, only 1 subject (6%) showed normal values for MEP at all 4 sites.
Diagnostic accuracy of MEP
All of our subjects reported that their bowel dysfunction developed after the SCI. Therefore, the pre-test clinical suspicion that all subjects had a neurologic injury was used as the gold standard for assessing the diagnostic utility of our tests. The area under the curve (AUC) was taken as an overall measure of the diagnostic accuracy and the individual MEP data are shown in table 3. The AUC data for the lumbar and sacral MEPs and at both sides ranged from 0.71 to 0.94 showing good to excellent diagnostic accuracy. If spino-anorectal neuropathy is defined as an abnormal and prolonged nerve conduction in any one of the eight spino-anorectal MEPs that were examined, we found that overall the MEP had a higher diagnostic accuracy (p<0.001) than PNTML for the detection of neuropathy. The MEP test was also abnormal in 16/17 subjects with a normal PNTML.
Table 3.
| Area Under the Curve | ||||
|---|---|---|---|---|
| Left | 95% CI | Right | 95% CI | |
| TL-rMEP | 0.771 | 0.620–0.884 | 0.843 | 0.702–0.935 |
| TL-aMEP | 0.929 | 0.809–0.984 | 0.943 | 0.829–0.990 |
| TS-rMEP | 0.714 | 0.558–0.840 | 0.757 | 0.604–0.874 |
| TS-aMEP | 0.814 | 0.668–0.915 | 0.857 | 0.719–0.944 |
Discussion
Bowel symptoms and anorectal dysfunction are both common in subjects with SCI, but the underlying neurophysiological mechanisms are incompletely understood. It has been proposed that the inability to control the voluntary sphincter muscles may cause a variety of dysfunctions including difficulty with defecation, constipation and fecal incontinence. Likewise, in subjects with lesions affecting the cauda equina there may be a loss of parasympathetic control and innervation of IAS. This in turn may lead to weak resting or squeeze tone and cause fecal incontinence. Although such mechanisms have been proposed, there is little objective evidence in humans, regarding the precise neuropathophysiological changes. This is largely because of the inability to examine the spino-anorectal neurological pathways within the intact body. Also, anorectal function and spino-anorectal neurophysiology, particularly at multiple levels, and in the same individual has not been evaluated systematically. Although MR imaging can detect structural neurological defects, currently there is no technique for evaluating the neurophysiological abnormalities that underpin bowel dysfunction in subjects with SCI.
We found that the translumbar anal MEPs obtained following translumbar magnetic stimulation were abnormal and significantly prolonged on both sides in subjects with SCI when compared to healthy controls. Likewise, the rectal MEPs obtained from lumbar stimulation were also significantly prolonged on both sides in subjects with SCI. These differences remained significant even after controlling for multiple comparisons between groups and sites suggesting that although only one or more tracts may be involved there are clear differences between the SCI group and controls. These findings demonstrate that the neurological conduction through the pelvic and spinal nerves that innervate the rectum and the anal canal were significantly prolonged secondary to nerve damage.
Similarly, the sacro-rectal MEPs and the sacro-anal MEPs obtained after transsacral magnetic stimulation were also significantly prolonged on both sides, further attesting to the spino-anal neuropathy, and an injury to the pelvic neuronal tracts. It is likely that the bowel dysfunction experienced by these subjects such as fecal incontinence, constipation or mixed problems were a consequence of these neurological injuries. Although suspected, an underlying neuropathy had remained largely undetected, because routine structural evaluation with tests such as magnetic resonance imaging was unrevealing in these patients.
Furthermore, although manometric tests of anorectal function had revealed some dysfunction such as weaker squeeze sphincter tone, and sensory dysfunction, in most subjects, these data by itself could not define the underlying neuropathophysiology. A new observation was the detection of dyssynergia in a significant proportion of subjects, most likely due to an acquired behavioral disorder of defecation secondary to the neurological insult. Recognition of this dysfunction will enable some of these individuals to receive biofeedback therapy and thereby improve their bowel dysfunction. The pudendal nerve terminal motor latency (PNTML) was also prolonged in approximately one half of these subjects suggesting that the neuropathy also affected the terminal portion of this nerve. However, the agreement between PNTML and MEP was only slight to moderate. This was in large part due to the higher sensitivity of the translumbar and transsacral MEPs for the detection of neuropathy when compared to the PNTML.
