Abstract
Intrinsic cardiac aging is an independent risk factor for cardiovascular disease and is associated with structural and functional changes that impede cardiac responses to stress and to cardio-protective mechanisms. Although systemic insulin resistance and the associated risk factors exacerbate cardiac aging, cardiac-specific insulin resistance without confounding systemic alterations, could prevent cardiac aging. Thus, strategies aimed to reduce insulin/insulin-like growth factor (IGF) signaling in the heart prevent cardiac aging in lower organisms and in mammals but the mechanisms underlying this protection are not fully understood. In this review, we describe the impact of aging on the cardiovascular system and discuss the mounting evidence that reduced insulin/IGF signaling in the heart could alleviate age-associated alterations and preserve cardiac performance.
Keywords: Insulin signaling, cardiac function, cardiac aging, mitochondria, reactive oxygen species, autophagy, fibrosis
INTRODUCTION
The number of elderly (≥65 years) people in the United States is projected to grow from 12.9% to 19.7% by 2050 [1]. Unfortunately, increased life expectancy does not come without cost, it is estimated that 80% of individuals of 65 years of age and older will have one chronic disease and 50% of them will have two chronic diseases which directly impact the overall health care expenditure. Among the many conditions associated with advance age, cardiovascular diseases (CVDs) are the most prevalent and the leading cause of death in this age group [2–3], Although altered cardiac function with age can be exacerbated by several risk factors such as hypertension, dyslipidemia, diabetes, insulin resistance and physical inactivity, intrinsic cardiac aging is an independent risk factor for the development of CVDs [4–6], The age-dependent alterations in cardiovascular function are characterized by structural and functional abnormalities impeding the heart’s responsiveness to stress and to cardio-protective interventions such as pre-conditioning and post-conditioning. The mechanisms for intrinsic cardiac aging have been extensively studied and involve (a) structural changes dominated by the development of cardiac hypertrophy, accumulation of interstitial fibrosis and arterial stiffness; (b) alterations in calcium homeostasis; (c) neuro-hormonal alterations; (d) mitochondrial dysfunction; (e) oxidative stress; (f) endothelial dysfunction and (g) impaired cellular degradation pathways. Several strategies aimed to normalized one or more of these alterations have shown promise in slowing cardiac aging; however its complete prevention has not yet been achieved.
Among the most conserved signaling pathways involved in life span extension is the insulin/insulin-like growth factor-1 (IGF-1) signaling pathway. Indeed, mutations in insulin or IGF-1 receptors or their downstream targets extended lifespan in vertebrates and invertebrates (Reviewed by Kenyon [7]). Most importantly, these signaling pathways were recently shown to impact cardiac autonomous aging in Drosophila [8] as well as in mice [9]. The mechanisms involved in the prevention of cardiac aging by suppression of cardiac insulin/IGF-1 signaling are not well understood but might involve increased stress resistance and induction of autophagy. In this review, mechanisms linking mitochondrial dysfunction, oxidative stress, insulin resistance and their impact on cardiac aging will be reviewed. Subsequently, the impact of impaired insulin signaling on cardiac aging and the underlying mechanisms will be discussed.
STRUCTURAL AND FUNCTIONAL CHANGES IN THE AGED HEART
The heart is a vital organ in the body responsible for pumping blood throughout the blood vessels via continuous rhythmic contractions. The proper functioning of the heart is entirely dependent on a constant supply of oxygen and energy [10]. Therefore, it is not surprising that the heart will have an expiration date based on its usage throughout a life span. Indeed, an intrinsic cardiac aging in the absence of other cardiovascular risk factors has become a reality and is now proven to exist in many species. Cardiac senescence is a slow and heterogeneous process characterized by the inability of the heart to sustain proper function in response to higher workload or stress such as exercise and ischemia [11–12]. Furthermore, the aged heart is either totally refractory to ischemic pre-conditioning (IPC)-induced cardio-protection or requires longer IPC stimulus to achieve the protection [13–14], suggesting that cardio-protective pathways are modified or impaired by aging. Although the heart as a whole is affected by aging, specific changes occur in the myocardium, the vascular system and the extra-cellular matrix.
1. Changes in the Myocardium
One of the most common characteristics of the aged heart is enhanced left ventricular hypertrophy (LVH) evidenced by thickening of the ventricular walls. Indeed, in the Framingham Heart Study and the Baltimore Longitudinal Study on Aging, an age-dependent increase in LVH was evident in healthy adults free of clinical hypertension, a known risk factor for LVH [15]. Enhanced mechanical load and reduced cardyomyocyte number and elevated interstitial fibrosis participate in the development of LVH with age. Another characteristic of the aged-myocardium is reduced, early diastolic filling rate, so that by 80 years of age, the rate is diminished by almost 50% [15], This reduction in diastolic filling rate triggers a secondary filling in late diastole, provoked by a robust arterial contraction, thus producing an exaggerated “A” wave. Increased arterial contraction plays a causal role in the development of arterial hypertrophy and increased the risk for arterial fibrillation, known to increase with age [15–16]. Furthermore, the ratio between early (E) and late (A) diastolic filling (E/A ratio) was reduced by age both in humans [17] and mice [18]. Decreased early diastolic filling and increased late diastolic filling are an indication of diastolic dysfunction and a predisposition to diastolic heart failure, both of which are highly prevalent in older individuals [19]. In contrast to the well identified diastolic dysfunction at rest, systolic function as measured by ejection fraction is preserved with age [20]. However, contractile reserve representing the increase in systolic function and heart rate in response to stress or to exercise is blunted with age [9, 21–22].
2. Changes in the Vascular System
In addition to the myocardium, aging also affects the vascular system and is associated with arterial stiffening and remodeling, impaired angiogenesis, age-associated atherosclerosis and endothelial dysfunction, a topic recently reviewed by Wang et al. [23]. Endothelial dysfunction is a generalized term that encompasses structural changes in the endothelium, reduced endothelial cell regeneration and repair, endothelial cell death, endothelial dilation and endothelial permeability, all of which contribute to vascular senescence. Senescent endothelial cells become flattened, enlarged and accumulate inflammatory markers, which contribute to their inability to regenerate and increased their susceptibility to apoptosis [24–26]. Furthermore, age-dependent reduction in nitric oxide (NO), caused by either a reduction in NO production or its inactivation by superoxide, lead to impaired NO-mediated endothelium relaxation in response to acetylcholine [27]. In addition, endothelial cells play a key role in angiogenesis, a process known to be reduced in advancing age in animal models [28], mainly due to reduced proangiogenic factors (such as vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1 (HIF-1)) that stimulate endothelial migration and proliferation [29]. In the other hand, increased permeability of the endothelium to plasma macromolecules is also known to increase with age [30] which further exacerbates the pathogenesis of arterial disease. Arterial stiffness of the large arteries such as the aortic tree, significantly contributes to cardiovascular disease in older individuals and is positively associated with systolic hypertension [31]. Moreover, intimal thickening is often observed in aged arteries even in the absence of atherosclerotic disease [32–33], which along with elastin depletion and collagen deposition, exacerbates arterial stiffness and promotes atherosclerosis. Indeed, atherosclerosis is enhanced with advanced age, which increases the risk of developing myocardial infarction, stroke and ischemic heart disease in elderly people. Although several factors are known to increase the incidence of atherosclerosis including hypertension, diabetes, serum total and low-density lipoprotein (LDL) cholesterol and smoking, age is an important risk factor for atherosclerosis [34].
