Abstract
Currently, numerous patients who receive targeted chemotherapy for cancer suffer from disabling skin reactions due to cutaneous toxicity, which is a significant problem for an increasing number of patients and their treating physicians. In addition, using inappropriate personal hygiene products often worsens these otherwise manageable side-effects. Cosmetic products for personal hygiene and lesion camouflage are part of a patients’ well-being and an increasing number of physicians feel that they do not have adequate information to provide effective advice on concomitant cosmetic therapy. Although ample information is available in the literature on pharmaceutical treatment for cutaneous side-effects of chemotherapy, little is available for the concomitant use of dermatological skin-care products with medical treatments. The objective of this consensus study is to provide an algorithm for the appropriate use of dermatological cosmetics in the management of cutaneous toxicities associated with targeted chemotherapy such as epidermal growth factor receptor inhibitors and other monoclonal antibodies. These guidelines were developed by a French and German expert group of dermatologists and an oncologist for oncologists and primary care physicians who manage oncology patients. The information in this report is based on published data and the expert group’s opinion. Due to the current lack of clinical evidence, only a review of published recommendations including suggestions for concomitant cosmetic use was conducted.
Introduction
Targeted chemotherapy is associated with cutaneous side-effects, which is becoming more and more a problem for an increasing number of patients and their treating physicians. However, with appropriate skin care, in association with pharmaceutical treatment, these reactions can be adequately managed.
In recent years, the improved survival outcome and the superior safety profile of targeted molecules in chemotherapy have revolutionized the treatment of haematological malignancies and solid tumours of head and neck, breast, lung, liver, kidney or colorectal origin and more recently, melanoma.1–4 Despite the improved systemic tolerability towards chemotherapeutic agents, they are nevertheless associated with adverse cutaneous reactions. If not managed appropriately, these reactions can become uncomfortable and disfiguring.5–12 Although not life threatening, they are becoming an increasingly important preoccupation for both patients and their treating physicians as patient survival rates improve. Skin, ocular, nail and hair toxicity have been comprehensively described in the literature as common side-effects to expect.5–15 Typical reactions include folliculitis (skin rash), xerosis, pruritus, hand and foot erythema and an increased sensitivity to ultraviolet radiation. Symptoms usually appear early in treatment and although usually mild at onset, can become severe and maybe considered as a class effect.16 They can lead to serious morbidities that impair quality of life.17–18 As symptoms are dose dependent and considered a validated measure for efficacy, patients are encouraged to manage adverse cutaneous reactions as a part of their treatment.3–20
Conversely, one recent retrospective survey of oncologists showed an alarming number of dose reductions and treatment discontinuations due to skin rash.13 Such dose reductions may be considered as potentially detrimental to the treatment outcome. Consequently, appropriate management of dermatological toxicity is an important issue throughout treatment.
The primary role of skin-care products is to provide exogenous support that maintains the epidermal barrier intact.21 Skin hydration relieves symptoms associated with dry skin and reduces further aggravation associated with pruritus and leading to secondary infections.22–23 In addition to their role of barrier function maintenance, skin-care products are intended for cleaning the skin. As personal hygiene is part of most cultures today, patients do need advising on appropriate skin care.24 Although many products are appropriate, a certain number hygiene products are not, as they may aggravate symptoms.9–24 Some dermatological skin-care products are formulated for and tested on fragile, pathological and sensitive skin. Such products may be considered as more appropriate for concomitant management of cutaneous side-effects.9
With an increasing overall survival, primary care providers are playing an increasingly important role in managing oncology patients, and may therefore need some guidance in managing adverse cutaneous reactions.25
Therefore, the objective of this study is to provide oncologists and primary care physicians managing cancer patients with a therapeutic algorithm based on an extensive literature review of cosmetics associated with targeted therapy for cancer as well as the expert’s opinion based on their experience with the use of dermatological skin-care products and cosmetics.
Methodology
The present recommendations focusing on skin, mucosa and nail disorders following oncology treatments were developed following proposals and conclusions reached during a consensus meeting held in November 2011. The working group consisted of six independent European dermatologists and one oncologist.
Prior to the meeting, an ad hoc literature review (using Pubmed and BIOSIS) was performed. The key words chosen were emollient + cancer + skin, sunscreen + cancer + skin, hygiene + cancer, make-up + cancer.
During the meeting, the literature concerning different cutaneous toxicities related to targeted therapy and chemotherapy as well as to quality of life was reviewed. The working group discussed appropriate dermatological products for each cutaneous symptom according to the available literature, and completed their recommendations with current practices in France and Germany and personal experience.
