Abstract
We present the results of the treatment of infected primary or delayed spine wounds after spinal surgery using negative pressure wound therapy. In our institution (University Hospital Zurich, Switzerland) nine patients (three women and six men; mean age 68.6, range 43-87 years) were treated in the period between January to December 2011 for non-healing spinal wounds. The treatment consisted of repeated debridements, irrigation and temporary closure with negative pressure wound therapy system. Three patients were admitted with a spinal epidural abscess; two with osteoporotic lumbar fracture; two with pathologic vertebra fracture and spinal cord compression, and two with vertebra fracture after trauma. All nine patients have been treated with antibiotic therapy. In one case the hardware has been removed, in three patients laminectomy was performed without instrumentation, in five patients there was no need to remove the hardware. The average hospital stay was 16.6 days (range 11-30). The average follow-up was 3.8, range 0.5-14 months. The average number of negative pressure wound therapy procedures was three, with the range 1-11. Our retrospective study focuses on the clinical problems faced by the spinal surgeon, clinical outcomes after spinal surgery followed by wound infection, and negative pressure wound therapy. Moreover, we would like to emphasize the importance for the patients and their relatives to be fully informed about the increased complications of surgery and about the limitations of treatment of these wounds with negative pressure wound therapy.
Key words: spinal infections, vacuum assisted therapy, infection, spinal surgery
Introduction
Infection of the spine after instrumentation is a common complication causing a challenge for the spinal surgeon. Various treatment options for debridement, soft-tissue management and antibiotic therapy have been recommended with mixed results. Accurate diagnosis and therapy is essential in order to effectively treat this condition. The reported incidence in the literature following posterior spinal instrumentation is from 2.6% to 3.8%.1-3 The clinical diagnosis is often difficult. In case of very unspecific presentation, a physical examination for neurological deficits, laboratory changes, and proper radiological imaging is crucial. In many cases the first clinical presentation is unspecific pain, increased laboratory infection parameters and discharge from the postoperative wound. In general, it is very important to recognize the possible risk factors which may influence the outcome of the treatment.
Some authors reported that age greater than 60 years, smoking, diabetes, previous infection, increased body mass index (BMI) and alcohol abuse were significant preoperative risk factors in patients after spinal surgery procedures.4 In contrary, a previous surgery and steroid use did not increase the infection rate. The application of negative pressure wound therapy (NPWT) causes the formation of granulation tissue, and increases debridement of necrotic tissue. In addition, it works as a dressing by covering the wound. Recently, we observe increased application of this type of therapy for different non healing wounds.5,6 In our retrospective study we evaluated the clinical outcome, preoperative risk factors, and bacterial specimens causing infection after vacuum-assisted therapy of spinal wounds in older patient’s population.
Materials and Methods
We have retrospectively reviewed all patients’ records out of our electronic hospital patient registration system that underwent surgery for acute spinal infection (within two months after primary surgery), and delayed deep spinal wound infection (signs of infection later than two months after surgery) between January to December 2011. There were nine patients (three women and six men; mean age 68.6, range 43-87 years).
Three patients were transferred to our department with epidural abscess (in thoracic, lumbar spine and thoracolumbar junction) and six were treated because of delayed wound infections after previous spinal surgeries. Three patients presented with spinal epidural abscess, two with osteoporotic lumbar fracture, two with pathologic vertebra fracture and spinal cord compression, one with burst fracture and the last with distraction injury. The established standard treatment protocol, in our institution includes multiple surgical debridement, irrigation, and temporary closure with NPWT dressing using the polyurethane foam.
In one case removal of hardware was necessary (patient no. three – after thoracic instrumentation). The decision for removal of the implant was based on experience of the spinal surgeon. The diagnosis of infection was made based on clinical presentation (e.g., persistent back pain, fever, discharge from the wound, increased inflammatory parameters, and radiographic imaging). The microbiological sampling was performed in the theatre during surgical procedure in all cases. All patients were treated with intravenous antibiotics based on microbial sensitivity tests according to the protocol of the department of infectious diseases in our hospital. The time between spinal surgery and occurrence of infection has been assessed. Moreover, operative reports, duration of the surgery, number of procedures, and hospital stay were recorded. In addition, the duration of the postoperative antibiotics, time before the secondary wound closure and patients outcome were evaluated. Six patients presented with deep subfascial infection conformed by positive microbiology reports. The treatment included repeated debridements, irrigation with jet-lavage, and removal of the hardware if necessary, until the negative microbiology reports were available before secondary wound closure was performed. The follow-up included clinical assessment of the wound, inflammatory parameters, and patient’s general condition.