3. Changes in the Extra-Cellular Matrix
The most recurrent and well characterized change associated with cardiac aging is increased collagen deposition within cardiomyocytes and around the blood vessels [35–39]. Moreover, deposition of collagen fibers or fibrosis is associated with stiffer ventricles and diastolic dysfunction in the senescent heart. Fibrotic remodeling of cardiac interstitium is often associated with matrix metalloproteinase (MMP) activation and enhanced matrix degradation [40], In addition to collagen deposition, modifications and cross-linking of both collagen and elastin contribute to the development of vascular calcification, which increases with aging [41–42]. These extracellular matrix modifications participate in the development of valvular sclerosis, which is present in 30% to 80% of old individuals. Indeed, a recent study showed that MMP-mediated degradation of elastin contributed to valve mineralization and calcification by inducing calcium deposition onto fragmented elastin in humans [43].
MECHANISMS OF CARDIAC AGING
The molecular mechanisms that underlie cardiac aging are complex and multi-factorial and include mitochondrial dysfunction and turnover, deregulation of redox homeostasis, exaggerated neuro-hormonal response, altered calcium homeostasis, perturbed metabolism, impaired gene expression, reduced regeneration and altered degradation pathways such as autophagy and the ubiquitinproteasome system (UPS) (Fig. 1). In this article, we will review the role of mitochondrial dysfunction, oxidative stress, altered antioxidant defense mechanisms and impaired autophagy in cardiac aging.
Fig. (1). The role of mitochondrial dysfunction, oxidative stress and impaired autophagy in cardiac aging.
Consequences of impaired mitochondrial function, increased oxidative stress and reduced autophagy on the cardiovascular system that occur during aging. mtDNA: mitochondrial DNA; NO: nitric oxide.
1. Mitochondrial Dysfunction
The early observations related to mitochondrial structural alterations in the heart during aging were made in Drosophilla in the 70s, with the appearance of enlarged mitochondria and the accumulation of intra-mitochondrial glycogen particles [44]. These so-called “giant” mitochondria were also observed in senescent cardiomyocytes in culture [45–46]. Moreover, mitochondrial volume per cardiac cells increased and mitochondrial inner-membrane area and cristae number per mitochondria decreased in the heart of old Syrian hamsters [47]. In contrast, ultra-structural examination of interfibrillar or subsarcolemmal mitochondria from old Fischer 344 rats did not show any effect of age on cristae density or shape [48– 49]. Another structural change that characterize the senescent mitochondria is the appearance of myelin-like structures inside the mitochondria that could be attributed to an age-dependent metabolic change such as the inability to oxidize long-chain fatty acids [50]. In support of this hypothesis, the same structures were also observed in the first postnatal weeks, where fatty oxidation is reduced. Consistent with reduced mitochondrial fatty acid (FA) oxidation, several studies have reported an age-dependent decline in FA oxidation in the heart of humans and rodents [51–52]. One of the mechanisms responsible for the age-associated decline in FA oxidation is reduced mRNA level of beta-hydroxyacyl-CoA dehydrogenase (HOAD), a key enzyme in the beta-oxidation pathway, and reduced mitochondrial carnitine palmitoyl-transferase I (CPT-I) activity [53–55]. In addition, the activity of several other mitochondrial enzymes and translocases such as citrate synthase, pyruvate translocator and adenine nucleotide translocase was reduced in the senescent heart [56–58].
The effect of age on oxidative phosphorylation capacity and mitochondrial electron transfer complexes activity has been extensively studied and reviewed [3, 59–62] but it is still a subject of debate [63]. Thus, reduced state 3 respiration with unchanged state 4 respiration was observed in the hearts of old rats paralleled with reduced F0F1 ATP synthase activity [64–66] and complex I activity [67]. Furthermore, the enzymatic activity of other complexes of the electron transport chain (ETC) was also reduced in the senescent heart [68–71]. In contrast, other studies found either no difference or subtle changes in complexes content, their activity or the overall oxidative capacity of the ETC in the aged myocardium [56, 72–75], The inconsistency between these studies is probably related to the different experimental design, animal species, age and substrate used. In addition to changes in the oxidative machinery, age-related changes in mitochondrial permeability transition, mitochondrial calcium sensitivity, mitochondrial membrane composition and mitochondrial pro-apoptotic signaling were identified [66, 68, 76–78]. . Indeed, increased ROS and Ca2+ in the senescent heart can directly influence the opening of mitochondrial permeability transition pore (mPTP), enhancing the succeptibility to ischemia and impairing cardio-protection [79–80]. As direct evidence linking aging to mPTP opening in the myocardium, the Tezic group [66] showed that Ca2+-induced mPTP opening was enhanced in isolated heart mitochondria of old Fischer 344 rats. At that time, the pore components were not completely identified and the mechanism for increased mPTP triggering was not known. Currently, the mPTP components are partially characterized such as the voltage-dependent ion channel (VDAC), the adenine nucleotide translocator (ANT) and the cyclophilin D (CypD) [81]. In addition, progress has been made to elucidate the mechanisms of mPTP opening in the senescent myocardium including ROS-mediated oxidation/depletion of cardiolipin [82] and reduced SIRT3-dependent deacetylation of CypD [76], all of which increase Ca2+-induced mPTP opening.
Several other mechanisms have been proposed to explain the age-associated alterations in mitochondrial structure and function in the heart including reduced mitochondrial biogenesis, accumulation of mitochondrial DNA (mtDNA) mutations/deletions and oxidative damage. Direct evidence of decreased mitochondrial biogenesis in the senescent myocardium is currently missing while it is evident in skeletal muscle of old individuals as shown by decreased both mDNA copy number and reduced peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-lα) content, a key regulator of mitochondrial biogenesis and function [83–84]. However, a recent study showed that age-dependent accumulation of shorter telomeres directly represses PGC-lα and PGC-1β via a p53-dependent mechanism, thus reducing mitochondrial content and OXPHO capacity in the heart [85–86]. Furthermore, the accumulation of mtDNA mutations, deletions and damage in the heart has been shown to correlate with age [87–88]. Indeed, Cortopassi and Arnheim [89] were among the first to show a low level of mtDNA deletions in the hearts of adult humans, a study that was followed by a series of investigations showing an age-dependent increase in 8-hydroxy-deoxyguanosine (8-OH-dG), a product of free radical damage to deoxyguanosine, in mtDNA in the heart but not in the liver [90–92]. A direct evidence for the causal role of mtDNA/mutations/deletions in cardiac aging came from recent studies using mtDNA mutator mice. Thus homozygous mice expressing defective mtDNA polymerase α or γ exhibit elevated mtDNA mutations/deletions and develop dilated cardiomyopathy associated with enhanced apoptosis [93–94]. Interestingly, and despite higher mtDNA mutations/deletions, the levels of ROS and ROS-mediated damage in the heart was not observed. Thus, the age-related cardiac alterations are solely related to decreased respiratory chain capacity mediated by the instability of the normally synthesized mitochondrial complexes in the mutated mice [95], a topic that was recently reviewed [96].
2. Oxidative Stress and Altered Antioxidant Defense Mechanisms
Several systems generating reactive oxygen species (ROS) exist in the heart including mitochondria, nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, xanthine oxidoreductase (XO), monoamine oxidase (MOA) and nitric oxide synthase (NOS). Indeed, age-dependent increase in XO was found in homogenates of aortic wall and myocardium of old rats and was associated with higher superoxide production and reduced antioxidant enzymes content [97–98]. Similarly, MAO activity and content as well as MAO-dependent hydrogen peroxide production increased with age [99–102], and mice over-expressing MAO exhibit progressive loss of cardiomyocytes and ventricular failure associated with the accumulation of the senescence marker p53 [103]. Furthermore, uncoupling of eNOS generates superoxide through oxidation of L-arginine leading to reduce NO bioavailability, endothelial dysfunction and arterial stiffness in the aged heart [104–105]. Whereas these enzymes play a substantial role in ROS generation specifically in the vessel wall, the majority of ROS in the senescent heart derive from NADPH oxidase and the mitochondrial electron transport chain (ETC). Indeed, an age-dependent increase in NADPH oxidase subunits expression and activity was reported in the aging heart of rodents, and inhibition of this enzyme attenuated age-related abnormalities [106–107]. It is believed that NADPH oxidase subunit 4 (NOX4) is the main player in the heart and its role in cardiac aging has recently been reviewed [108].
