Abstract
Biphenotypic acute leukemias (BAL) account for less than 4% of all cases of acute leukemia. Philadelphia chromosome and 11q23 rearrangement are the most frequently found cytogenetic abnormalities. Since t(15;17) is almost always associated with acute promyelocytic leukemia, t(15;17) in BAL cases is extremely uncommon. We report here a rare and instructive case of BAL with t(15;17) and the successful treatment approach adopted. A 55-year old woman was referred to our hospital for an examination of elevated white blood cell (WBC) counts with blasts (WBC 13.4×109/L; 76% blasts). The blasts with acute lymphoblastic leukemia (ALL-L2, FAB) morphology co-expressed B-lymphoid and myeloid lineages, and a cytogenetic study revealed 4q21 abnormalities and t(15;17). However, promyelocytic-retinoid acid receptor α rearrangement was not detected by fluorescence in situ hybridization on interphase nuclei. Our patient was treated with chemotherapy for ALL and gemtuzumab ozogamicin without all-trans-retinoic acid, and has remained in hematologic first complete remission for more than 3.7 years.
Key words: biphenotypic acute leukemias, t(15;17), PML/RARα rearrangement, chemotherapy for acute lymphoblastic leukemia, gemtuzumab ozogamicin
Introduction
Biphenotypic acute leukemias (BAL) are rare and account for less than 4% of all cases of acute leukemia.1 In 1995, the European Group for the Immunological Classification of Leukemias (EGIL) proposed immunological criteria for the classification of acute leukemias, including a scoring system for the definition of BAL.2 The most frequent cytogenetic abnormalities described in BAL patients include the Philadelphia chromosome and the presence of 11q23 rearrangement.1,3
The t(15;17)(q22;q12) is almost always associated with the morphological picture of acute promyelocytic leukemia (APL). At the genetic level, this translocation creates the promyelocytic (PML)-retinoid acid receptor α (RARα) and RARα-PML fusion genes.4 In clinical practice, the identification of the t(15;17) translocation predicts sensitivity to all-trans-retinoic acid (ATRA).5 The t(15;17) in BAL cases is extremely uncommon.
We encountered an extremely rare case of BAL with t(15;17) lacking PML/RARα rearrangement. The treatment was effective with chemotherapy for ALL and gemtuzumab ozogamicin (GO) without ATRA. We report here this suggestive BAL case of uncommon disease condition and successful treatment.
Case Report
A 55-year old Japanese woman was referred to our hospital with elevated white blood cell (WBC) counts with blasts. Her laboratory data on admission showed rising WBC counts (13.4×109/L; 76% blasts, 1% band, 6% segmented, 16% lymphocytes, 1% monocytes) with anemia and thrombocytopenia (hemoglobin 9.3 g/dL and platelets 110×109/L). Coagulation studies were normal. Lactate dehydrogenase was slightly raised to 367 IU/L (normal range 120-245). Bone marrow aspiration revealed replacement of normal marrow by blasts (94%). We detected differences in the size of the blasts, corresponding to an abnormal lymphocyte-like cell population with a high nuclear/cytoplasmic (N/C) ratio, but without granules in the cytoplasm (Figure 1). These blasts were negative for myeloperoxidase (MPO) and esterase. Morphological findings were compatible with acute lymphoblastic leukemia (ALL-L2, FAB classification).
Figure 1.
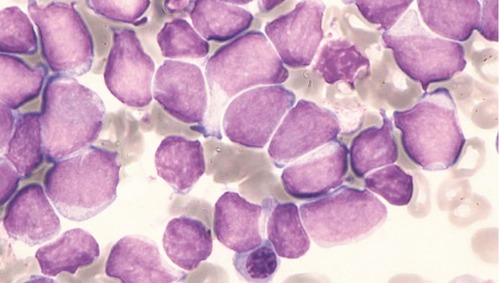
Bone marrow aspiration revealed morphological findings compatible with ALL-L2 (May-Giemsa staining, 1000×).
