Abstract
In clinical practice viscosupplementation with hyaluronic acid (HA) is common for the treatment of degenerative osteoarthritis (OA). Both molecular weight and concentration of HA have significant impact on its rheological properties, which in turn affects its therapeutic effects. The objective of this study is to evaluate the effectiveness of a double HA preparation for the treatment of knee osteoarthritis with respect to pain reduction, joint function improvement and concomitant medication consumption reduction. One thousand and fourteen patients (521 males and 693 females) with a mean age of 62.4 years old, suffering from OA of the knee, were enrolled into this study. All patients received two intra-articular injections one week apart and a third injection one month after the second one. Concomitant medication was recorded and evaluated at follow up visits. Evaluation was performed at baseline, day 30 and day 180, on several parameters: knee pain by visual analog scale (VAS) 0-10 cm, Lequesne Index, and consumption of concomitant medications including non-steroidal anti-inflammatory drugs, analgesics and chondoprotective supplementations. A statistically significant reduction in pain VAS score was recorded at D30 (38.01±17.68; P<0.01) before the third injection, and D180 (25.91±15.33; P<0.01) check-points comparing to baseline (67.12±15.99). Similarly, remarkable reduction in Lequesne Index was shown at D30 (5.91±4.01; P<0.01) in 1214 patients before the third injection, and D180 (3.59±3.45; P<0.01) (with 938 patients) when compared to the baseline (11.60±5.13). Patients also consumed less concomitant medications after the treatment course. The beneficial effects were maintained for up to six months. Intra-articular injection of a double HA preparation of low molecular weight and high molecular weight of different concentrations was well tolerated, and generated satisfactory results in terms of pain control, joint function improvement and concomitant medication reduction for the management of knee OA.
Key words: intraarticular, hyaluornic acid, knee osteoarthritis
Introduction
Osteoarthritis (OA) is the most common form of arthritis. Epidemiological studies have estimated that symptomatic radiographic knee osteoarthritis (OA) affects 10% of adults over 55 years old.1 According to the World Health Organization (WHO), OA is likely to become a greater cause of concern amongst physicians due to the global trend of aging population and increasing life expectancy. On the other hand, OA is a great burden on healthcare resources due to both morbidity and treatment costs. It was reported that average direct cost of OA is approximately 2600 US Dollars per year per person in US in 1997,2 while average annual cost for osteoarthritis-related treatment for newly diagnosed patients were 6811 per year in 2012.3
According to the treatment guidelines on knee OA from the American College of Rheumatology (ACR), and the European League Against Rheumatism (EULAR), analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) are the first line options. However, given the fact that NSAIDs may cause potential systemic side effects, caution must be taken when using NSAIDs especially for the elderly population which is also the largest population of OA patients. Viscosupplementation with HA is indicated for patients who do not respond to non-pharmacological therapy or pain control of analgesics including acetaminophen.4 The short-term and long-term therapeutic effects of intra-articular HA preparations in pain relief and joint function improvement in the knee OA patients have been repeatedly shown in various clinical trials compared to placebo, or to intra-articular injection of corticosteroids.5-7
Both molecular weight and concentration of HA have significant impact on its rheological properties, which play an important role on therapeutic effects of the viscosupplementation therapy. Petrella et al.8 reported a new preparation of double HA with two molecular weights and concentrations to be effective in controlling symptoms and improving joint mobility, with shortened treatment cycle. In clinical settings, physicians may require further support in considering the attributes of this double preparation of HA for osteoarthritis. The objective of this study was to evaluate the effectiveness of the double HA preparation with two molecular weights and concentrations on pain control, joint function improvement and concomitant medication reduction in osteoarthritis of the knee. The double HA preparation is a sequential injection of two different HA solutions, of low molecular weight (LMW) at 2.2% concentration and high molecular weight (HMW) at 1.0% concentration in a single administration.
Study design
More than 50 physicians from different regions in Italy participated in this study. All investigators used identical case report forms to record data. At the first visit, patients were evaluated to set up baseline data and received the first intra-articular injection of HA. After one week, patients received the second injection without evaluation of efficacy parameters. At day 30 after the baseline, patients received a third injection and underwent an assessment of pain reduction joint function and consumption of concomitant medication. The last assessment was scheduled six months from the baseline. Assessment was performed on joint pain, joint function and concurrent medication; knee pain by visual analog scale (VAS) 0-10 cm, Lequesne Index,9 and consumption of concomitant medication including NSAIDs, analgesics and chondoprotective (Supplementary Table 1 and Supplementary Figure 1).
Table 1.