Dermatological skin care was defined as cleansing, moisturizing, personal hygiene and photoprotection using products having a good tolerance profile, tested on pathological skin.
There are different classifications for the degree of skin toxicity. However, the working group referred to The National Cancer Institute Common Toxicity Criteria (CTCAE) version 4, which is a widely known and accepted scale for the assessment of adverse events.26 This scale provides objective criteria that reflect the current management of cutaneous toxicities associated with targeted therapy.
Literature review
Evidence-based support for the use of dermatological cosmetics and make-up as adjunctive therapy remains scarce. Practice is based on anecdotal reports or studies with limited control. Table 1 provides an overview of key studies conducted recommending the use of cosmetics as part of side-effect management.
Table 1.
List of key references recommending the use of cosmetic agents in combination with chemotherapy
| Author | Study design | Title | Comments | ||
|---|---|---|---|---|---|
| Lacouture9 | Review | Mechanisms of cutaneous toxicity | |||
| Segaert S et al.7 | Review article | Clinical signs, pathophysiology and management skin toxicity | Adequate sun protection | ||
| Avoid skin drying cosmetic products | |||||
| An emollient on hands and limbs to prevent fissures | |||||
| Segaert S et al.6 | Review article | Skin toxicities of targeted therapy | Maximize skin hydration | ||
| Sun protection | |||||
| Rash: Avoid retinoids | |||||
| Xerosis: oil-in-water creams, 5-10% urea | |||||
| Paronychia: Topical antiseptic | |||||
| Robert C et al.5 | Review article | Cutaneous side-effects of kinase inhibitors and blocking antibodies | Camouflage cosmetics for folliculitis | ||
| Xerosis: prescribe 5–10% urea | |||||
| Ouwerkerk, J et al.30 | Review article | Anti EGFR for metastatic colorectal cancer | Sunscreen >15+ | ||
| Avoid cleaning detergents | |||||
| Mild body cleansers | |||||
| Moisturizers | |||||
| Avoid alcohol-based products | |||||
| Cosmetics to conceal rash | |||||
| Gentle non-alcohol-based cleansers | |||||
| Perez-Soler et al.14 | Guideline | HER1/EGFRI assoc rash: future directions for mgt and outcomes from the HER1/EGFRI rash management forum | Cover rash with make-up | ||
| Use a skin-friendly make-up remover | |||||
| Use emollients to prevent skin dryness | |||||
| Use a good sunscreen | |||||
| Avoid over-the-counter acne medication | |||||
| Burtness et al.1 | Guideline | Task force report. Management of dermatological and other toxicities associated with EGFRI in patients with cancer. | Initiate treatment early | ||
| Avoid using antiacne medication | |||||
| Thick emollients | |||||
| Mild soap | |||||
| Bernier et al.36 | Guideline | Consensus guidelines for the mgt of radiation dermatitis and acne-like rash in patients receiving radiotherapy+ EGFRI for the treatment of head and neck squamous cell carcinoma | Use gentle cleansers | ||
| Topical cosmetics for symptomatic relief | |||||
| Avoid sun exposure (mineral sunblocks or clothing) | |||||
| Avoid perfumes and alcohol-based lotions. | |||||
| Lynch TJ et al.6 | Guideline | EGFRI dermatologic toxicity overview of outcomes. | Expert opinion | ||
| Initiation of treatment, moisturize dry areas twice daily | |||||
| Thick alcohol-free emollient | |||||
| Broad-spectrum sunscreen 15+ or higher | |||||
| Add Medical treatment if severity increases. | |||||
| Infections | |||||
| Grenader T et al23 | Case report | Staph aureus on culture following erlotinib treatment | |||
| Author | Study Design | Population | Intervention | Sample size | Outcome measurement | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluhr 21 | 3-week, controlled, monocentric, study | Experiencing dry, sensitive skin during chemotherapy | Application of acidic pH 5.5 washing emulsion and body lotion | 30 | Skin-surface pH | Improved skin physiology | |||||||
| TEWL | Sig increase in stratum corneum hydration P < 0.001 | ||||||||||||
| Corneometer | Reduced TEWL P > 0.007 | ||||||||||||