Flow cytometry analysis
Immunophenotype with double-color flow cytometry showed positivity (>30%) for CD19 (90.8%), CD22 (45.4%), CD79a (92.4%), CD13 (40.9%), CD33 (94.6%), CD34 (95.8%) and HLA-DR (93.8%). MPO was negative (2.0%). Double staining for CD19xCD33 was strongly positive (96.6%). The use of the EGIL scoring system revealed 5 points for B-lymphoid lineage and 2 points for myeloid lineage. The immunophenotype analysis was conclusive for a diagnosis of BAL.
Cytogenetic analysis
Chromosomal analysis with G-banded karyotype of the bone marrow cells showed 46, XX, t(4;12)(q21;p11), t(15;17)(q22;q12) in all 24 metaphase spreads (Figure 2A). Fluorescence in situ hybridization (FISH) performed on interphase nuclei using an LSI PML/RARα dual color, dual fusion translocation probe (Vysis, USA) showed two separate PML and RARα signals (Figure 2B) in 1000 interphase nuclei and all metaphases analyzed. No PML/RARα fusion signal was identified.
Figure 2.
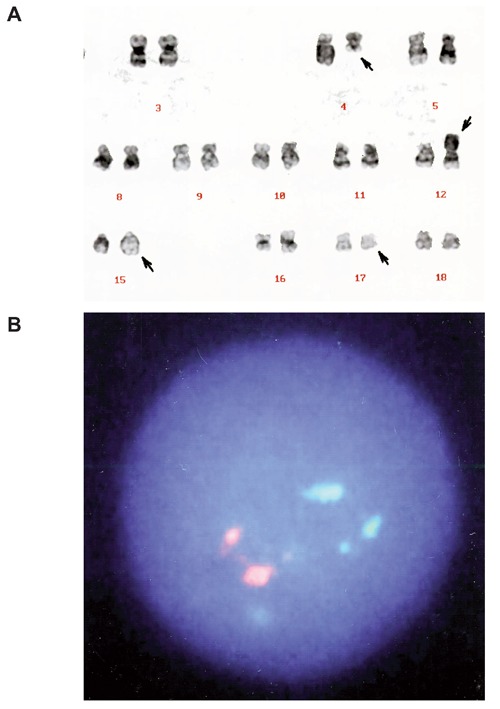
A) G-banded karyotype of the bone marrow cells showing t(4;12)(q21;p11) and t(15;17)(q22;q21). Arrows indicate the derivative chromosomes. B) FISH analysis with PML/RARα-specific probes showing two orange (PML) and two green (RARα) signals. No PML/RARα fusion signal (which should appear yellow) was detected.
Clinical course
The patient was initially treated with the Japan Adult Leukemia Study Group (JALSG)-ALL202 chemotherapy protocol (Table 1) and induction for ALL treatment was completed. The bone marrow aspirate at the end of the induction phase revealed hematologic complete remission (CR). After the consolidation phase I (with a high dose of cytarabine), the patient experienced the complication of septic shock with acute phlegmonous gastritis. Thereafter, we administered a single dose (9 mg/m2) of gemtuzumab ozogamicin (GO), an anti-CD33 antibody conjugate, independent of the JALSG protocol, and subsequently, completed the course until consolidation phase V. Furthermore, we continued maintenance phase therapy using the JALSG protocol until two years after the onset of disease, during which time we administered GO (6 mg/m2) twice. At present, 1.7 years after the end of chemotherapy, the patient has remained in hematologic first CR during the over 3.7 years follow up.
Table 1.
JALSG-ALL202 chemotherapy protocol.