In the heart, nearly 90% of ROS in cardiomyocytes is produced by leakage of electrons from complex I and III of the ETC [109–110]. Isolated heart mitochondria from old rats generated higher amount of superoxide in the absence of superoxide dismutase [111]. A direct involvement of the ETC-mediated ROS generation in cardiac aging has been lacking because most studies reported either a reduction or no change in oxidative phosphorylation. However, an inhibition of complex I with age could lead to higher reverse electron transfer from complex II and lead to higher superoxide generation. A direct role of mitochondrial ROS in cardiac aging has recently emerged from work in the Rabinovitch Laboratory showing that over-expression of mitochondria targeted catalase attenuated cardiac aging in part via reduction of mitochondrial protein carbonyls accumulation [18]. However, the same strategy partially rescued the age-associated cardiac hypertrophy and dilation in mtDNA mutator mice despite the fact that these mice do not exhibit increased ROS levels [112–113], suggesting a role for mitochondrial catalase in cardiac aging that is independent of ROS. Despite a higher capacity of ROS production in the heart, this organ is well equipped with antioxidant enzymes capable of scavenging free radicals. However, the literature has been controversial about the changes that occur in the antioxidant reserve during cardiac aging, with studies showing enhanced content and activity [114–115] and others reporting no change [116]. In support of a role of antioxidant enzymes in the maintenance of proper cardiac function with age, are studies examining cardiac aging using mouse models of reduced or enhanced antioxidant capacity. Indeed, mice bearing a heterozygous deletion of manganese superoxide dismutase (MnSOD) develop age-associated endothelial dysfunction and aortic stiffening as a result of ROS-mediated vascular wall remodeling and enhanced smooth muscle cells apoptosis [117–118]. Similarly, muscle-specific deletion of MnSOD resulted in the development of progressive congestive heart failure in mice, indicating the important role of this enzyme in detoxifying mitochondrial superoxide, which builds up in the heart during aging [119]. Finally, interventions aimed to increase antioxidant capacity in the heart through over-expression of catalase were able to reverse age-associated alterations and heart failure caused by G-protein Gα-q over-expression in mice [120–122].
Elevated oxidative stress in the senescent myocardium has several consequences such as enhanced protein oxidation/nitration reduced NO bio-availability, lipofuscin formation, the activation of the inflammatory response, anti-oxidative stress response, apoptosis and endoplasmic reticulum (ER) stress. Indeed, elevated oxidative stress along with deregulation of calcium homeostasis lead to impaired protein folding in the ER and the accumulation of unfolded proteins that activate the adaptive (pro-survival) and the maladaptive (pro-apoptotic) pathways of the unfolded protein response (UPR) [123–125]. Whereas activation of the UPR and ER stress by pathophysiological stimuli is involved in the development of cardiovascular disease, its role in cardiac senescence has not been confirmed. Thus, Jiao et al. [126] found no change in cardiac mRNA level of key components of ER stress between young (5 months) and old (18 months) mice. Other studies that somehow relate to cardiac aging come from work performed on patients with hypertrophic cardiomyopathies or patients with heart failure. Indeed, proliferation of the ER tubules, detected by electron microscopy, was observed in degenerated cardiomyocytes of hypertrophic hearts [127]. Furthermore, ER stress is enhanced in patients with heart failure as suggested by the existence of the spliced X box-binding protein 1 (XBP1) and increased glucose-regulated protein 78kDa (GRP78) expression, suggesting that UPR activation is associated with the pathophysiology of heart failure in humans [128–129] but it remains to be determined whether ER stress and UPR activation are enhanced in the heart of old individuals that are free of confounding risk factors such as hypertension, the metabolic syndrome and diabetes. Finally, since increased cardiac hypertrophy is well established characteristic of the senescent myocardium, it is plausible to speculate that ER stress and UPR will be elevated when cardiac hypertrophy is present. Indeed, UPR activation can be seen in pressure overload-induced hypertrophic hearts [128], which is considered initially as an adaptive mechanism that helps alleviate the burden of excessive protein synthesis associated with increased heart size. However, if ER stress is prolonged, the ER-initiated apoptosis signal CCAAT/ enhancer binding protein (C/EBP) homologous protein (CHOP) is activated as seen in the failing mouse heart subjected to pressure overload [126]. Additional work is needed to determine the role of ER stress in the structural and functional changes associated with cardiac aging. This is a promising area of research that could potentially identify the UPR pathway as a therapeutic target for cardiac senescence.
3. Impaired Degradation Pathways
Many theories have proposed that aging could result from progressive accumulation of protein transcription errors and somatic DNA mutations that lead to improper function of an organ [130–131]. Indeed, post-mitotic cells such as neurons, cardiomyocytes and retinal pigment epithelial cells accumulate damaged macro-molecules and organelles referred to as “biological garbage or waste”[132]. However, an organ such as the heart with higher mitochondrial content and oxidation capacity will be more prone to accumulate biological garbage overtime as opposed to some other organs. For these reasons, the heart is equipped with multiple degradation pathways that can eliminate this waste such as the UPS, autophagy/lysosomal degradation pathway and the calpain system. Although not the focus of this review, a handful of studies have shown that cardiac proteasomal activity decreases with age, which participate in the accumulation of ubiquitinated, oxidized and sumuoylated proteins [133–135] however, whether this impainnent contributes to age-associated cardiac dysfunction is and the underlying mechanisms are not completely understood but might involve increased apoptosis and cardiomyocytes loss [136].
In contrast to UPS, autophagy, which is a conserved cellular degradation process responsible for the turnover of unnecessary or dysfunctional organelles and cytoplasmic proteins, is well characterized but its involvement in cardiac aging has just began to emerge (see review by Dutta et al. [137]). Some studies reported a reduction in autophagy in the senescent heart [138], whereas others showed either no change [9,139] or an increase [140]. Nonetheless, the inhibition of autophagy in the heart via autophagy-related 5 (Atg5)-deficiency at adulthood causes cardiac hypertropgy, left ventricular dilation and contractile dysfunction [141]. In contrast, when ATg5 was deleted out of embryogenesis mice had normal phenotype up to 10 weeks of age but had an accelerated age-dependent cardiac dysfunction that became evident at 10 months of age [138]. Furthermore, genetic deficiency in lysosomal-associated membrane protein 2 (LAMP-2) that characterizes the Danon disease is associated with cardiomyopathy and the aberrant accumulation of autophagic vacuoles in the heart [142–143]. Similarly, increased autophagic vacuoles containing mitochondria were observed in the myocardium of heart failure patients, suggesting a defect in autophagic clearance that lead to intracellular garbage accumulation, a process known to increase cell death [144–145]. In the other hand, impairing autophagic flux and lysosomal function by reducing cardiac lysosomal deoxyribonuclease (DNase) II increased mortality, caused severe myocarditis and dilated cardiomyopathy after pressure overload-induced cardiac hypertrophy in mice. The mechanism involved in the development of cardiac dysfunction in these mice is mediated through increased inflammation and the accumulation of mitochondrial DNA deposits in the autophagosomes [146]. It is thus suggested that up-regulation of autophagy during cardiac hypertrophy can serve as an adaptive response for protecting cells from cell death caused by hemodynamic stress. Indeed, autophagy is increased by aortic banding and its reduction in beclin 1 heterozygous mice enhanced their resistance to pressure-induced cardiac remodeling. Conversely, beclin 1 over-expression increased autophagy and promoted pathologic cardiac remodeling [147]. Finally, autophagy has been proposed to play a protective role during the development of atherosclerosis as evidenced by the abundance of autophagosomes in the atherosclerotic plaques, which prevent smooth muscle cell death and the stabilization of the plaques [148].