| Sebumeter | Significant reduction of skin symptoms P < 0.001 | ||||||||||||
| Clinical evaluation | |||||||||||||
| Roé E et al29 | Prospective, uncontrolled study of toxicity | Cetuximab or erlotinib | Severity graded | 30 | Application of an emollient for treatment of xerosis | Good control of symptoms. | No description of which cosmetic products used when. | ||||||
| Antiseptic soaps | |||||||||||||
| Pre-emptive skin-care management reduces incidence and severity of skin rash | |||||||||||||
| Wohlrab34 | Monocentre POC | Breast cancer chemotherapy | Application of an emollient | 13 | DLQI – quality-of-life questionnaire | Maintains quality of life and reduces frequency of adverse events | Poster | ||||||
| Ocvirk J et al 22 | Prospective, monocentre, uncontrolled study | cetuximab Review of local practice for management of skin toxicity | Application of an emollient after the first documented cutaneous toxicity | 31 | Skin toxicity Classification using NCI CTCAE v3.0 | 29.03% grade 1 – advised emollients | Recommend prompt application of topical emollients. Add topical of systemic medication for increasing severity. | ||||||
| Xerosis | Grade 1 were treated with emollients | 51.61% grade 2 – advised emollients + topical antibiotic | |||||||||||
| fissures | Grade 2 emollients+ antibiotics | 19.36% grade 3 – interruption of treatment then emollients + topical antibiotics | |||||||||||
| Grade 3 emollients+ systemic antibiotics | Xerosis – advised emollients 2–5% urea | ||||||||||||
| Fissures – creams + dexpanthenol | |||||||||||||
| Use of beauty care and cosmetics improves quality of life and management of side-effects | |||||||||||||
| Boone SL et al13 | Qualitative Survey | Oncology providers | 51 item, open-ended questionnaire pertaining to incidence of rash among patients on EGFRI, treatment practices, patient perceptions, outcome treating rash | 110 | 71–90% of patients experience rash. | ||||||||
| 8% of providers surveyed obtained a dermatology consult. | |||||||||||||
| Grade 3 and 4 rash interfered with EGFRI therapy (3%). | |||||||||||||
| Taggart L et al39 | Pilot study | Cancer patients | Participation at a look-good–feel-better workshop | 20 | Self-image NSC | Improved self-image (P < 0.005) | |||||||
| Anxiety STAI | Reduced anxiety (P < 0.01) | ||||||||||||
| Haley et al18 | Monocentre, uncontrolled study | Cancer patients receiving either cytotoxic chemotherapy, targeted, or hormonal and/or radiotherapy | Applied three test products after first toxicity visit skin and face moisturizer and face wash | 99 | Q of L: Skindex questionnaire | Significant decrease (P = 0.0003) from baseline in mean overall skindex score | |||||||
| Merial-Kieny C et al52 | Multicentre qualitative survey | Visible cutaneous side-effects following chemotherapy | Participated in a make-up training seminar | 90 | Self-completed questionnaire on tolerance, quality of life and the make-up techniques | 95,4% very or satisfied with tolerance | |||||||
| Applied a hypoallergenic make-up | 81,2% improved their quality of life. | ||||||||||||
| Amiel P et al40 | Multicentre, qualitative survey | Cancer patients | Survey of patient experience receiving various beauty-care services | 60 | Observation and semistructured interviews | 18/40 appreciated information on treating skin problems | |||||||
| 23/40 appreciated information on make-up techniques | |||||||||||||
| Titeca G et al41 | Prospective, randomized, controlled multicentre study | Breast cancer Antitumor chemotherapy | 2H cosmetic care interview | 27 | VQ dermatological questionnaire | less discouraged (P = 0.032) | |||||||
| more self-confident (P = 0.032) | |||||||||||||
| Williams S et al46 | Outpatient chemotherapy clinics | First chemotherapy Breast cancer patients | Informational audiotapes for self care | 70 | STAI | Treatment group increased use of management techniques | |||||||
| Symptoms decreased | |||||||||||||
TEWL, Trans Epidermal Water Loss; NCI-CTC, National Cancer Institute cutaneous toxicity Criteria.
Table 2.