Reduction in pain VAS score and Lequense Index.
| Total number of patient | Lequesne Index baseline | Lequesne Index L D30 | Lequesne Index D180 | VAS pain baseline | VAS pain D30 | VAS pain D180 |
|---|---|---|---|---|---|---|
| 1214 | 11.60±5.13 | 5.91 ±4.01 | 66.38±16.14 | 38.21±17.21 | ||
| 938 | 3.59±3.45 | 25.91±16.33 |
Figure 1.
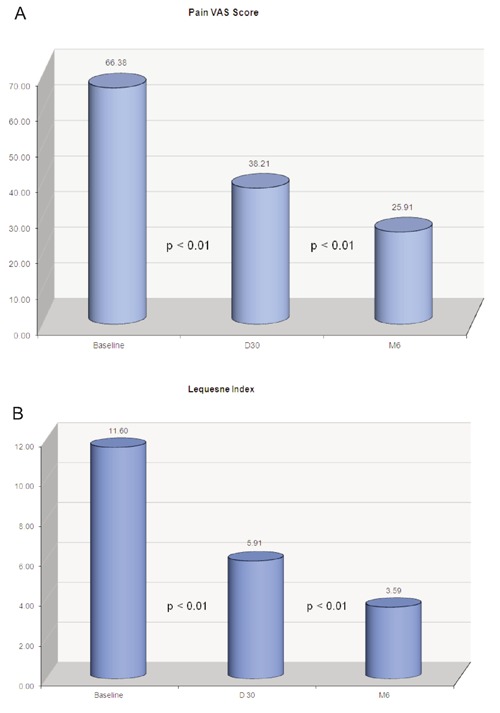
A) Pain VAS Score; B) Lequesne Index: 1214 patients followed up at D30; 938 patients followed up at M6.
Materials and Methods
Between February 2011 and September 2012, 1214 patients of both sexes suffering from OA of the knee were enrolled into this study, with 1129 (93%) cases of grade I-III of knee OA according to Kellegren-Lawrence classification scale. There were 521 males (43%) and 693 female (57%), with a mean age of 62.4 years old (for patients characteristics see Supplementary Table 2).
Table 2.
Subscores of Lequense Index.
| Night rest | Morning stiffness | Pain increase | Pain at movement | Pain sitting up | Max Dist | Max Dist with help | Climb stairs | Descend stairs | Crouch | Irregular surface walk | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1.01 | 1.45 | 0.63 | 1.17 | 0.65 | 2.47 | 0.20 | 0.38 | 0.47 | 0.68 | 0.39 |
| D30 | 0.48 | 0.46 | 0.26 | 0.60 | 0.29 | 1.34 | 0.12 | 0.19 | 0.22 | 0.28 | 0.19 |
| D180 | 0.31 | 0.29 | 0.14 | 0.40 | 0.16 | 1.03 | 0.06 | 0.07 | 0.09 | 0.18 | 0.08 |
Patients over 18 years old with clinically confirmed diagnosis of knee OA for at least four months, and available for the duration of the study were screened and enrolled. Exclusion criteria included patients receiving anticoagulants; with previous fracture of the knee; with prosthesis or fixation of the knee; systemic neuromuscular disorders; use of regular NSAIDs for four months prior to entry; history of drug or alcohol abuse; pregnant or lactating, currently using other intra-articular HA injections; or having received other investigational product within 30 days of the entry.
The HA preparation (RenehaVisTM) under study was a clear solution consisting of 0.7 mL of sterile 2.2% LMW (1000 KDaltons) HA and 0.7 mL of sterile 1% HMW (2000 KDaltons) HA, separated by a bypass stopper within a pre-filled sterile syringe.
The study was carried out under real life practice settings. All topical or general concomitant medications were allowed during the study period if it was considered appropriate for the best interests of the patient by the treating physician. These topical or general concomitant medications included and were not limited to: rest, assistive devices, exercise, manual therapy, hydrotherapy, NSAIDs, COX-2 inhibitors, analgesics, chondroitin sulfate, glucosamine sulfate. Concomitant medication was recorded in the case report form (CRF).
Statistical analysis
The Microsoft Windows programs were used for data management and statistical analysis. The level of significance was set at 0.05 for all statistical tests. The comparison of parametric values (VAS, Lequense Index, concomitant consumption) was made with the Student t-test.