One of the consequences of defective or inefficient autophagy along with increased ROS formation is the accumulation of cross-linked proteins and lipids within the lysosomal lumen, forming a granular pigment called lipofuscin, which is often seen in the senescent myocardium [149–150]. It is believed that these deposits further cause an impairment of the autophagsomal-lysosomal machinery, which triggers a vicious cycle in which defective autophagy and the accumulation of damaged mitochondria influence each other, causing further oxidative stress and enhanced lipofuscin deposits [150].
INSULIN/IGF-1 SIGNALING AND CARDIAC AGING
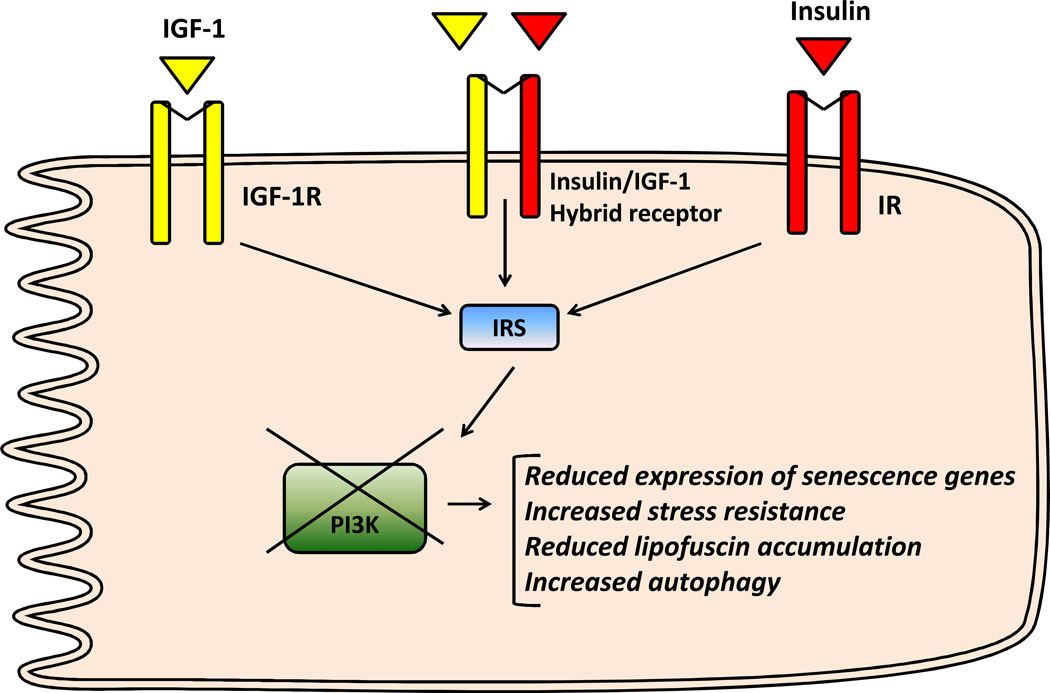
As illustrated in (Fig. 2), insulin and IGF-1 signaling share common intracellular protein substrates, including members of the insulin receptor substrate (IRS) family, and the Src homology and collagen domain protein (Shc). Additionally, insulin and IGF-1 activate many of the same downstream signaling molecules, such as phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) [151–152]. Despite these similarities, the insulin receptor (IR) and the IGF-1 receptor have significantly different affinities to their cognate ligand [153]. The role of insulin/IGF-1 signaling in heart function was determined through the use of genetic animal models with restricted deletion/over-expression of insulin and/or IGF-1 receptors. While insulin receptor (IR) deletion in the heart regulated cardiac growth and metabolism, absent IGF-1 receptor (IGF-1R) in cardiac cells had no effect on cardiac development under basal condition but prevented exercise-induced cardiac hypertrophy [154–155]. Furthermore, mice lacking both receptors in the heart develop early-onset dilated cardiomyopathy and die from heart failure within the first month of life despite having normal glucose homeostasis [156]. In this study, the deletion of IGF-1R alone did not affect cardiac function but when combined with a heterozygous deletion of the IR, cardiac function was impaired along with mitochondrial function, highlighting the important role of IR in the heart.
Fig. (2). Insulin/insulin-like growth factor 1 (IGF-1) signaling and cardiac aging in the mammalian system.
A simplified insulin/IGF-1 signaling pathway in cardiac cells and the effects associated with reduced phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signaling on markers of cardiac aging. IR: insulin receptor; IGF-1R: IGF-1 receptor; IRS: insulin receptor substrate.
As for cardiac aging, the role of IR and IGF-1R signaling in this process has begun with the pioneer work in the Bodmer Laboratory [8], showing that cardiac-specific over-expression of insulin-IGF receptor resulted in premature cardiac aging in Drosophila. Interestingly, these age-dependent alterations were significantly reduced by antagonizing the insulin-IGF signaling through expression of the tumor-suppressor phosphatase and tensin homologue (dPTEN) or the forkhead box O transcription factor (dFOXO). In the same year, a study that somehow contradicted the findings by the Bodmer’s group was published, in which the authors used cardiac-restricted IGF-1 over-expressing mice and showed a protection from cardiac aging through preservation of cardiac stem cell function [157–158]. However, these transgenic mice had elevated levels of plasma IGF-1, making it difficult to distinguish between systemic and cardiac effects of IGF-1 [159]. Indeed, serum IGF-1 levels are inversely correlated with the risk for congestive heart failure in elderly people and plasma IGF-1 deficiency was sufficient to reduce cardiac aging in mice [160–161]. In order to confirm the protective role of IGF-1 on cardiac aging, studies that will use cardiac-specific IGF-1 knockout mice, which do not exhibit any abnormalities in cardiac growth and development under basal condition [155], are necessary. Such studies were recently performed by Moellendorff et al. [162] who showed that inducible inactivation of IGF-1R in cardiac cells had no effect on age-associated cardiac dysfunction in mice, suggesting that the protective effect of cardiac IGF-1 over-expression was rather due to increased circulating levels of IGF-1. In support of this hypothesis, studies that enhanced locally acting IGF-1 peptide promotes functional recovery of heart after myocardial infarction, at least in part, by enhancing sirtuin 1 (SIRT1) expression and increasing cellular oxidative stress resistance [163]. Thus, it is necessary to distinguish between the systemic versus the cardiac effects of IGF-1 on cardiac aging, a topic that has recently been reviewed [164]. The role of insulin receptor signaling on cardiac aging is discussed bellow.
INSULIN RESISTANCE AND CARDIAC AGING
The focus of this section is to dissociate the impact of systemic versus cardiac insulin resistance on cardiac aging.