Spectrum of dermatological reactions to EGFR inhibitors39
| Adverse Event | Description | Frequency | Time Course |
|---|---|---|---|
| Rash (follicular-pustular) | Monomorphous erythematous maculopapular, follicular or pustular lesions, which may be associated with mild pruritis | 60–80% | Onset: treatment week 1. |
| Maximum: treatment week 3–5. | |||
| Resolution: within 4 weeks of treatment cessation, may wax and wane or resolve spontaneously | |||
| Paronychia and fissuring | Painful periungual granualtion-type or friable pyogenic granuloma-like changes, associated with erythema, swelling and fissuring of lateral nailfolds and/or distal finger tufts. | 6–12% | Onset: treatment month 2–4 |
| Dry skin | Diffuse fine scaling | 4–35% | Occurs after appearance of rash |
Side-effects associated with targeted chemotherapeutic agents
Cutaneous toxicity with chemotherapeutic agents is common. Although designed to target specific molecular tumour growth factors, they also target growth factors in the skin and its appendages.6–9 To date, the exact mechanisms involved in the development of cutaneous symptoms are only partly understood.16 However, the molecular, histological and clinical observations suggest that targeted therapies ultimately disturb skin barrier function.9 Clinical symptoms include disruption of the pilosebaceous follicle causing folliculitis (skin rash), alteration of the skin barrier with xerosis, cracked skin and pruritus (itchy skin). Other common reactions include hand and foot erythema, increased sensitivity to ultraviolet radiation, hyperpigmentation and finally, alteration of phaneres with paronychia.7–15 In addition to disturbed epidermal barrier function, the skin is more sensitive to allergens and open to infection.7,15
Rash (folliculitis)
The most common reaction reported is skin rash,1 which appears in 43–85% of patients treated with epidermal growth factor receptor inhibitors (EGFRI)s.5 This rash follows a typical, chronological pattern that peaks in severity during the first 1–2 weeks.6–7 Although it is not associated with death, reports of serious morbidity have been identified.27 In the current absence of consistent clinical trials, patients are therefore advised to use mild skin care and photoprotection.16–28
Grade 1 rash was successfully managed with emollients and adapted skin cleansers in a local practice review.22 In a similar observational study, Grade 1 rash was also shown to be managed with topical antibiotics and antiseptic soap.29
Non-occlusive make-up with a high pigment concentration to adequately cover scars and lesions has been repeatedly suggested to cover grade 1 and 2 rash. 5,8 Furthermore, appropriate skin care and corrective make-up were shown to be tolerated by patients receiving chemotherapy in one multicentre study, and avoiding allergenic over-the-counter products is recommended.6–14 Make-up should be removed with a dermatologist-approved, low-irritant, non-alcoholic hypoallergenic remover.14,30 Over-the-counter acne products have been repeatedly contraindicated, including products containing benzoyl peroxide and topical retinoids such as tretinoin, adapalene or tazarotene. These agents generally are considered as drying the skin and causing sensations of burning, stinging and irritation, while not having shown clinical benefit in the treatment of rashes.1,8
A number of authors have discussed the growing evidence for rash severity as a surrogate marker of efficacy with certain products.19 Although further evidence is required to quantify these observations, authors advise continuing epidermal growth factor receptor (EGFR) inhibitor therapy in association with appropriate psychosocial support.
Xerosis
Xerosis appears several weeks after the first EGFR treatment in up to 35% of patients.28–32 The review articles examined unanimously recommended applying emollients to ensure maximal skin hydration.7,28, Some authors found emollients containing 5–10% urea useful, while a recent monocentric proof-of-concept study suggested that the supportive application of an emollient containing niacinamide maintains quality of life and reduces the frequency of adverse events.34 Three international expert groups support the general use of emollients for dry skin despite the lack of prospective data.1,8 Segaert presents his clinical experience of switching topical treatments to oil-in-water formulations and for limbs, water-in-oil formulations for moderate-to-severe xerosis on the first sign of dryness.7
One single-centre, controlled, assessment of skin function in chemotherapy patients showed a significant increase in the stratum corneum hydration (P < 0.001) and a decrease in transepidermal water loss (P < 0.03) following prophylactic treatment with an acidic (pH 5.5) skin-care system (emollient and cleanser).21 Roé et al., in an uncontrolled trial of 30 patients also reported that moisturizers were a useful treatment for xerosis.29
Nursing reviews recommend proactive management of rash and xerosis.18–30 Consensus articles state treating fissures with liquid bandages or thick emollients containing 5–10% urea,5,35 _ENREF_43 and the use of antiseptic cream to prevent infection.1–7
Paronychia
Paronychia is a painful inflammatory reaction of the nail folds.37 It is difficult to treat and causes the nail folds to become sensitive to infection. Antiseptic creams and drying pastes have been reported to be useful to prevent infection of the nail fold.6,7 Fissures in the nails have also been treated by liquid bandages and glue.1–36
Hand–foot skin reaction
In addition to practical measures to avoid friction, mild reactions have been treated successfully with urea or salicylic acid ointment. Xerosis cutis has also been managed with specifically formulated hand or foot emollients.7–29
Mucosal disorders
There is little mention in the literature on the treatment of oral and nasal aphthae, (mucositis) and dry anal and vaginal mucosae. Symptomatic treatment available consists of oral gels, nasal and vaginal creams.32