Results
One thousand and fourteen patients were recruited and completed the three injection treatment course at D30; 938 patients were further followed up at Month 6 according to the study protocol.276 patients completed the three injection treatment and were followed up at D30 but did not complete the Month 6 follow up. A statistically significant reduction in pain VAS score was recorded at D30 (38.01±17.68; P<0.01) and D180 (25.91±15.33; P<0.01) check-points comparing to baseline (67.12± 15.99). Similarly a remarkable reduction in Lequesne Index was shown at D30 (5.91±4.01; P<0.01), with total 1214 patients and D180 (3.59±3.45; P<0.01) with 938 patients if compared to the baseline (11.60±5.13) (Table 1). More importantly, there is a remarkable improvement in all the sub-scores in the three sub-sets of Lequesne Index: pain, maximum distance and daily life activities (Table 2).
Further investigation of the patients’ subgroups to the OA grade shows a statistically significant decrease in both Lequesne index and pain VAS score. These were recorded in all the subgroups at D30 and D180 checkpoints, compared to baseline (P<0.01). A comparison between groups showed grades 3 and 4 OA groups had slightly better improvement in scoring compared to grade 2 OA group, which in turn was better than the grade 1 group, although these differences were not statistically significant. It is worth noticing that the grade 4 OA group responded extremely well to the intra-articular injections in this study, which is contrary to the general impression that IA HA is effective in mild and moderate OA management.
The correlation between the severity of disease and improvement of pain and joint function after HA injections was not clearly demonstrated in this study due to the limited data obtained (Figures 1 and 2). There were much less patients in the early stage or late stage of OA (10% grade 1, and 7% grade 4) recruited in this study (Table 3). Another limitation of the study is radiological data were not recorded in this study therefore the OA classification is hard to verify.
Table 3.
Subgroup breakdown by OA grade.
| Lequesne Index baseline | Lequesne Index D30 | Lequesne IndexD180 | VAS pain baseline | VAS pain D30 | VAS pain D180 | |
|---|---|---|---|---|---|---|
| Grade 1 | 5.10 | 2.74 | 1.34 | 53.88 | 32.13 | 24.00 |
| SD | 3.55 | 3.54 | 3.25 | 12.70 | 15.69 | 16.54 |
| Grade 2 | 6.30 | 2.85 | 2.06 | 58.93 | 33.50 | 22.71 |
| SD | 4.40 | 3.20 | 2.50 | 13.68 | 15.68 | 13.66 |
| Grade 3 | 8.40 | 3.94 | 2.61 | 76.40 | 42.25 | 28.23 |
| SD | 7.62 | 4.33 | 3.80 | 10.57 | 18.23 | 17.29 |
| Grade 4 | 10.52 | 6.38 | 3.00 | 85.92 | 51.13 | 36.06 |
| SD | 8.56 | 5.83 | 4.45 | 10.63 | 16.78 | 20.18 |
Consumption of concomitant medication was recorded and evaluated as one of the key parameters at all end points. It consisted of three indexes: number of patients who took concomitant medication; absolute number of tablets/capsules consumed; and the frequency of medicine intake. NSAIDs, analgesics and chondroitin sulfate were the most commonly concurrent medications that were recorded in this study (for more details see Supplementary Table 3). A remarkable reduction in all three sub-scores was registered. At baseline, there were 427 patients taking concurrently NSAIDs, while only 214 patients remained at D30. This number went down to 104 six months after the baseline. A similar trend of decrease was shown in terms of total number of tablets/capsules consumed at D30 and D180 compared to baseline. The total of 1775 tablets of NSAIDs consumed per day was recorded at baseline versus 811 tablets at D30, and 338 tablets at D180 (Table 3). The average frequency of concurrent medicine intake also decreased at D30 and D180 compared to baseline, but this less obvious.