Systemic Insulin Resistance and Cardiac Aging
Insulin resistance, characterized by decreased rates of insulin-stimulated glucose uptake, is accompanied by hyperinsulinemia and adverse changes in cardiovascular risk factors, such as high triglycerides, low HDL cholesterol, and hypertension [165–166], and is an independent risk factor for coronary heart disease in elderly men [167–169]. Furthermore, insulin resistance increased the risk of type 2 diabetes, which is known to increase with age both in men and in women and predisposes to coronary heart disease in the elderly [170–171]. Some of the mechanisms underlying insulin resistance-induced cardiac dysfunction with age could be related to decreased telomeres length of leukocytes [172] and the development of endothelial dysfunction in response to insulin, all of which plays an important role in the development of age-related hypertension [173]. In the contrary, separate reports found that fasting glucose levels but not insulin resistance (measured by the homeostatic model assessment of insulin resistance (HOMA-IR)), was the strongest predictor of heart failure in the elderly [174]. Similarly, components of the metabolic syndrome including insulin resistance, did not predict arterial fibrillation attacks in older individuals [175], which was further confirmed in a community based study showing that insulin resistance alone was not associated with incident arterial fibrillation [176]. Finally, certain factors contributing to cardiac fibrosis and dysfunction such as plasminogen activator inhibitor type 1 (PAI-1) are known to increase with age independently of insulin resistance in mice [177]. All these studies suggest that insulin resistance alone is probably not the driver for age-dependent coronary heart disease. More work is needed to dissociate the effects of insulin resistance from the effects of other risk factors on cardiac dysfunction in older humans or animals.
Cardiac Insulin Resistance and Aging of the Heart
Cardiac insulin resistance is defined as ablated insulin signaling or insulin-stimulated glucose uptake in the heart in the absence of systemic insulin resistance or known risk factors for coronary heart disease such as obesity, hyperglycemia, hyperinsulinemia, hypercholesterolemia or hypertension. In contrast to the extensive work with IGF-1/IGF-1R, only few studies that examined the role of insulin receptor (IR) and its downstream signaling on cardiac aging are available. The contribution of IR signaling in the development of cardiovascular disease and microvascular complications outside of aging has been recently reviewed [178]. Our own work using mice with cardiac-specific deletion of IR (CIRKO mice) demonstrated that cardiac insulin resistance resulted in reduced cardiac and mitochondrial function that was associated with enhanced oxidative stress [179]. The mechanism for increased oxidative stress in the heart of CIRKO mice is not completely understood but could be related to either enhanced fatty acid oxidation or to mitochondrial dysfunction. Beside cardiomyocytes, IR plays an important role in the vascular wall as evidenced by altered expression of vasoactive mediators (eNOS and endothelin 1) and the inability to maintain vascular tone and insulin sensitivity in response to changes in dietary salt intake in mice lacking IR specifically in endothelial cells [180]. Furthermore, conditional knockout of IR in endothelial cells increased the atherosclerotic lesion size in the apolipoprotein E-null mice, independently of changes in glucose tolerance, insulin sensitivity, cholesterol levels or blood pressure [181]. It is worth nothing that these mice have higher angiogenesis that is related to the absence of IR and not to changes in glucose homeostasis or insulin sensitivity. In contrast to the importance of endothelial IR in atherosclerosis, high fat diet-induced vascular dysfunction did not result from absent IR in the heart, as evidenced by normal basal endothelial function and eNOS phosphorylation in mice with total IR knockout (TIRKO mice) [182], Finally, absent IR in macrophages modestly increased atherosclerotic lesions and promoted formation of necrotic core in atherosclerotic plaque, a condition that precedes plaque rupture and thrombosis [183]. The contribution of cell type specific IR deletion on cardiac aging has not been explored yet. While waiting for these studies to be performed, other mouse models with impaired downstream components of insulin/IGF-1 signaling have been explored. Thus, suppression of PI3K prevented cardiac aging, attenuated expression of senescence and inflammatory markers and reduced lipofuscin accumulation, changes known to increase in the aged heart [9]. In contrast, when the same group used mice with persistent activation of PI3K in the heart, senescent markers such as beta-galactosidase activity, inflammatory and senescent markers as well as lipofuscin accumulation were not different from age-matched wild-type mice [184]. Furthermore, when the dominant negative PI3K (dnPI3K) mice were crossed to a cardiac-specific transgenic mouse model of dilated cardiomyopathy, cardiac function was further depressed [185], suggesting that the lack of PI3K in a condition of increased cardiac stress is rather deleterious. These results are consistent with the inability of CIRKO mice to respond to stress created by pressure overload or myocardial infarction [186–187]. One of the proposed mechanisms responsible for protecting the heart of dnPI3K mice from aging is increased cardiac autophagy. The involvement of autophagy induction in the reduction of cardiac aging of dnPI3K mice was confirmed was confirmed by the observation, inhibiting the mammalian target of rapamycin (mTOR) was able to reduce lipofuscin accumulation in aged-wild-type mice [9]. In support of these findings, a recent study showed that the age-associated decrease in autophagy was exacerbated in hearts of transgenic mice over-expressing the hemagglutinin (HA)-tagged Akt with src myristoylation (activated Akt) in the heart [188]. However, the precise signaling proteins and transcription factors that act downstream of PI3K in cardiac aging remain unknown but might involve Akt or its downstream target FoxO. Indeed, under starvation, FoxO 1 and FoxO 3 translocate to the nucleus and bind to the promoter of certain autophagy genes to promote their expression in isolated cardiomyocytes [189]. On the other hand sirtuins are known to regulate cardiac aging because moderate over-expression of SIRT1 in the heart retards cardiac aging through enhanced resistance to stress and reduced apoptosis, an effect that is dependent on FoxO [190]. In addition, SIRT3 plays an important role in cardiac aging as SIRT3 whole body knockout mice exhibit an age-dependent increase in mitochondrial swelling due to increased mPTP opening and cardiac hypertrophy [76]. Beside cardiomyocytes, sirtuins and FOXO play an important role in vascular senescence through inhibition of telomere erosion, reduction of oxidative stress, promotion of DNA repair, prevention of prolonged AMPK activation, suppression of p66SHC (an adaptor protein whose inactivation protects mice from aging-associated vascular disease [191]) and increased NO bioavailability through modulation of eNOS in endothelial cells [192].
CONCLUSION AND PERSPECTIVES
Studies of cardiac aging are important and timely considering the rising number of elderly people in the United States. The ability of reduced insulin signaling to prevent cardiac aging is fascinating and intriguing taking in consideration its role in the maintenance of cardiac growth and function. Nonetheless, the clinical implications of such studies are significant because pharmacological strategies that block insulin signaling pathway components could be used to prevent cardiac aging. Although, reducing insulin signaling in the heart at young ages could lead to disastrous consequences, such interventions in the aged myocardium would be beneficial. Thus, additional research is needed to identify the intracellular mediators, downstream of the insulin signaling pathway that could be used as a treatment for cardiac aging.
ACKNOWLEDGEMENTS
This work was supported by grants 09SDG2220218 from the American Heart Association and P30-HL-101310 from the National Institutes of Health (NIH) to Sihem Boudina. S.B. researched the literature, wrote, constructed and edited the manuscript.