Alteration of patient quality of life
The pain and morbidity associated with targeted chemotherapy can be difficult for cancer patients to bear and have been shown to impact quality of life as well as interpersonal relationships.9,11 The use of cosmetics and appropriate skin-care management has shown objective improvements on quality of life. A pilot study found significantly improved self-image (P < 0.005 compared with baseline) on the Self-image Non melanoma skin cancer scale and anxiety (P < 0.01) on the STAI scale.39 An uncontrolled, monocentre study showed a decrease from baseline (P < 0.0003) on the Skindex questionnaire.18 A multicentre qualitative survey reported patient appreciation for beauty-care services for treating skin problems that helped patients cope better throughout the treatment period.40 Recent prospective studies have shown that proactive education on self-care behaviours significantly reduced anxiety (P = 0.032) on the VQ dermato-scale, contributing to better symptom management.41 Training seminars that teach both men and women appropriate skin care, camouflage and dressing techniques have been proposed to restore self-esteem, particularly for those patients with pre-existing low esteem.42–43
Skin-care options
Proactive treatment
Proactive treatment is critical as toxicity has been reported to arise as early as 2 days after the first treatment.7,9 There is no clear evidence which patients may be more susceptible.7 However, Gallimont-Collen et al. reported a correlation between both older age and atopic predisposition and higher incidence of xerosis.32 A retrospective survey of skin-toxicity management found that proactive intervention was warranted to obtain maximum benefit from EGFRI treatment and prevent dose change or interruption.13 The NCCN task force report also recommended initiating treatment even for mild reactions in case they become dose limiting.1 Early education and continued encouragement throughout treatment have been shown to benefit quality of life.44–45
Skin cleansing
In the process of skin cleansing, dirt or cosmetics are removed along with the sebum associated with it, thus further drying damaged skin.24–46 This has been shown to be particularly detrimental to skin affected by chemotherapy where the skin barrier is already disturbed.21 Without professional guidance, patients tend to experiment with inappropriate self-care behaviours that aggravate the situation or irritate their sensitive skin.18–45 In the lack of evidence, authors recommend that patients avoid washing with soap.7–31 Recently, some authors have started producing helpful evidence. Fluhr et al. reported that combining an acidic cleanser and emollient (pH 5.5) improved barrier function, stratum corneum hydration and skin surface lipids.21 Roé et al. reported good control of secondary infections in a 30-patient, prospective, study of the management of cutaneous side-effects with the use of antiseptic soaps.29
Skin hydration
Chemotherapy reduces the skin’s tolerance to cosmetic products.21 This distinctive reaction that cancer patients experience, has been attributed to an imbalance in the stratum corneum that ultimately results in a disruption of skin barrier function.9–21 General measures to prevent further deterioration of the barrier topical treatments should be continued, with care not to apply occlusive creams. The role of emollients is to protect the epidermal barrier.9 Topical application of moisturizers or emollients binds water with the stratum corneum, providing partial surface hydration. This has been shown repeatedly to improve epidermal barrier function and reduce the stinging, scaling, redness and cracks associated with chemotherapy-induced xerosis.21 Adequate hydration improves barrier function, reduces pruritus and prevents secondary infections due to scratching.6–18
Therefore, skin care with moisturizers, low-irritant cleansers and make-up is effective in improving skin hydration and controlling or covering up some cutaneous reactions.
Photoprotection
Lacouture highlights observations that EGFRI toxicity often occurs in sun-exposed areas, which have later been further supported by clinical and experimental data.47–48 Daily photoprotection is important as the skin becomes more sensitive to UV radiation and in certain cases can lead to pigmentation changes.49–50 Symptom management and supportive care forums on dermatological-toxicity management recommend applying a broad-spectrum sunscreen [Sun protection factor(SPF) or UVA-PF, SPF 15 or higher] depending on the patient’s phototype and on the photosensitivity induced.8,16
Deodorants
The use of antiperspirants or deodorants is a controversial topic as the effect of chemotherapy on the eccrine glands eliminates their need. However, the working group felt that in the interest of the patients’ well-being, deodorants and non-irritant perfumes may be used as part of maintaining a daily routine.
Skin sensitivity
Individuals treated with EGFRIs often complain of having sensitive skin that stings, burns and itches, all of which may be due to cutaneous inflammation.9 Several authors recommend avoiding allergenic or irritant products such as alcohol, topical retinoid and benzoyl peroxide.6–8
Dermatologist referral
Most symptoms either resolve spontaneously, or can be managed by the treating physician. However, dermatologist consultation is recommended when lesions are uncharacteristic, blistering, petechial or necrotic.8
Discussion and recommendations
The association of cutaneous side-effects with the use of targeted chemotherapy is now well accepted. However, evidence-based support for the use of dermatological cosmetics as adjunctive therapy to manage these problems remains scarce. Practice is based on anecdotal reports, personal experience or studies with limited control, and currently, no standard recommendation on how to treat cutaneous side-effects of oncology treatments exists.