In case of this retrospective analysis of electronic patients’ records, no approval of our institutional board was necessary. The analysis was performed in compliance with the Helsinki Declaration.
Results
Between January and December 2011, nine patients (three with primary infection of the spine, and six with delayed wound infection (Figure 1), (time from primary spinal surgery to manifestation of infection – mean was 41.8, range 11-150 days) were treated with NPWT. Three patients presented with epidural abscess were treated with laminectomy and drainage. One patient with osteoporotic lumbar fracture and one with thoracic vertebral fracture after trauma were treated with instrumentation. Four patients with myelon compression caused by; metastatic disease (two patients – metastatic breast, and colon carcinoma, respectively), one with osteoporotic fracture, and one with distraction injury of the spine after trauma (Table 1). Our cohort presented with following risk factors and comorbidities: smoking – two, alcohol abuse – one, arterial hypertension – three, acute kidney failure – one, coronary heart disease – one, pulmonary fibrosis – one, tumor – three (two metastatic – breast, and colon, one cutaneous T-cell lymphoma), steroids – two, previous chemotherapy – two, hepatitis C, and HIV – one (Table 2). The mean time from surgery to diagnosis of delayed infection in all six cases was 41.8 days (range 11-150). In one case the implant were removed. After microbiological sampling, cephalosporin antibiotics (the second generation) was used as initial intra-operative antibiotic therapy in all patients. Antibiotic therapy was adjusted after sensitivity results were available, according to the protocol of the department of infectious diseases. The mean time of antibiotic therapy after first debridement and irrigation was 51.4, range 8-150 days.
Figure 1.
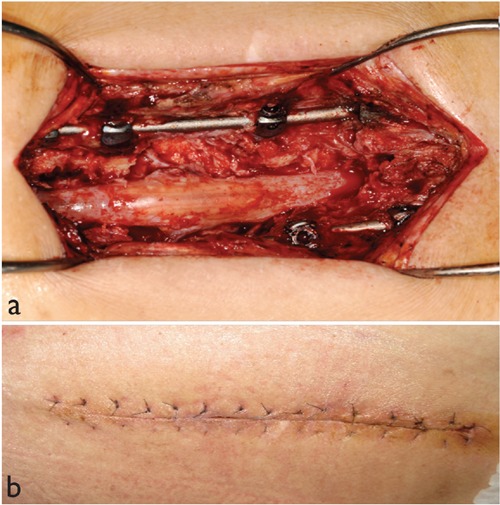
Clinical pictures demonstrate deep subfascial infection after spinal surgery (a), delayed primary closure of the same wound after negative pressure wound therapy (b).
Table 1.
Indication for spinal surgery and performed procedure.
| Patient | Indication for surgery | Surgery |
|---|---|---|
| 1 | Epidural abscess L3-S1 | Laminectomy |
| 2 | Osteoporotic fracture L2 with myelon compression | Laminectomy , kyphoplasty , and posterior instrumentation |
| 3 | Pathologic fracture Th5-Th7 and L5with myelon compression- breast ca. | Laminectomy, kyphoplasty, and posterior instrumentation |
| 4 | Burst Fracture Th2 | Posterior instrumentation |
| 5 | Epidural abscess Th2-Th8 | Laminectomy |
| 6 | Osteoporotic fracture L2 | Posterior intrumentation |
| 7 | Pathologisc fracture L3 with myelon compression- colon ca | Laminectomy and posterior instrumentation |
| 8 | Epidural abscess at thoracolumbar junction | Laminectomy |
| 9 | Distraction injuriy Th12 with myelon compression | Laminectomy and posterior instrumentation |
Table 2.
Risk factors, demographic data, number of procedures, and the duration of negative pressure wound therapy.