| Phase | Drug | Dosage | Days |
|---|---|---|---|
| Induction | Cyclophosphamide | 1200 mg/m2 | 1 |
| Daunorubicine | 60 mg/m2 | 1-3 | |
| Vincristine | 1.3 mg/m2* | 1, 8, 15, 22 | |
| L-Asparginase | 3000 U/m2 | 9, 11, 13, 16, 18, 20 | |
| Prednisolone | 60 mg/m2 | 1-21** | |
| Consolidation Phase I & IV | Cytarabine | 2000 mg/m2 | 1-3 (twice a day) |
| Etoposide | 100 mg/m2 | 1-3 | |
| Dexamethasone | 40 mg/bodies | 1-3 | |
| Consolidation Phase II & V | Methotrexate | 1500 mg/m2 | 1, 15 |
| Vincristine | 1.3 mg/m2* | 1, 15 | |
| Mercaptopurine (6-MP) | 25 mg/m2 | 1-21 | |
| Consolidation Phase III | Vincristine | 1.3 mg/m2* | 1, 8, 15 |
| Doxorubicin | 30 mg/m2 | 1, 8, 15 | |
| Dexamethasone | 10 mg/m2 | 1-8, 15-22 | |
| Cyclophosphamide | 1000 mg/m2 | 29 | |
| Mercaptopurine (6-MP) | 60 mg/m2 | 29-42 | |
| Cytarabine | 75 mg/m2 | 29-33, 36-40 | |
| Maintenance (repeat until 2 years from onset) | Vincristine | 1.3 mg/m2* | 1 |
| Prednisolone | 60 mg/m2 | 1-5 | |
| Methotrexate | 20 mg/m2 | 1, 8, 15, 22 | |
| Mercaptopurine (6-MP) | 60 mg/m2 | 1-28 |
*Max 2 mg
**tapered in 1 week, days 22-28.
Discussion and Conclusions
According to the 2001 WHO classification and EGIL scores,1,2 which are the established diagnostic criteria for BAL, a diagnosis of BAL in our patient was confirmed. This is due to scoring (>2 points) that includes blasts of B-lymphoid lineage consistent with morphological ALL, as well as myeloid lineages such as CD13 and CD33. However, in the 2008 WHO classification, the criteria for myeloid lineage were revised so that MPO or monocytic differentiation was a necessary condition. Acute leukemia with dual phenotype was classified in a new category called mixed phenotype acute leukemia (MPAL).6 According to these diagnostic criteria, our case would not fall under MPAL.
About one-third of cases of BAL have the Philadelphia chromosome, and some cases are associated with the t(4;11)(q21;q23) or other 11q23 abnormalities.1 We observed 4q21 abnormalities (AF4 gene) in our patient, but could not confirm 11q23 abnormalities. Although a case of APL by the G-banding method with the insertion of PML-RARα fusion gene in 4q21 was previously reported,7 we could find no evidence of a relationship between 4q21 abnormalities and t(15;17) in our patient.
Although t(15;17) and PML/RARα fusion are regarded as highly specific for APL, they have only been reported in rare cases of AML that were neither morphologically nor immunophenotypically consistent with APL.8,9 Moreover, BAL with t(15;17) is extremely rare. Scolnik et al. reported a case of a 7-year old girl who received a combined therapy; she was first treated with ALL protocols, changing to AML protocols in combination with ATRA in a second instance. She showed a good response and achieved hematologic CR.10
The localization of breakpoints at PML/RARα had not been clearly defined, being variously identified as 15q22-q24 and 17q11-q21. More exactly, the precise location of the RARα breakpoint had been the subject of variable reports in the literature. The PML/RARα breakpoint was unified to 15q22 and 17q12 according to the 2001 WHO classification.1 Before 2001, 3 other cases of t(15;17) translocations similar to our case had been described, where the PML/RARAα rearrangement was absent by FISH, although the chromosomal breakpoints were 15q22-q24 and 17q11-q21.11-13 In these cases (including ours), the break- points are considered to be subtly different from PML/RARα. The previously reported 3 cases were non-APL (M2, M2, M5a), with low sensitivity to chemotherapy (ATRA was administered in 2 cases without effect), and all the patients had died within 1.5 years.
Recently, chemotherapy for ALL has been shown to be effective for MPAL.14 The prognosis appears to be unfavorable, particularly in adults; the occurrence of the t(4;11) or the Philadelphia chromosome are particularly unfavorable prognostic findings.3,15 On the other hand, Arabi et al. noted CR rates of 78% and an overall survival probability at two years of 60% in 31 adult BAL patients, excluding t(9;22)(q34;q11)-positive cases, undergoing mainly Hyper-CVAD therapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone combined with high-dose cytarabine and methotrexate).16 On the basis of the morphology and immunophenotype of the blasts at onset, we determined our case to be BAL, also consistent with CD33 strongly positive ALL. As such, we initially managed the patient with induction chemotherapy for ALL and achieved CR. We considered CD33-positive minimal residual disease and added GO to the standard JALSG-ALL protocol as consolidation and maintenance treatment. There was no evidence of recurrence, and this management approach achieved and maintained a first CR of over 3.7 years. We believe that not only chemotherapy for ALL, but also a low, divided dose of GO, was effective in treatment.