Most authors agree that skin care is an essential part of well-being and dermatologists should not deprive patients of this habit. The consistency of evidence and reported experience allowed the expert group making realistic suggestions on a treatment algorithm.
The literature analysed consistently supports the use of emollients and mild soaps, and a controlled study demonstrated significantly improved skin physiology and appearance with combined use of mild soap and emollients.21
To treat skin rash, the most common reaction reported to appear within the first 2 days of treatment, all authors unanimously recommended the use of non-occlusive emollients.
Sun exposure has been reported to worsen rash, hence the need for photo protection.50
The sensitive nature of the skin has often been described, leading numerous authors to suggest avoiding use of irritant products.9
Parenchyma and fissures were reported to be difficult to treat. Glue and liquid bandages as well as antiseptic creams were considered to be the most useful.
The use of antiperspirants is a controversial topic as the effect of chemotherapy on the eccrine glands eliminates their need. However, the working group felt that in the interest of patient well-being, deodorants and non-irritant perfumes may be used as part of maintaining a daily routine.
In terms of quality of life, studies showed significant improvements on anxiety and self-image when patients received adequate skin-care advice.39–41
To provide physicians with practical information, the following treatment algorithm was built from the available data and expert opinion. The algorithm proposes a baseline treatment followed by additional suggestions according to symptom severity.
The working group considers that all symptoms including folliculitis, xerosis, fissures, as well as hand and foot syndrome are linked to a skin barrier dysfunction. Maintaining skin barrier function using appropriate cosmetic products can control the severity of these symptoms. At the beginning of treatment, patients should receive information about dermatological skin-care products and education on appropriate use.44 This should be continued and encouraged throughout treatment.51 Symptoms should be evaluated all along the therapy and topical or systemic treatments may be added according to existing guidelines, if necessary. Dermatologist referral should be considered whenever symptoms worsen.
The following strategies are illustrated in the algorithm Fig. 1.
Figure 1.
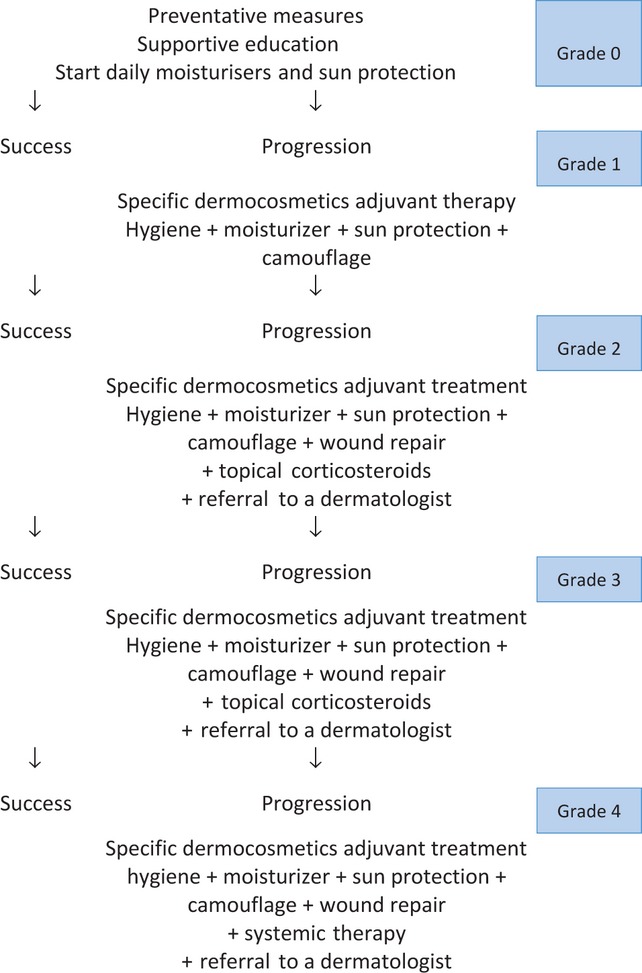
Proposed algorithm for the management of cutaneous toxicity associated with targeted therapies.
There is no evidence to suggest that skin cleansing should be avoided. Syndets with a pH of 5.5 are well tolerated and may be considered for use.
Daily application of a non-comedogenic moisturizing cream on both the face and body, irrespective of the chemotherapeutic agent prescribed, controls rash and xerosis. Consider oil-in-water vehicles for medical treatments and emollients containing humectants such as urea 5–10% or niacinamide.
Apply broad-spectrum sunscreen to the face and other exposed areas (i.e. neck and arms). SPF 15+/UVA-PF level according to phototype or expected photosensitivity
Well-being was improved by covering disfiguring erythema and pallor with non-comedogenic make-up. Avoid occlusive make-up if folliculitis is severe.