| Patient | Sex/Age | Surgery | Risk factors | Procedures | Duration, days | Hospital stay | Final wound closure |
|---|---|---|---|---|---|---|---|
| 1 | M/43 | Laminectomy | Smoking, HCV, HIV | 3 | 6 | 21 | Delayed primary closure |
| 2 | F/83 | Laminectomy, kyphoplasty, and posterior instrumentation | Art. hypertension | 2 | 3 | 9 | Delayed primary closure |
| 3 | F/75 | Laminectomy, kyphoplasty, and posterior instrumentation | Art. hypertension, kidney tranplant, steroids, metastatic breast cancer | 2 | 10 | 30 | Delayed primary closure |
| 4 | M/73 | Posterior instrumentation | Smoking, alcohol, cardiomyopathy, pulmonary fibrosis | 11 | 15 | 17 | Latissimus muscle flap, and than local rotation flap with mesh grafting |
| 5 | M/44 | Laminectomy | None | 2 | 5 | 13 | Delayed primary closure |
| 6 | M/87 | Posterior intrumentation | Coronary heart disease | 1 | 3 | 14 | Delayed primary closure |
| 7 | M/65 | Laminectomy and posterior instrumentation | Matastatic colon cancer, chemotherapy, steroids | 1 | 3 | 11 | Delayed primary closure |
| 8 | F/80 | Laminectomy | Acute kidney failure, art. hypertension | 2 | 19 | 19 | Delayed primary closure |
| 9 | M/68 | Laminectomy and posterior instrumentation | Cutaneous T-cell- lymphoma | 3 | 7 | 16 | Delayed primary closure |
The primary and secondary infecting organisms from sampling, and the antibiotic therapy have been summarized in Table 3. Gram positive bacteria caused mostly the wound infections, one patient presented with methicillin-resistant Staphylococcus aureus. In eight patients we had to change antibiotic therapy (Table 3). All patients were treated with repeated surgical debridements, irrigation with saline and NPWT. The mean time of all surgical procedures was 145.5 min, range 45-450 min. The total number of NPWT was mean three, range 1-11 (Table 2). All NPWT changes were performed in the theatre. The mean of the total time of NPWT during hospital stay was 7.8 range 3-19 days. The median duration of antibiotic therapy was 51.1, range 9-150 days. In eight patients, secondary wound closure could be achieved (Table 2). In one case, a free vascular muscle flap (latissimus dorsi muscle flap) was needed for closure. Unfortunately during the postoperative course the flap became necrotic, and definitive wound closure was achieved using a local rotation flap in combination with mesh grafting. The mean initial C-reactive protein (CRP) value was 149, range 0.4-367 mg/dL. The mean follow-up for all patients was 3.8, range 0.5-14 months. The clinical outcome of the vacuum assisted therapy was as follows: one patient (80 year old) died during hospital stay because of sepsis and multi-organ failure caused by MRSA infection, one patient with metastatic colon carcinoma (65 year old) died during hospital stay because of acute pulmonary decompensation, another patient (75 year old) with metastatic breast carcinoma died two months after NPWT in rehabilitation clinic because of urosepsis, two patients (73 and 87 year old) with infect after posterior instrumentation died about 3.5 months after hospital discharge in rehabilitation clinic because of pneumonia, two patients were already retired before NPWT therapy, one patient was able to return to his previous job as IT specialist, one patient receives disability pension. In our cohort, only one patient died due to ongoing MRSA infection, despite the wound was closed. All mortalities were related to severe co-morbidity of the patients, despite of absence of local signs of infection after NPWT.
Table 3.
Microbiological sampling, antibiotic treatment, and duration of antibiotic therapy.
| Patient | Bacterial specimen, initial sampling | Bacterial specimen, sampling during antibiotic therapy | Initial antibiotic therapy | Final antibiotic therapy | Duration of antibiotic therapy (days) |
|---|---|---|---|---|---|
| 1 | Enterococcus faecalis, Staphylococcus aureus | Enterococus faecalis, Staphylococcus aureus | Flucloxacillin,gentamicin, amoxicillin | Amoxicillin, sulfamethoxazole and trimethoprim | 150 |
| 2 | Enterococcus sp. | Enterococcus sp. | Amoxicillin + clavulanic acid | Ciprofloxacin, rifampicin | 55 |
| 3 | Enterococcus faecalis, Staphylococcus aureus | Enterococcus faecalis, Staphylococcus aureus | Ciprofloxacin, Meropenem daptomycin | 15 | |
| 4 | Coagulase-negative Staphylococcus | Coagulase-negative Staphylococcus | Vancomycin | Linezolid | 62 |
| 5 | Staphylococcus aureus | Staphylococcus aureus | Flucloxacilin, meropenem | Ciprofloxacin, flucloxacilin | 61 |
| 6 | Negative | Negative | Vancomycin | Ciprofloxacin, rifampicin | 25 |
| 7 | Coagulase-negative Staphylococcus | Coagulase-negative Staphylococcus | Vancomycin | Ciprofloxacin | 9 |
| 8 | Methicillin-resistant staphylococcus aureus | Methicillin-resistant staphylococcus aureus | Ciprofloxacin, daptomycin | Ciprofloxacin, rifampicin | 20 |
| 9 | Staphylococus aureus | Staphylococus aureus | Amoxicillin + clavulanic acid | Ciprofloxacin, rifampicin | 67 |
Discussion