Abnormal MEPs on one or both sides were found in 37/39 (95%) subjects whereas an abnormal pudendal neuropathy was identified in 19/36 subjects (53%). All patients with an abnormal PNTML also had an abnormal MEP. However, in 16/17 subjects with a normal PNTML, the MEP was abnormal. Thus, PNTML is less sensitive than translumbar/transsacral MEPs for the detection of neuropathy that is secondary to SCI. Hence, translumbar and transsacral evaluations of the spino-rectal and spino-anal MEPs not only provide an accurate localization of the site and the magnitude of neurological injury but also a superior evaluation of the neuropathy that occurred following SCI.
The magnetic stimulation over L3-L4 spinal level most likely stimulated the proximal portion of the cauda equina i.e. the lumbar nerve roots, which provides sympathetic innervation to the colon and anorectum. These nerves carry information regarding the visceral afferent input27 as well as the inhibitory tracts28. The magnetic stimulation over the S1–S3 levels most likely stimulated the sacral nerve roots that supply the parasympathetic and sensori-motor innervations of the rectum, anal canal and pelvic floor muscles29 including the pudendal nerves. However, the precise nerves that are involved remain to be further characterized. Nevertheless, together, the translumbar and transsacral MEPs can provide comprehensive information regarding the peripheral neurological innervation of the anorectum.
Although lumbar and sacral nerve stimulations have been described previously14–18, 21,30, there are significant methodological differences compared to our study. For example, the data from transcutaneous electrical spinal stimulation have revealed longer nerve latencies 31 than those found in our study. In contrast, the normative data for the lumbo-anal MEP found in our study were similar to those described by others using magnetic stimulation and anal plug EMG21,30. Furthermore some investigators have assessed only the lumbar region30 or sacral region17 and either with surface electrode or with needle electrode17,18. Our technique of stimulating the lumbar and sacral nerve pathways separately and record MEPs simultaneously at both the rectal and anal sites was novel, and in part was facilitated by the use of a specially designed probe for recording MEP, and the use of a focal coil. This probe consisted of 2 pairs of bipolar steel ring electrodes. Unlike previous electrodes that were made from platinum or silver32, the steel electrodes are less expensive and appear to be more durable from our experience. Also the electrodes seem to provide a better contact at both the rectal and anal sites unlike unidirectional electrodes.
Through a better characterization of the neurological injury, it would be possible to provide an accurate diagnosis of the neurological problems that underpin bowel symptoms in SCI subjects. A lack of such objective testing has hampered decision-making particularly in disability claims following SCI. Moreover, given the minimal invasive nature of the study, it can be repeated to assess either recovery or progression of nerve injury. Also the receiver operating characteristics for the MEP tests were excellent with a high area under the curve. The technique is simple, objective, and painless21. It may also be useful for the evaluation of subjects with other disorders of pelvic floor, such as fecal incontinence33. Given the complex innervation of the rectum and the anal canal and the patchy nature of neurological injury, we believe that it is prudent to evaluate MEP at all 8 sites in order to obtain optimal information.
There are a few limitations of this test. Firstly, the test is contraindicated in subjects with implants such as pacemakers, sacral nerve stimulators, metallic implants, and central intravenous catheter. Secondly, owing to technical reasons such as spinal deformity, difficulty with positioning patient or obesity, sometimes it was not possible to obtain MEP responses. This occurred in two subjects. Thirdly, the equipments used, namely anorectal probe and the magnetic stimulator are not widely available, but most components are available in a standard neurophysiology lab and can be adapted for this study. Finally, because of the exploratory nature of the study, our sample size was not adequately powered to differentiate all of the tested parameters between patients and controls.
In conclusion, our study showed that the assessment of translumbar and transsacral MEPs can provide a better delineation of the spino-rectal and spino-anal pathways. Also it revealed significant neuropathy in SCI subjects by using a minimally invasive technique. The test was well tolerated, safe, inexpensive, reproducible20 and served as an objective method of evaluating peripheral brain-gut pathways. It provided hitherto unknown information regarding pelvic floor neuropathy that could aid further management of these patients.