Discussion
Degenerative OA of the knee is one of the most frequent diseases of the joints with an age dependent occurrence of 4% in 16 -24 year old patients up to 85% in 75-79 year old patients.10 HA is a naturally occurring biological substance representing an unbranched, high molecular weight polysaccharide as a major component of ligament, tendon, cartilage and synovial structure. In histopathological animal models, cartilage structure protection effect was demonstrated by high molecular weight HA (Suvenyl).11
HA viscosupplementation is commonly used in clinical practice for the management of OA of synovial joints, such as the knee, shoulder, hip and small joints in the hand. Its effectiveness for these indications was demonstrated by extensive clinical trials,12,13 and it is recommended by different scientific advisory bodies like EULAR, OARSI, and ACR.3,14,15 The Cochrane review analyzed the efficacy of intra-articular hyaluronic acid derivatives in the treatment of osteoarthritis of the knee. Overall efficacy from 76 placebo-controlled trials was reported as being comparable to that with NSAIDs and corticosteroid injections. However, the hyaluronic acid products were more efficacious from 5 to 13 weeks with regard to pain, range of motion, and WOMAC and Lequesne scores when compared with corticosteroid injections.16
Numerous studies on HA preparations with different concentrations and molecular weights showed different but generally positive clinical results.17 A randomized controlled study, high MW HA (hylan G-F 20,) showed that higher molecular weight HA might be more efficacious in WOMAC pain and stiffness scoring in treating knee OA compared to lower molecular weight HA.18 However, other meta-analyses found non-superiority results between high MW HA versus low MW HA preparations. There was also no evidence of a clinically relevant benefit of one or another.19 A recent study made head-to-head comparison between two different HA formulations, of intermediate MW (800-1500 kD, 25 mg/2.5 mL vs low MW (MW 500-730 kD, 20 mg/2 mL). The study showed that intermediate MW HA had higher proportion of OARSI/OMERACT responders than with low MW HA (73.3% vs 58.4%, P=0.001).20 Other literatures showed a trend towards a higher incidence of local adverse reactions of chemically modified high MW HA compared with lower MW products, which may be due to peptide contaminants, formaldehyde, or crystal-induced inflammation.21
Based on existing evidences, it might be concluded that both low MW and high MW HA are effective in the management of OA to certain extent, based on different rheological features. Moreover, it was demonstrated that the rheological variables characterizing the elastoviscosity of the synvial fluid is dependent on the interaction of hyaluronate molecules, its concentration and average molucular weight.22 Furthermore, it was reported that the concentration of HA might have a greater bearing on its viscosity than its molecular weight.23 Based on this, it could be postulated that giving a combination of HA solutions with different MWs and concentrations could generate better therapeutic effects than a low MW or high MW HA alone.
OA patients with different degrees of severity and character of symptoms have different elastic-viscous ratios in the synovial joint. The knee in dynamic motion requires elastic composition at an optimal molecular weight and yet, has to be kept in balance with viscous needs. For example, high frequency loading through synovial fluid is dissipated through a dynamic rheological change in HA toward more elastic modulus compared to more viscous properties when the load to HA is of low frequency.3 Since most commercial HA preparations have a limited range of molecular weight and concentration, there is no study evaluating the benefits of giving a combination of two different HA solutions at the same time. In accordance to the contents of a healthy and dynamic knee, it might be necessary to provide both a low molecular weight and high molecular weight HA in order to improve both elasticity and viscosity properties of the synovial fluid.
In a randomised placebo controlled trial, Petrella et al. compared a double HA preparation with LMW HA, HMW HA and placebo. The authors demonstrated that the double HA solutions with different molecular weight ranges and different concentrations, could generate statistically significantly lower activity-related pain, and fewer concomitant therapeutic modalities compared to either HA of low molecular weight or high molecular weight alone. Maximum improvement in VAS pain was achieved after two weekly intra-articular injections.8 In the current study, 1214 patients received a series of intra-articular injections of a double HA preparation of LMW and HMW and of different concentrations. The treatment was well tolerated. Side effects, including injection site pain, swelling, effusion were mild and transient, dissolving without intervention. No severe adverse events or systemic side effects were recorded throughout the study period. The treatment generated satisfactory results in terms of pain control, joint function improvement and concomitant medication reduction. The therapeutic effects were maintained for up to six months. This double HA preparation might provide two complementary compositions of HA that mimics the needs of the active patient with an osteoarthritic knee joint. It is worth noting that more than 50 physicians from different clinics participated in and contributed to this trial, therefore their experiences under daily life practice settings can be generalised.
Despite the fact that the study product is marketed as two weekly injections per treatment course, in this study, a third injection after one month was given to each patient after the first two injections one week apart. This was based on the assumption that a third maintenance dose would further enhance the efficacy that was achieved by the two initial doses.
This study was a questionnaire based survey. Comparing to the clinical trial, which is often designed with specific profile of patients and well defined therapeutic strategies, this was a non-interventional study defining health benefits under real-life conditions more broadly than traditional endpoints in pre-market trials. The treatment strategy adopted in this study was strictly under the physician’s responsibility as per usual medical practice, and the intra-articular injection is clearly separated from the inclusion of the patient into this study. This survey involved approximately 60 practitioners from different regions of Italy with different treatment habit. There were over 1200 patients registered in the study, a population much broader than in the relevant clinical trials performed on the product. Whilst the RCTs demonstrated substantial clinical benefits of the intra-articular injection of combined HA preparation, this study further confirms the findings from a broader patient population of larger environment in Italy.
Conclusions
The findings of this study confirmed the positive results reported from controlled trials, and suggest that the double HA of low and high ranges of MW may provide patients with a more physiologically dynamic viscosupplementation and hence a more responsive synovial rheology that controls pain and improves joint function in knee osteoarthritis.