Fruit acids, antibacterials or benzoyl peroxide are not helpful to treat rash. Furthermore, they may cause irritation and be harmful.
Antiseptics and wound-healing creams maybe helpful in managing fissures and parenchyma.
The authors recognize that no systematic review was performed on available literature and hence relevant studies may not be cited. However, the authors feel that with this recommendation, a first attempt was made to provide guidance to the physicians who are dealing daily with skin-care problems in patients undergoing chemotherapy.
Conclusion
The present guidelines are intended to support optimization of therapeutic management of cutaneous side-effects and to improve the quality of life of oncology patients.
However, the authors recognize that further research is needed to test skin-care products in this population suffering from particularly sensitive skin.
Acknowledgments
The authors acknowledge Amy Whereat for her editorial assistance in writing this manuscript and Carole Rade and the CRIC for technical assistance.
References
- Burtness B, Anadkat M, Basti S, et al. NCCN Task Force Report: Management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl 1):S5–S21. doi: 10.6004/jnccn.2009.0074. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Pérez-Soler R, Chachoua A, Hammond L, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Senderowicz A, Johnson J, Sridhara R, et al. Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas. Oncology. 2007;21:1696–1709. [PubMed] [Google Scholar]
- Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- Segaert S, Chiritescu G, Lemmens L, et al. Skin toxicities of targeted therapies. Eur J Cancer. 2009;45(Suppl 1):295–308. doi: 10.1016/S0959-8049(09)70044-9. [DOI] [PubMed] [Google Scholar]
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425–1433. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- Perez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- Fernandez-Torres R, Martinez Gomez W, Cuevas Santos J, et al. Rosaceiform eruption induced by cetuximab. Eur J Dermatol. 2010;20:392–393. doi: 10.1684/ejd.2010.0900. [DOI] [PubMed] [Google Scholar]
- Jacot W, Bessis D, Jorda E, et al. Acniform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004;151:238–241. doi: 10.1111/j.1365-2133.2004.06026.x. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Boone SL, Rademaker A, Liu D, et al. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72:152–159. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- Perez-Soler R, Saltz L. Cutaneous adverse events with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol. 2006;155:852–854. doi: 10.1111/j.1365-2133.2006.07452.x. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- Jatoi A, Nguyen PL. Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist. 2008;13:1201–1204. doi: 10.1634/theoncologist.2008-0149. [DOI] [PubMed] [Google Scholar]
- Haley A, Calahan C, Gandhi M, et al. Skin care management in cancer patients: an evaluation of quality of life and tolerability. Support Care Cancer. 2011;19:545–554. doi: 10.1007/s00520-010-0851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3912–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- Wolf M, Swaisland H, Averbuch S, et al. Development of the novel biologically targeted anticancer agent gefitinib: determining the optimum dose for clinical efficacy. Clin Cancer Res. 2004;10:4607–4613. doi: 10.1158/1078-0432.CCR-04-0058. [DOI] [PubMed] [Google Scholar]
- Fluhr J, Miteva M, Primavera G, et al. Functional assessment of a skin care system in patients on chemotherapy. Skin Pharmacol Physiol. 2007;20:253–259. doi: 10.1159/000104423. [DOI] [PubMed] [Google Scholar]
- Ocvirk J, Cencelj S. Management of cutaneous side-effects of cetuximab therapy in patients with metastatic colorectal cancer. J Eur Acad Dermatol Venereol. 2010;24:453–459. doi: 10.1111/j.1468-3083.2009.03446.x. [DOI] [PubMed] [Google Scholar]
- Grenader T, Gipps M, Goldberg A, et al. Staphylococcus aureus bacteremia secondary to severe erlotinib skin toxicity. Clin Lung Cancer. 2008;9:59–60. doi: 10.3816/CLC.2008.n.010. [DOI] [PubMed] [Google Scholar]
- Wolf R, Wolf D, Tüzün B, et al. Soaps, Shampoos, Detergents. Clin Dermatol. 2001;19:393–397. doi: 10.1016/s0738-081x(01)00193-6. [DOI] [PubMed] [Google Scholar]
- Skolarus T, Homes-Rovner M, Northouse L, et al. Primary care perspectives on prostate cancer survivorship: Implications for improving quality of care. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.06.002. Jul 18. [Epub ahead of print] PMID:21775171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health NIo. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4. [11 November 2011]; Available from: ctep.cancer.gov/reporting/ctc.html.