Supplementary Material
Table 4.
| TL-rMEP | TL-aMEP | TS-rMEP | TS-aMEP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal (>3.5 msec) | Normal | Abnormal (>4.15 msec) | Normal | Abnormal (>4.05 msec) | Normal | Abnormal (>4.5 msec) | ||
| Left PNTML | Normal | 9 | 12 | 11 | 9 | 14 | 7 | 11 | 9 |
| Abnormal (>2.2 msec) | 4 | 9 (k=0.11, p=0.48) | 2 | 11 (k=0.36, p=0.023) | 6 | 6 (k=0.16, p=0.346) | 5 | 7 (k=0.13, p=0.465) | |
| Right PNTML | Normal | 6 | 13 | 8 | 11 | 11 | 8 | 9 | 10 |
| Abnormal (>2.2 msec) | 6 | 7 (k=0.13, p=0.403) | 3 | 10 (k=0.17, p=0.266) | 5 | 9 (k=0.22, p=0.208) | 5 | 7 (k=0.53, p=0.756) | |
What is current knowledge
The neuropathophysiology of ambulatory subjects with spinal cord injury (SCI) and bowel problems remains unclear.
Although structural defects can be identified through tests such as magnetic resonance imaging, currently there is no objective method of defining the neurological deficits that cause bowel dysfunction in SCI subjects.
What is new here
We describe a new technique for neurophysiological evaluation that consists of simultaneous assessment of rectal and anal motor evoked potentials (MEP) by using transspinal magnetic stimulation at both the lumbar and sacral regions.
We found that the MEP at one or more regions (lumbar or sacral), and at one or more sites (anal or rectal) and at one or more sides (right or left) were significantly abnormal in a majority of subjects with SCI when compared to healthy controls.
Transspinal MEP evaluation is relatively noninvasive, safe, has a high diagnostic yield and can objectively identify the neuropathophysiological features that underpin bowel dysfunction in subjects with SCI.
Acknowledgments
Dr. SSC Rao was supported by NIH grant No. 2R01 KD57100-05A2. Portions of this manuscript were presented at the ACG annual meeting, in Philadelphia and published as an abstract, Am J Gastro 2007;102(s2):S426-58. Dr. Kasaya Tantiphlachiva was supported by research fellowship grant from Chulalongkorn University, Bangkok, Thailand. We thank Dr. Shaheen Hamdy for his advice and assistance and Mrs. Stephanie Chapman for her secretarial support.
Footnotes
Conflict of interest: None
Guarantor: Satish SC Rao– Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, important intellectual content and final approval.
Contributing Authors:
Kasaya Tantiphlachiva - study recruitment, data acquisition, data collection, data analysis, manuscript preparation, critical revision
Ashok Attaluri - Data acquisition, data collection, data analysis, data interpretation, critical revision
Jessica Valestin- Data acquisition, data collection and study recruitment.
Thoru Yamada - Study concept and design, data interpretation, manuscript preparation
References
- 1.National Spinal Cord Injury Statistical Center. Spinal cord injury: facts and figures at a glance. J Spinal Cord Med. 2005;28(4):379–80. [PubMed] [Google Scholar]
- 2.McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215–24. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347:1651–3. doi: 10.1016/s0140-6736(96)91487-7. [DOI] [PubMed] [Google Scholar]
- 4.Craggs MD, Balasubramaniam AV, Chung EAL, Emmanuel AV. Aberrant reflexes and function of the pelvic organ following spinal cord injury in man. Auton Neurosci. 2006;126–127:355–70. doi: 10.1016/j.autneu.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39:193–203. doi: 10.1038/sj.sc.3101119. [DOI] [PubMed] [Google Scholar]
- 6.Krogh K, Nielsen J, Djurhuus JC, Mosdal C, Sabroe S, Laurber S. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997;40:1233–9. doi: 10.1007/BF02055170. [DOI] [PubMed] [Google Scholar]
- 7.De Looze D, Van Laere M, De Muynck M, Beke R, Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36:63–6. doi: 10.1038/sj.sc.3100531. [DOI] [PubMed] [Google Scholar]
- 8.Enck P, Greing I, Klosterhalfen S, Wietek B. Upper and lower gastrointestinal motor and sensory dysfunction after human spinal cord injury. Progr Brain Res. 2006;152:373–84. doi: 10.1016/S0079-6123(05)52025-9. [DOI] [PubMed] [Google Scholar]
- 9.Remes-Troche JM, Rao SC. Neurophysiological testing in anorectal disorders. Expert Rev Gastroenterol Hepatol. 2008;2:323–35. doi: 10.1586/17474124.2.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blank A, Magora A. Electromyographic investigation of the superficial sphincter ani muscle (SSAM) in spinal cord injury. Electromyogr Clin Neurophysiol. 1975;15:261–8. [PubMed] [Google Scholar]