ABBREVIATIONS
- CVDs
Cardiovascular diseases
- IGF-1
Insulin-like growth factor 1
- LVH
Left ventricular hypertrophy
- E/A
Early (E)/late (A) diastolic filling
- NO
Nitric oxide
- VEGF
Vascular endothelial growth factor
- HIF-1
Hypoxia-inducible factor 1
- LDL
Low density lipoprotein
- MMP
Matrix metalloproteinase
- UPS
Ubiquitin-proteasome system
- HOAD
Hydroxyacyl-CoA dehydrogenase
- FA
Fatty acid
- CPT-I
Carnitine palmitoyl-transferase I
- CoA
Coenzyme A
- ATP
Adenosine triphosphate
- ETC
Electron transport chain
- VDAC
voltage-dependent ion channel
- ANT
adenine nucleotide translocator
- CypD
cyclophilin D
- PGC-lα
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PGC-1β
Peroxisome proliferator-activated receptor gamma coactivator 1-beta
- P53
Protein 53 or tumor protein 53
- mtDNA
Mitochondrial DNA
- 8-OH-dG
8-hydroxy-deoxyguanosine
- ROS
Reactive oxygen species
- NADPH
Nicotinamide adenine dinucleotide phosphate
- XO
Xanthine oxidase
- MOA
Monoamine oxidase
- NOS
Nitric oxide synthase
- eNOS
Endothelial nitric oxide synthase
- NOX4
Nicotinamide adenine dinucleotide phosphate oxidase 4
- MnSOD
Manganese superoxide dismutase
- ER
Endoplasmic reticulum
- UPR
Unfolded protein response
- XBP1
X box-binding protein 1
- GRP78
Glucose-regulated protein 78kDa
- CHOP
CCAAT/enhancer binding protein (C/EBP) homologous protein
- Shc
SHC-adaptor protein
- IRS
Insulin receptor substrate
- MAPK
Mitogen-activated protein kinase
- IR
Insulin receptor
- IGF-1R
Insulin-like growth factor 1 receptor
- dFOXO
Drosophila forkhead box O
- dPTEN
Drosophila tumor-suppressor phosphatase and tensin homologue
- SIRT1
Sirtuin 1
- SIRT3
Sirtuin 3
- HDL
High density lipoprotein
- HOMA-IR
Homeostatic model assessment of insulin resistance
- PAI-1
Plasminogen activator inhibitor type 1
- CIRKO
Cardiomyocyte-insulin receptor knockout
- TIRKO
Total insulin receptor knockout
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- dnPI3K
Dominant negative Phosphatidylinositol-4,5-bisphosphate 3-kinase
- HA
Hemagglutinin
- Akt or PKB
Protein kinase B
- mPTP
Mitochondrial permeability transition pore
- SERCA
Sarcoplasmic/endoplasmic reticulum calcium ATPase
- FOXO1
Forkhead box O 1
- FOXO3
Forkhead box O 3
- Atg5
Autophagy related 5
- AMPK
AMP-activated protein kinase
- P66SHC
Src homology 2 domain-containing transforming protein C 1
Footnotes
CONFLICT OF INTEREST
The author confirms that this article content has no conflicts of interest
REFERENCES
- 1.Projection. NP. 2012. Apr 2, [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary KR, El-Sikhry H, Seubert JM. Mitochondria and the aging heart. J Geriatr Cardiol. 2011;8:159–167. doi: 10.3724/SP.J.1263.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG. Cardiovascular aging in health. Clin Geriatr Med. 2000;16:419–444. doi: 10.1016/s0749-0690(05)70021-5. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG. Heart aging: a fly in the ointment? Circ Res. 2001;88:984–986. doi: 10.1161/hh1001.091963. [DOI] [PubMed] [Google Scholar]
- 7.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 9.Inuzuka Y, Okuda J, Kawashima T, et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. doi: 10.1161/CIRCULATIONAHA.109.871137. [DOI] [PubMed] [Google Scholar]
- 10.Yaniv Y, Juhaszova M, Nuss HB, et al. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann NY Acad Sci. 2010;1188:133–142. doi: 10.1111/j.1749-6632.2009.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleg JL, Schulman S, O'Connor F, et al. Effects of acute beta-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–2341. doi: 10.1161/01.cir.90.5.2333. [DOI] [PubMed] [Google Scholar]
- 12.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 14.Bartling B, Hilgefort C, Friedrich I, Silber RE, Simm A. Cardioprotective determinants are conserved in aged human myocardium after ischemic preconditioning. FEBS Lett. 2003;555:539–544. doi: 10.1016/s0014-5793(03)01342-5. [DOI] [PubMed] [Google Scholar]
- 15.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin EJ, Levy D, Anderson KM, et al. Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study) Am J Cardiol. 1992;70:508–515. doi: 10.1016/0002-9149(92)91199-e. [DOI] [PubMed] [Google Scholar]
- 18.Dai DF, Santana LF, Vermulst M, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnaiyan KM, Alexander D, Maddens M, McCullough PA. Curriculum in cardiology: integrated diagnosis and management of diastolic heart failure. Am Heart J. 2007;153:189–200. doi: 10.1016/j.ahj.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Fraticelli A, Josephson R, Danziger R, Lakatta E, Spurgeon H. Morphological and contractile characteristics of rat cardiac myocytes from maturation to senescence. Am J Physiol. 1989;257:H259–H265. doi: 10.1152/ajpheart.1989.257.1.H259. [DOI] [PubMed] [Google Scholar]
- 21.Fleg JL, O'Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 22.Correia LC, Lakatta EG, O'Connor FC, et al. Attenuated cardiovascular reserve during prolonged submaximal cycle exercise in healthy older subjects. J Am Coll Cardiol. 2002;40:1290–1297. doi: 10.1016/s0735-1097(02)02132-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Khazan B, Lakatta EG. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz SM, Benditt EP. Aortic endothelial cell replication I. Effects of age and hypertension in the rat. Circ Res. 1977;41:248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- 25.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vase Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 26.Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol. 2001;36:1327–1347. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
- 27.Hongo K, Nakagomi T, Kassell NF, et al. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- 28.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 29.Rivard A, Berthou-Soulie L, Principe N, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 30.Belmin J, Corman B, Merval R, Tedgui A. Age-related changes in endothelial permeability and distribution volume of albumin in rat aorta. Am J Physiol. 1993;264:H679–H685. doi: 10.1152/ajpheart.1993.264.3.H679. [DOI] [PubMed] [Google Scholar]
- 31.Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;31:1267–1276. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high , low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Metter EJ, Fleg JL. Increased carotid artery intimal-medial thickness: risk factor for exercise-induced myocardial ischemia in asymptomatic older individuals. Vasc Med. 1999;4:181–186. doi: 10.1177/1358836X9900400309. [DOI] [PubMed] [Google Scholar]
- 34.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 35.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 36.de Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- 37.Orlandi A, Francesconi A, Marcellini M, Ferlosio A, Spagnoli LG. Role of ageing and coronary atherosclerosis in the development of cardiac fibrosis in the rabbit. Cardiovasc Res. 2004;64:544–552. doi: 10.1016/j.cardiores.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Lopez EF, Jin Y, et al. Age-related cardiac muscle sarcopenia: Combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn MA, Graham HK, Richards MA, et al. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson J. Age-related medial elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 43.Nassimiha D, Aronow WS, Ahn C, Goldman ME. Association of coronary risk factors with progression of valvular aortic stenosis in older persons. Am J Cardiol. 2001;87:1313–1314. doi: 10.1016/s0002-9149(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 44.Sohal RS. Mitochondrial changes in the heart of Drosophila repleta, Wollaston with age. Exp Gerontol. 1970;5:213–216. doi: 10.1016/0531-5565(70)90040-9. [DOI] [PubMed] [Google Scholar]
- 45.Terman A, Brunk UT. On the degradability and exocytosis of ceroid/lipofuscin in cultured rat cardiac myocytes. Mech Ageing Dev. 1998;100:145–156. doi: 10.1016/s0047-6374(97)00129-2. [DOI] [PubMed] [Google Scholar]
- 46.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann N Y Acad Sci. 2004;1019:70–77. doi: 10.1196/annals.1297.015. [DOI] [PubMed] [Google Scholar]
- 47.Sachs HG, Colgan JA, Lazarus ML. Ultrastructure of the aging myocardium: a morphometric approach. Am J Anat. 1977;150:63–71. doi: 10.1002/aja.1001500105. [DOI] [PubMed] [Google Scholar]
- 48.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 49.Riva A, Tandler B, Lesnefsky EJ, et al. Structure of cristae in cardiac mitochondria of aged rat. Mech Ageing Dev. 2006;127:917–921. doi: 10.1016/j.mad.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cano J, Machado A. Intramitochondrial inclusions in maturing and senescent muscle cells of rat myocardium. Experientia. 1984;40:206–207. doi: 10.1007/BF01963602. [DOI] [PubMed] [Google Scholar]