In conclusion, we report an extremely rare case of BAL with t(15;17) lacking PML/RARα rearrangement. The clinical course of this patient is proceeding satisfactorily with chemotherapy as for ALL and GO.
References
- 1.Brunning RD, Matutes E, Borowitz M, et al. Acute leukemias of ambiguous lineage. : Jaffe ES, Harris NL, Stein H, Vardiman JW, World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp 106-107 [Google Scholar]
- 2.Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995;9:1783-6 [PubMed] [Google Scholar]
- 3.Legrand O, Perrot JY, Simonin G, et al. Adult biphenotypic acute leukaemia: an entity with poor prognosis which is related to unfavourable cytogenetics and P-glycoprotein over-expression. Br J Haematol 1998;100:147-55 [DOI] [PubMed] [Google Scholar]
- 4.Rowley JD, Golomb HM, Dougherty C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet 1977;1:549-50 [DOI] [PubMed] [Google Scholar]
- 5.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 1997;337:1021-8 [DOI] [PubMed] [Google Scholar]
- 6.Borowitz MJ, Bene M-C, Harris NL, et al. Acute leukaemias of ambiguous lineage. : Swerdlow SH, Campo E, Harris NL, World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. pp 150-155 [Google Scholar]
- 7.Haraguchi K, Ohno N, Tokunaga M, et al. Masked t(15;17) APL with the insertion of PML-RARalpha fusion gene in 4q21. Leuk Res 2009;33:1552-5 [DOI] [PubMed] [Google Scholar]
- 8.Allford S, Grimwade D, Langabeer S, et al. Identification of the t(15;17) in AML FAB types other than M3: evaluation of the role of molecular screening for the PML/RARalpha rearrangement in newly diagnosed AML. The Medical Research Council (MRC) Adult Leukaemia Working Party. Br J Haematol 1999;105:198-207 [PubMed] [Google Scholar]
- 9.Virchis A, Massey E, Butler T, et al. Acute myeloblastic leukaemias of FAB types M6 and M4, with cryptic PML/RARalpha fusion gene formation, relapsing as acute promyelocytic leukaemia M3. Br J Haematol 2001;114:551-6 [DOI] [PubMed] [Google Scholar]
- 10.Scolnik MP, Aranguren PN, Cuello MT, et al. Biphenotypic acute leukemia with t(15;17). Leuk Lymphoma 2005;46:607-10 [DOI] [PubMed] [Google Scholar]
- 11.Di Bona E, Montaldi A, Guercini N, et al. A (15;17) translocation not associated with acute promyelocytic leukaemia. Br J Haematol 1996;95:706-9 [DOI] [PubMed] [Google Scholar]
- 12.Zhang XX, Robinson LJ, Stenzel TT, Qumsiyeh MB. Translocation (15;17) (q22;q21) as a secondary chromosomal abnormality in a case of acute monoblastic leukemia with tetrasomy 8. Cancer Genet Cytogenet 1999;113:9-13 [DOI] [PubMed] [Google Scholar]
- 13.Kwan Ma ES, Au Wy, Kong Wan TS, et al. Translocation (15;17)(q22;q21) not associated with acute promyelocytic leukemia and negative for PML/RARa rearrangement. Haematologica 2000;85:768-9 [PubMed] [Google Scholar]
- 14.Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood 2011;117:3163-71 [DOI] [PubMed] [Google Scholar]
- 15.Killick S, Matutes E, Powles RL, et al. Outcome of biphenotypic acute leukemia. Haematologica 1999;84:699-706 [PubMed] [Google Scholar]
- 16.Aribi A, Bueso-Ramos C, Estey E, et al. Biphenotypic acute leukaemia: a case series. Br J Haematol 2007;138:213-6 [DOI] [PubMed] [Google Scholar]