- 11.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience. Am J Gastroenterol. 2002;97:232–40. doi: 10.1111/j.1572-0241.2002.05450.x. [DOI] [PubMed] [Google Scholar]
- 12.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–60. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 13.Rao SSC. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99(8):1585–604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 14.Opsomer RJ, Caramia MD, Zarola F, Pesce F, Rossini PM. Neurophysiological evaluation of central-peripheral sensory and motor pudendal fibres. Electroencephalogr Clin Neurophysiol. 1989;74:260–70. doi: 10.1016/0168-5597(89)90056-7. [DOI] [PubMed] [Google Scholar]
- 15.Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:27. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci. 1997;149:69–72. doi: 10.1016/s0022-510x(97)05388-4. [DOI] [PubMed] [Google Scholar]
- 17.Morren GL, Walter S, Lindehammar H, Hallbook O, Sjodahl R. Evaluation of the sacroanal motor pathway by magnetic and electric stimulation in patients with fecal incontinence. Dis Colon Rectum. 2001;44:167–72. doi: 10.1007/BF02234288. [DOI] [PubMed] [Google Scholar]
- 18.Snooks SJ, Swash M, Henry MM. Abnormalities in central and peripheral nerve conduction in patients with anorectal incontinence. J R Soc Med. 1985;78:294–300. doi: 10.1177/014107688507800405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jost WH, Schimrigk K. Magnetic stimulation of the pudendal nerve. Dis Colon Rectum. 1994;37:679–9. doi: 10.1007/BF02054414. [DOI] [PubMed] [Google Scholar]
- 20.Remes-Troche JM, Attulari A, Paulson J, Rao SSC. Test of brain-gut axis in humans using cortical evoked potentials and trans-cranial, trans-lumbar, and transsacral magnetic stimulation and its reproducibility. Am J Gastroenterol. 2007;1083:S515. [Google Scholar]
- 21.Herdmann J, Bielefeldt K, Enck P. Quantification of motor pathways to the pelvic floor in humans. Am J Physiol. 1991;260(5.1):G720–3. doi: 10.1152/ajpgi.1991.260.5.G720. [DOI] [PubMed] [Google Scholar]
- 22.Maertens de Noordhout AM, Rothwell JC, Thompson PD, Day BL, Marscen CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry. 1988;51:174–81. doi: 10.1136/jnnp.51.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamdy S, Enck P, Aziz Q, Uengoergil S, Hobson A, Thompson DG. Laterality effects of human pudendal nerve stimulation on corticoanal pathways: evidence for functional asymmetry. Gut. 1999;45:58–63. doi: 10.1136/gut.45.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao SSC, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Mot. 2002;14:553–9. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 25.Rao SSC, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 26.Rao SC, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469–75. [PubMed] [Google Scholar]
- 27.Gladman MA, Dvorkin LS, Lunniss PJ, William NS, Scott SM. Rectal hyposensitivity: a disorder of the rectal wall or the afferent pathway? An assessment using the barostat. Am J Gastroenterol. 2005;100:106–14. doi: 10.1111/j.1572-0241.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 27.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brading AF, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res. 2006;152:345–58. doi: 10.1016/S0079-6123(05)52023-5. [DOI] [PubMed] [Google Scholar]
- 29.Agur AMR, Lee MJ. Grant’s atlas of anatomy. 9. Maryland: Williams & Wilkins; 1991. [Google Scholar]
- 30.Loening-Baucke V, Read NW, Yamada T, Barker AT. Evaluation of the motor and sensory components of the pudendal nerve. Electroencephalogr Clin Neurophysiol. 1994;93:35–41. doi: 10.1016/0168-5597(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 31.Merton PA, Hill DK, Morton HB, Marsden CD. Scope of a technique for electrical stimulation of human brain, spinal cord, and muscle. Lancet. 1982;2(8298):597–600. doi: 10.1016/s0140-6736(82)90670-5. [DOI] [PubMed] [Google Scholar]
- 32.Sheldon R, Kiff ES, Clarke A, Harris ML, Hamdy S. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. Br J Surgery. 2005;92:1423–31. doi: 10.1002/bjs.5111. [DOI] [PubMed] [Google Scholar]
- 33.Rao SS, Tantiphlachiva K, Attaluri A, Paulson JA, Remes-Troche JM, Yamada T. S1826. Translumbar and Transsacral Magnetic Stimulation-A novel test of assessing anorectal neuropathy in fecal incontinence. Gastroenterology. 2008;134:A-278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.