- Jatoi A, Green EM, Rowland KM, Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in north central cancer treatment group study N0147. Oncology. 2009;77:120–123. doi: 10.1159/000229751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657–670. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Roe E, Garcia Muret MP, Marcuello E, et al. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol. 2006;55:429–437. doi: 10.1016/j.jaad.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk J, Boers-Doets C. Best practices in the management of toxicities related to anti-EGFR agents for metastatic colorectal cancer. Eur J Oncol Nurs. 2010;14:337–349. doi: 10.1016/j.ejon.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Moore S. Managing treatment side effects in advanced breast cancer. Semin Oncol Nurs. 2007;23(4 Suppl 2):S23–S30. doi: 10.1016/j.soncn.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Galimont-Collen AF, Vos LE, Lavrijsen AP, et al. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43:845–851. doi: 10.1016/j.ejca.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–326. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Wohlrab J, Luftner D, Johne A, et al. The advantage of a proactive, barrier-protective, supportive skin care in patients with brest cancer on chemotherapy. Oncology. 2011;34:62. [Google Scholar]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- Bernier J, Bonner J, Vermorken J, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19:142–149. doi: 10.1093/annonc/mdm400. [DOI] [PubMed] [Google Scholar]
- Rigopoulos D, Larios G, Gregoriou S, et al. Acute and chronic paronychia. Am Fam Physician. 2008;77:339–346. [PubMed] [Google Scholar]
- Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116:3916–3923. doi: 10.1002/cncr.25090. [DOI] [PubMed] [Google Scholar]
- Taggart L, Ozolins L, Hardie H, et al. Look Good Feel Better Workshops: A “big lift” for women with cancer. J Cancer Educ. 2009;24:94–99. doi: 10.1080/08858190802664594. [DOI] [PubMed] [Google Scholar]
- Amiel P, Dauchy S, Bodin J, et al. Evaluating beauty care provided by the hospital to women suffering from breast cancer. Support Care Cancer. 2009;17:839–845. doi: 10.1007/s00520-009-0620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeca G, Poot F, Cassart D, et al. Impact of cosmetic care on quality of life in breast cancer patients during chemotherapy and radiology. J Eur Acad Dermatol Venereol. 2007;21:771–776. doi: 10.1111/j.1468-3083.2006.02080.x. [DOI] [PubMed] [Google Scholar]
- Anderson M, Johnson J. Restoration of body image and self-esteem fro women after concer treatment: a rehabiliatative survey. Cancer Pract. 1994;2:345–349. [PubMed] [Google Scholar]
- Fawzy N, Secher L, Evans S, et al. The Positive Appearance Center: an innovative concept in comprehensive psychosocial cancer care. Cancer Pract. 1995;3:233–238. [PubMed] [Google Scholar]
- Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Schreier A. The effect of education in managing side effects in women receiving chemotherapy for treatment of breast cancer. Oncol Nurs Forum. 2004;31:E16–E23. doi: 10.1188/04.ONF.E16-E23. [DOI] [PubMed] [Google Scholar]
- Thune P. The effects of Detergents on Hydration and Skin Surface Lipids. Clin Dermatol. 1996;14:29–33. doi: 10.1016/0738-081x(95)00105-o. [DOI] [PubMed] [Google Scholar]
- El-Abaseri T, Hansen L. EGFR activation and ultraviolet light-induced skin carcinogenesis. J Biomed Biotechnol. 2007;2007:97939. doi: 10.1155/2007/97939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Gasparro FP, Jensen PJ, et al. Keratinocyte apoptosis induced by ultraviolet B radiation and CD95 ligation – differential protection through epidermal growth factor receptor activation and Bcl-x(L) expression. J Invest Dermatol. 2001;116:860–866. doi: 10.1046/j.1523-1747.2001.01356.x. [DOI] [PubMed] [Google Scholar]
- Funke A, Kulp-Shorten C, Callen J, et al. Subacute cutaneous lupus erythematosus exacerbated or induced by chemotherapy. Arch Dermatol. 2010;146:1113–1116. doi: 10.1001/archdermatol.2010.258. [DOI] [PubMed] [Google Scholar]
- Luu MLS, Patel J, Guitart J, et al. Photosensitive rash due to the epidermal growth factor receptor inhibitor erlotinib. Photodermatol Photoimmunol Photomed. 2007;23:42–45. doi: 10.1111/j.1600-0781.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- Battie C, Verschoore M. Dermatology, cosmetic and well being. Ann Dermatol Venerol. 2001;138:294–301. doi: 10.1016/j.annder.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Merial-Kieny C, Nocera T, Mery S, et al. Medical corrective make-up in post chemotherapy. Ann Dermatol Venereol. 2008;1:25–28. doi: 10.1016/S0151-9638(08)70094-2. [DOI] [PubMed] [Google Scholar]