NPWT is today after its introduction in the late Nineties of the last Century, a very widely used treatment modality, which promotes granulation of the tissue, results in removal of fluid edema, and improves blood supply to the granulation tissue.7,8 The wound healing can be subdivided into three phases: inflammation, proliferation and tissue remodeling in the wound environment. In the inflammation phase, the complement system plays the most important role.9,10 It can be activated through different cellular pathways. Activated complement system increases the phagocytosis, and removal of the damaged cells by macrophages. These cells stimulate endothelial cells in blood vessels, and fibroblasts in the wound environment to release vascular endothelial growth factors (VEGF), and fibroblast growth factors (FGF) to stimulate specific pathways, which leads to angiogenesis (formation of new blood vessels), and cell proliferation.11,12 It is of great importance to understand these very complex mechanisms, in order to treat properly infected wounds. Currently, the NPWT is an established treatment option for this challenging entity, not only for spinal surgeon. Moreover, it reduces postoperative complications, hospital stay, medical costs, and morbidity of the patients.13 The NPWT is a very useful technique in older patients with multiple comorbidities, since it may decrease time of treatment, which can allow faster ambulation and rehabilitation. In addition, patients treated with this system may not need hardware removal or complex reconstructive procedures done to cover the wound defect. Some publications propose leaving the infected wound open at the time of debridement and jet-lavage, and to close it in a delayed-staged fashion.14
Others suggested that infected wound can be successfully treated by repeated dressing changes and healing by secondary intention.15-17 Lehner et al.18 showed results of the treatment of infected orthopedic implants with negative pressure wound therapy with instillation (NPWTi). Thirty two patients were treated with this system after diagnosis of early or late implant infection. This multi-center prospective, non-randomized study demonstrated that 86.4% patients with acute implant infection, and 80% with chronic infection were successfully treated, and were able to retain their implant at the six months follow-up. The mean duration time of NPWTi was 16.3 days, and mean number of procedures performed during hospital stay was 3.5. Our results are quite consistent with their results.
Moreover, some (demographic, epidemiologic and preoperative risk factors) play an important role in the clinical outcome after posterior spinal surgery. There are some risk factors which cannot be modified, and play a major role in recovery after surgery and healing of the postoperative wounds, such as age.3,19-22 Other authors demonstrated increased infection rate in patients with diabetes, alcohol abuse, nicotine abuse, obesity, and previous surgeries.4,23-25 Olsen et al.26 showed that diabetes and elevated level of glucose preoperatively was associated independently with increased risk of infected wound. In addition, the posterior spinal approach was associated with increased risk of postoperative wound infection by about 4%. On the other hand, complexity of the surgery e.g., posterior spinal instrumentation increases the risk up to 9%.27,28 All preoperative risk factors which play an important role in wound healing after spinal surgeries were present in our patients’ population. Some reports presented promising results of the treatment of infected wound after application of NPWT in adult population after spinal surgeries.5 The authors presented twenty patients treated with this mode of therapy, which had many risk factors such as; age, diabetes, smoking, tumor or immunodeficiency. Our patient population was significantly older; average age 68.6 vs 55 years. In addition, they reported that 2.2 procedures were required to achieve clean wounds with the time of NPWT (mean) seven days. Our series shows that we needed also about seven days (mean 7.8 days) to obtain clean wounds. However, in their cohort; twelve patients were treated with two or more debridements, and irrigations under general anesthesia before NPWT was applied. We performed on average three procedures before we were able to obtain clean wound, which is consistent with their results. As a clean wound, we considered a wound without any macroscopical signs of infection (inflammation). In addition, our patients were treated with NPWT just after first debridement and irrigation. Schimmel et al.29 showed that in his series of thirty six patients the most common organism cultured from infection site, was Staphylococcus aureus, which is consistent with our observation (five patients). Further the mean time from surgery to the diagnosis of infection was 13.5 days, in comparison to our series; it was longer (mean 41.8 days). In our cohort, we had three patients presented initially with spine infect (epidural abscess) without previous surgery, five patients with acute infect (within eight weeks from spinal surgery), and one patient with late infect (longer than eight weeks after surgery). The clinical outcome after NPWT in our series was not satisfactory. It was predominantly caused by many preoperative risk factors and significantly older patients’ population. In addition, one patient with epidural abscess has been admitted already in septic state, two other patients with metastatic cancer disease treated with NPWT died during rehabilitation because of progression of the cancer.
Conclusions
The weakness of our study is the small sample size of infected patients (nine), moreover, the relatively short follow-up time. However, we would like to demonstrate that in older patients’ population with many comorbidities the treatment of infected wounds is very challenging and difficult. In order to better investigate outcome and preoperative risk factors in older patients’ population, it is necessary to evaluate the effect of only one surgical procedure in larger population. Summarizing, authors would like to emphasize the importance of full information to the patients and their relatives regarding increased complications, and limitations of this type of surgery followed by NPWT in patients at risk.