Figure 2.
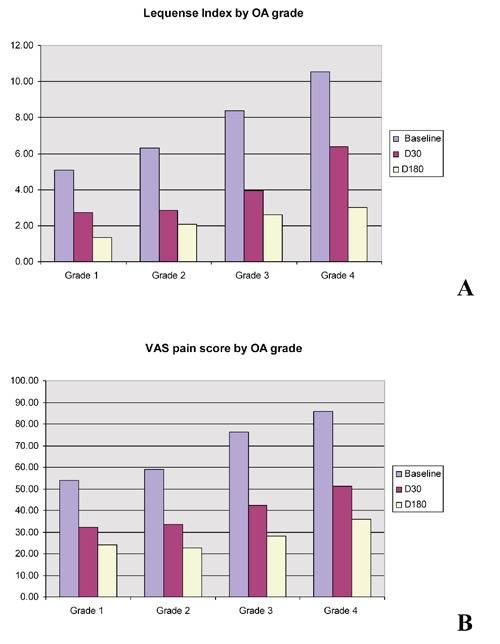
A) Lequense Index by OA grade; B) VAS Pain Score by OA grade: 1214 patients followed up at D30; 938 patients followed up at M6.
Acknowledgements
special thanks to Dr. R. Gatti for reviewing the study protocol and medical team of Athena Pharma for logistic assistance and data collection.
References
- 1.Peat G, McCarney R, Croft P.Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, Campion ME, et al. Direct medical costs unique to people with arthritis. J Rheumatol. 1997;24:719-25 [PubMed] [Google Scholar]
- 3.Le TK, Montejano LB, Cao Z, et al. Healthcare costs associated with osteoarthritis in US patients. Pain Pract. 2012;12:633-40 [DOI] [PubMed] [Google Scholar]
- 4.American College of Rheumatology Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 Update. American College of Rheumatology Subcommittee on osteoarthritis guidelines. Arthritis Rheum. 2000;43:1905-15 [DOI] [PubMed] [Google Scholar]
- 5.Balasz EA, Denlinger SL.Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3-9 [PubMed] [Google Scholar]
- 6.Dougados M, Nguyen M, Listrat V, Amor B.High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1-year placebo-controlled trial. Osteoarthritis Cartilage. 1993;1:97-103 [DOI] [PubMed] [Google Scholar]
- 7.Leopold SS, Redd BB, Warme WJ, et al. Corticosteroid compared with hyaluronic acid injections for the treatment of osteoarthritis of the knee: a prospective, randomized trial. J Bone Joint Surg Am. 2003;85:1197-203 [DOI] [PubMed] [Google Scholar]
- 8.Petrella R, Cogliano A, Decaria J.Combining two hyaluronic acids in osteoarthritis of knee: a randomized, double-blind, placebo-controlled trial. Clin Rheumatol. 2008;27:975-81 [DOI] [PubMed] [Google Scholar]
- 9.Lequesne MG.The algofunctional indices for hip and knee osteoarthritis. J Reumatology. 1997;24:779-81 [PubMed] [Google Scholar]
- 10.Dieppe P.Osteoarthritis. Acta Orthop Scand Suppl. 1998;281:2-5 [DOI] [PubMed] [Google Scholar]
- 11.Mihara M, Higo S, Uchiyama Y, et al. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthritis Cartilage. 2007;15:543-9 [DOI] [PubMed] [Google Scholar]
- 12.Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2: CD005328. [DOI] [PubMed] [Google Scholar]
- 13.Kotevoglu N, Iyibozkurt PC, Hiz O, et al. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26:325-30 [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-99 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:377-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;18: CD005321. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg DD, Stoker A, Kane S, et al. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage. 2006;14:814-22 [DOI] [PubMed] [Google Scholar]
- 18.Kotevoglu N, Iyibozkurt PC, Hiz O, et al. A prospective randomized controlled clinical trial comparing the efficacy of different molecular weight hyaluronansolutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26:325-30 [DOI] [PubMed] [Google Scholar]
- 19.Reichenbach S, Blank S, Rutjes AW, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57:1410-8 [DOI] [PubMed] [Google Scholar]
- 20.Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71:1454-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balazs EA.Viscosupplementation: a new concept in the treatment of osteoarthritis. Surg Technol Int. 2004;12:278-89 [PubMed] [Google Scholar]
- 23.Aviad AD, Houpt JB.The molecular weight of therapeutic hyaluronan (sodium hyaluronate): how significant is it? J Rheumatol 1994:21:297-301 [PubMed] [Google Scholar]