- 51.Kates AM, Herrero P, Dence C, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 52.Koonen DP, Febbraio M, Bonnet S, et al. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- 53.LeMoine CM, McClelland GB, Lyons CN, Mathieu-Costello O, Moyes CD. Control of mitochondrial gene expression in the aging rat myocardium. Biochem Cell Biol. 2006;84:191–198. doi: 10.1139/o05-169. [DOI] [PubMed] [Google Scholar]
- 54.McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc Res. 1993;27:2222–2228. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- 55.Odiet JA, Boerrigter ME, Wei JY. Carnitine palmitoyl transferase-I activity in the aging mouse heart. Mech Ageing Dev. 1995;79:127–136. doi: 10.1016/0047-6374(94)01552-w. [DOI] [PubMed] [Google Scholar]
- 56.Picard M, Wright KJ, Ritchie D, Thomas MM, Hepple RT. Mitochondrial function in permeabilized cardiomyocytes is largely preserved in the senescent rat myocardium. PLoS One. 2012;7:e43003. doi: 10.1371/journal.pone.0043003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paradies G, Ruggiero FM. Age-related changes in the activity of the pyruvate carrier and in the lipid composition in rat-heart mitochondria. Biochim Biophys Acta. 1990;1016:207–212. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- 58.Kim JH, Shrago E, Elson CE. Age-related changes in respiration coupled to phosphorylation. II. Cardiac mitochondria. Mech Ageing Dev. 1988;46:279–290. doi: 10.1016/0047-6374(88)90130-3. [DOI] [PubMed] [Google Scholar]
- 59.Marin-Garcia J, Goldenthal MJ. Understanding the impact of mitochondrial defects in cardiovascular disease: a review. J Card Fail. 2002;8:347–361. doi: 10.1054/jcaf.2002.127774. [DOI] [PubMed] [Google Scholar]
- 60.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–C1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- 62.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maklashina E, Ackrell BA. Is defective electron transport at the hub of aging? Aging Cell. 2004;3:21–27. doi: 10.1111/j.1474-9728.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 64.Guerrieri F, Capozza G, Kalous M, Zanotti F, Drahota Z, Papa S. Age-dependent changes in the mitochondrial F0F1 ATP synthase. Arch Gerontol Geriatr. 1992;14:299–308. doi: 10.1016/0167-4943(92)90029-4. [DOI] [PubMed] [Google Scholar]
- 65.Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 66.Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 67.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decrease in the cytochrome c oxidase activity and changes in phospholipids in rat-heart mitochondria. Arch Gerontol Geriatr. 1993;16:263–272. doi: 10.1016/0167-4943(93)90037-i. [DOI] [PubMed] [Google Scholar]
- 69.Lesnefsky EJ, Gudz TI, Moghaddas S, et al. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 70.Choksi KB, Nuss JE, DeFord JH, Papaconstantinou J. Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging (Albany NY) 2011;3:754–767. doi: 10.18632/aging.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Padrao AI, Ferreira R, Vitorino R, et al. Effect of lifestyle on age-related mitochondrial protein oxidation in mice cardiac muscle. Eur J Appl Physiol. 2012;112:1467–1474. doi: 10.1007/s00421-011-2100-3. [DOI] [PubMed] [Google Scholar]
- 72.Manzelmann MS, Harmon HJ. Lack of age-dependent changes in rat heart mitochondria. Mech Ageing Dev. 1987;39:281–288. doi: 10.1016/0047-6374(87)90067-4. [DOI] [PubMed] [Google Scholar]
- 73.Paradies G, Ruggiero FM, Dinoi P. Decreased activity of the phosphate carrier and modification of lipids in cardiac mitochondria from senescent rats. Int J Biochem. 1992;24:783–787. doi: 10.1016/0020-711x(92)90012-p. [DOI] [PubMed] [Google Scholar]
- 74.Serviddio G, Bellanti F, Romano AD, et al. Bioenergetics in aging: mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. 2007;12:91–95. doi: 10.1179/135100007X162112. [DOI] [PubMed] [Google Scholar]
- 75.Tatarkova Z, Kuka S, Racay P, et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–289. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- 76.Hafner AV, Dai J, Gomes AP, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 78.Ljubicic V, Menzies KJ, Hood DA. Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech Ageing Dev. 2010;131:79–88. doi: 10.1016/j.mad.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Crompton M. Mitochondria and aging: a role for the permeability transition? Aging Cell. 2004;3:3–6. doi: 10.1046/j.1474-9728.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 80.Di Lisa F, Bernardi P. Mitochondrial function myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res. 2005;66:222–232. doi: 10.1016/j.cardiores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 81.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340–346. doi: 10.1111/j.1600-079X.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 83.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 84.Finley LW, Haigis MC. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res Rev. 2009;8:173–188. doi: 10.1016/j.arr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res. 2012;110:1226–1237. doi: 10.1161/CIRCRESAHA.111.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 88.Szibor M, Holtz J. Mitochondrial ageing. Basic Res Cardiol. 2003;98:210–218. doi: 10.1007/s00395-003-0421-z. [DOI] [PubMed] [Google Scholar]
- 89.Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990;18:6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayakawa M, Sugiyama S, Hattori K, Takasawa M, Ozawa T. Age-associated damage in mitochondrial DNA in human hearts. Mol Cell Biochem. 1993;119:95–103. doi: 10.1007/BF00926859. [DOI] [PubMed] [Google Scholar]
- 91.Takasawa M, Hayakawa M, Sugiyama S, Hattori K, Ito T, Ozawa T. Age-associated damage in mitochondrial function in rat hearts. Exp Gerontol. 1993;28:269–280. doi: 10.1016/0531-5565(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 92.de Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver: 8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic Biol Med. 2001;30:916–923. doi: 10.1016/s0891-5849(01)00483-x. [DOI] [PubMed] [Google Scholar]
- 93.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 94.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 95.Edgar D, Shabalina I, Camara Y, et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–138. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 96.Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 97.Aranda R, Domenech E, Rus AD, et al. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res. 2007;41:1195–1200. doi: 10.1080/10715760701481461. [DOI] [PubMed] [Google Scholar]
- 98.Pushpalatha K, Nishanth K, Sathyavelu Reddy K. Myocardial antioxidant status and oxidative stress after combined action of exercise training and ethanol in two different age groups of male albino rats. Acta Biol Hung. 2007;58:173–185. doi: 10.1556/ABiol.58.2007.2.4. [DOI] [PubMed] [Google Scholar]
- 99.Meco M, Bonifati V, Collier WL, Ramacci MT, Amenta F. Enzyme histochemistry of monoamine oxidase in the heart of aged rats. Mech Ageing Dev. 1987;38:145–155. doi: 10.1016/0047-6374(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 100.Strolin Benedetti M, Thomassin J, Tocchetti P, Dostert P, Kettler R, Da Prada M. Species differences in changes of heart monoamine oxidase activities with age. J Neural Transm Suppl. 1994;41:83–87. doi: 10.1007/978-3-7091-9324-2_10. [DOI] [PubMed] [Google Scholar]
- 101.Saura J, Richards JG, Mahy N. Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol Aging. 1994;15:399–408. doi: 10.1016/0197-4580(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 102.Maurel A, Hernandez C, Kunduzova O, et al. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1460–H1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 103.Villeneuve C, Guilbeau-Frugier C, Sicard P, et al. p53-PGC-lalpha Pathway Mediates Oxidative Mitochondrial Damage and Cardiomyocyte Necrosis Induced by Monoamine Oxidase-A Upregulation: Role in Chronic Left Ventricular Dysfunction in Mice. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ .Physiol. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim JH, Bugaj LJ, Oh YJ, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rueckschloss U, Villmow M, Klockner U. NADPH oxidasederived superoxide impairs calcium transients and contraction in aged murine ventricular myocytes. Exp Gerontol. 2010;45:788–796. doi: 10.1016/j.exger.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 108.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2010;2:1012–1016. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ide T, Tsutsui H, Kinugawa S, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 110.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 111.Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 112.Dai DF, Chen T, Wanagat J, et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Trifunovic A, Hansson A, Wredenberg A, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci USA. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lammi-Keefe CJ, Swan PB, Hegarty PV. Copper-zinc and manganese superoxide dismutase activities in cardiac and skeletal muscles during aging in male rats. Gerontology. 1984;30:153–158. doi: 10.1159/000212623. [DOI] [PubMed] [Google Scholar]