References
- 1.Glassman SD, Dimar JR, Puno RM, Johnson JR.Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976). 1996;21:2163-9 [DOI] [PubMed] [Google Scholar]
- 2.Richards BS.Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:524-9 [DOI] [PubMed] [Google Scholar]
- 3.Pullter Gunne AF, Cohen DB.Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34:1422-8 [DOI] [PubMed] [Google Scholar]
- 4.Fang A, Hu SS, Endres N, Bradford DS.Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30:1460-5 [DOI] [PubMed] [Google Scholar]
- 5.Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14-7 [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Samartzis D, Heller JG, et al. The management of complex soft-tissue defects after spinal instrumentation. J Bone Joint Surg Br. 2006;88:8-15 [DOI] [PubMed] [Google Scholar]
- 7.Timmers MS, Le Cessie S, Banwell P, Jukema GN.The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665-71 [DOI] [PubMed] [Google Scholar]
- 8.Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086-96 [DOI] [PubMed] [Google Scholar]
- 9.Eming SA, Krieg T, Davidson JM.Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514-25 [DOI] [PubMed] [Google Scholar]
- 10.Neher MD, Weckbach S, Flierl MA, et al. Molecular mechanisms of inflammation and tissue injury after major trauma—is complement the bad guy? J Biomed Sci. 2011;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner S, Grose R.Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835-70 [DOI] [PubMed] [Google Scholar]
- 12.Cazander G, Jukema GN, Nibbering PH.Complement activation and inhibition in wound healing. Clin Dev Immunol. 2012;2012:534291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stannard JP, Gabriel A, Lehner B.Use of negative pressure wound therapy over clean, closed surgical incisions. Int Wound J. 2012;9:32-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szoke G, Lipton G, Miller F, Dabney K.Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18:727-33 [PubMed] [Google Scholar]
- 15.Mees J, Mardin WA, Senninger N, et al. Treatment options for postoperatively infected abdominal wall wounds healing by secondary intention. Langenbecks Arch Surg. 2012;397:1359-66 [DOI] [PubMed] [Google Scholar]
- 16.Lewis R, Whiting P, ter Riet G, et al. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention. Health Technol Assess. 2001;5:1-131 [DOI] [PubMed] [Google Scholar]
- 17.Jung JY, Roh HJ, Lee SH, et al. Comparison of secondary intention healing and full-thickness skin graft after excision of acral lentiginous melanoma on foot. Dermatol Surg. 2011;37:1245-51 [DOI] [PubMed] [Google Scholar]
- 18.Lehner B, Fleischmann W, Becker R, Jukema GN.First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop. 2011;35:1415-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill PJ, Warnick DE, Lee MJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976). 2010;35:1211-7 [DOI] [PubMed] [Google Scholar]
- 20.Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2011;36:556-63 [DOI] [PubMed] [Google Scholar]
- 21.Schuster JM, Rechtine G, Norvell DC, Dettori JR.The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976). 2010;35:125-37 [DOI] [PubMed] [Google Scholar]
- 22.Pull ter Gunne AF, van Laarhoven CJ, Cohen DB.Surgical site infection after osteotomy of the adult spine: does type of osteotomy matter? Spine J. 2010;10:410-6 [DOI] [PubMed] [Google Scholar]
- 23.Lonjon G, Dauzac C, Fourniols E, et al. Early surgical site infections in adult spinal trauma: a prospective, multicentre study of infection rates and risk factors. Orthop Traumatol Surg Res. 2012;98:788-94 [DOI] [PubMed] [Google Scholar]
- 24.Linam WM, Margolis PA, Staat MA, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol. 2009;30:109-16 [DOI] [PubMed] [Google Scholar]
- 25.Milstone AM, Maragakis LL, Townsend T, et al. Timing of preoperative antibiotic prophylaxis: a modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatr Infect Dis J. 2008;27: 704-8 [DOI] [PubMed] [Google Scholar]
- 26.Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62-9 [DOI] [PubMed] [Google Scholar]
- 27.Kurtz SM, Lau E, Ong KL, et al. Infection risk for primary and revision instrumented lumbar spine fusion in the Medicare population. J Neurosurg Spine. 2012;17:342-7 [DOI] [PubMed] [Google Scholar]
- 28.Memtsoudis SG, Vougioukas VI, Ma Y, et al. Perioperative morbidity and mortality after anterior, posterior, and anterior/posterior spine fusion surgery. Spine (Phila Pa 1976). 2011;36:1867-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J: Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19:1711-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