- 115.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 116.Gounder SS, Kannan S, Devadoss D, et al. Impaired transcriptional activity of nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wenzel P, Schuhmacher S, Kienhofer J, et al. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou RH, Vendrov AE, Tchivilev I, et al. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler Thromb Vase Biol. 2012;32:745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nojiri H, Shimizu T, Funakoshi M, et al. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 120.Ren J, Li Q, Wu S, Li SY, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mech Ageing Dev. 2007;128:276–285. doi: 10.1016/j.mad.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol. 2007;34:81–87. doi: 10.1111/j.1440-1681.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- 122.Qin F, Lennon-Edwards S, Lancel S, et al. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circ Heart Fail. 2010;3:306–313. doi: 10.1161/CIRCHEARTFAILURE.109.864785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 124.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 125.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 126.Fu HY, Okada K, Liao Y, et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 127.Maron BJ, Ferrans VJ, Roberts WC. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am J Pathol. 1975;79:387–434. [PMC free article] [PubMed] [Google Scholar]
- 128.Okada K, Minamino T, Tsukamoto Y, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 129.Sawada T, Minamino T, Fu HY, et al. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1280–1089. doi: 10.1016/j.yjmcc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Burnet FM. A genetic interpretation of ageing. Lancet. 1973;2:480–483. doi: 10.1016/s0140-6736(73)92074-6. [DOI] [PubMed] [Google Scholar]
- 131.Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci USA. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 133.Bulteau AL, Szweda LI, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304. doi: 10.1006/abbi.2001.2663. [DOI] [PubMed] [Google Scholar]
- 134.Voziyan PA, Fisher MT. Polyols induce ATP-independent folding of GroEL-bound bacterial glutamine synthetase. Arch Biochem Biophys. 2002;397:293–297. doi: 10.1006/abbi.2001.2620. [DOI] [PubMed] [Google Scholar]
- 135.Li F, Zhang L, Craddock J, et al. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech Ageing Dev. 2008;129:515–521. doi: 10.1016/j.mad.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Powell SR. The ubiquitin-proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 137.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taneike M, Yamaguchi O, Nakai A, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–666. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 139.Wohlgemuth SE, Julian D, Akin DE, et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 140.Boyle AJ, Shih H, Hwang J, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 142.Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 143.Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 144.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 145.Kostin S, Pool L, Elsasser A, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 146.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu H, Tannous P, Johnstone JL, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 149.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blakesley VA, Scrimgeour A, Esposito D, Le Roith D. Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling? Cytokine Growth Factor Rev. 1996;7:153–159. doi: 10.1016/1359-6101(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 152.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 153.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 154.Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim J, Wende AR, Sena S, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laustsen PG, Russell SJ, Cui L, et al. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27:1649–1664. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 158.Li Q, Wu S, Li SY, et al. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 159.Reiss K, Cheng W, Ferber A, et al. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vasan RS, Sullivan LM, D'Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 162.Moellendorf S, Kessels C, Peiseler L, et al. IGF-IR signaling attenuates the age-related decline of diastolic cardiac function. Am J Physiol Endocrinol Metab. 2012;303:E213–E222. doi: 10.1152/ajpendo.00538.2011. [DOI] [PubMed] [Google Scholar]
- 163.Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2010;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 166.Laakso M. Insulin resistance and coronary heart disease. Curr Opin Lipidol. 1996;7:217–226. doi: 10.1097/00041433-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 167.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100:123–128. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 168.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 169.Maggi S, Noale M, Gallina P, et al. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: the Italian Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2006;61:505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- 170.Wilson PW, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in the elderly. Am J Geriatr Cardiol. 2002;11:119–123. doi: 10.1111/j.1076-7460.2002.00998.x. 125. [DOI] [PubMed] [Google Scholar]
- 171.Kannel WB. Coronary heart disease risk factors in the elderly. Am J Geriatr Cardiol. 2002;11:101–117. doi: 10.1111/j.1076-7460.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 172.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 173.Li QX, Xiong ZY, Hu BP, et al. Aging-associated insulin resistance predisposes to hypertension and its reversal by exercise: the role of vascular vasorelaxation to insulin. Basic Res Cardiol. 2009;104:269–284. doi: 10.1007/s00395-008-0754-8. [DOI] [PubMed] [Google Scholar]
- 174.Kalogeropoulos A, Georgiopoulou V, Harris TB, et al. Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the health, aging, and body composition study. J Card Fail. 2009;15:593–599. doi: 10.1016/j.cardfail.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu YF, Chen SA, Yeh HI. Metabolic syndrome does not impose greater atrial fibrillation risk in elderly hypertensive patients. Acta Cardiol. 2010;65:653–659. doi: 10.1080/ac.65.6.2059862. [DOI] [PubMed] [Google Scholar]
- 176.Fontes JD, Lyass A, Massaro JM, et al. Insulin resistance and atrial fibrillation (from the Framingham Heart Study) Am J Cardiol. 2012;109:87–90. doi: 10.1016/j.amjcard.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kelbaek H, Godtrfedsen J. Effects of acute cardioselective and non-selective beta-adrenergic blockade on left-ventricular volumes and vascular resistance at rest and during exercise. Scand J Clin Lab Invest. 1991;51:161–166. [PubMed] [Google Scholar]
- 178.Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Symons JD, McMillin SL, Riehle C, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Han S, Liang CP, DeVries-Seimon T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 184.Niizuma S, Inuzuka Y, Okuda J, et al. Effect of persistent activation of phosphoinositide 3-kinase on heart. Life Sci. 2012;90:619–628. doi: 10.1016/j.lfs.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 185.Pretorius L, Du XJ, Woodcock EA, et al. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol. 2009;175:998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 187.Sena S, Hu P, Zhang D, et al. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. J Mol Cell Cardiol. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 189.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 191.Zhou S, Chen HZ, Wan YZ, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 192.Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110:1238–1251. doi: 10.1161/CIRCRESAHA.111.246488. [DOI] [PubMed] [Google Scholar]