Abstract
BACKGROUND
Current guidelines recommend that all gastric ulcers (GUs) be biopsied extensively to exclude underlying malignancy. However, many gastroenterologists opt to also perform surveillance endoscopy (EGD) to document ulcer healing. The purpose of this study was to examine frequency of utilization of surveillance EGD in patients found to have GUs using a national endoscopic database.
METHODS
The Clinical Outcomes Research Initiative (CORI) database was used to identify ambulatory patients diagnosed with a GU between 2001 and 2005. A surveillance EGD was defined as any EGD performed ≤3 months after index EGD. Results were stratified by patient demographic factors, index ulcer size and location, practice setting, and geographic region. Multivariate logistic regression was performed to identify independent predictors of surveillance EGD utilization.
RESULTS
In the database, 6,113 patients met our inclusion/exclusion criteria, of which 1,510 (24.7%) underwent surveillance EGD. Older patients were more likely to undergo surveillance than younger patients (P < 0.0001), though a substantial minority (15.2%) of patients <40 years of age underwent a surveillance examination. Index ulcer size ≥1 cm and care in a Veterans Affairs (VA) setting were also independent predictors of surveillance EGD utilization. Significant geographic variation was noted, with surveillance rates varying from 16.0% to 35.9% across the United States (P < 0.0001).
CONCLUSIONS
In contrast to guideline recommendations, approximately 25% of ambulatory patients diagnosed with GUs underwent surveillance EGD within 3 months. Notably, patients at low-risk for gastric cancer, including young patients, those with small index ulcers, and those with antral ulcers, underwent surveillance at higher than expected rates, which suggests overuse of surveillance EGD.
INTRODUCTION
A gastric ulcer (GU) is defined as a significant break in the mucosal lining of the stomach. GUs are a common endoscopic finding, with a point prevalence as high as 4% and a lifetime incidence of more than 10% in the general population (1–3). During endoscopy (EGD), a small percentage of these ulcers will appear grossly malignant, prompting biopsies and surveillance EGD to document healing. However, the vast majority of GUs (>90%) appear benign both endoscopically and histologically, the result of nonsteroidal antiinflammatory drugs (NSAIDs) and/or Helicobacter pylori (HP) infection (4, 5). Yet, approximately 5% of endoscopically benign-appearing GUs are in fact malignant (6–8).
Determining whether an endoscopically benign-appearing GU is truly benign or malignant can be challenging. One method for making this distinction is to obtain multiple biopsies of the ulcer at the time of the index EGD. This approach has been shown to be highly sensitive for detecting malignancy in both retrospective and prospective trials (8, 9). As a result, the combined American Society of Gastrointestinal Endoscopy and American College of Gastroenterology (ASGE/ACG) Task Force on Quality in Endoscopy recommends routinely performing biopsies of all GUs to exclude malignancy (10). Nevertheless, endoscopists frequently perform surveillance EGD to document GU healing in order to exclude underlying malignancy. In a survey of Canadian endoscopists, more than 60% reported that they perform surveillance EGD routinely in patients with a GU (11). Overuse of surveillance EGD in patients with GUs may increase procedure-related complications and costs and misappropriate limited endoscopic resources. Indeed, not one of the three major U.S. professional gastroenterology societies (ASGE, ACG, and American Gastroenterological Association [AGA]) specifically recommends performing endoscopic surveillance of benign-appearing GUs. Yet, no prior study has examined how often endoscopists across the United States perform GU surveillance. In addition, no study has identified risk factors for utilization of surveillance EGD. The purpose of this study was (1) to estimate the proportion of patients initially diagnosed with a GU who underwent surveillance EGD using a national endoscopic database and (2) to stratify these results by patient demographic factors, index EGD characteristics, and practice setting.
METHODS
Study Design and Dataset
We performed a retrospective cohort study using data from the Clinical Outcomes Research Initiative (CORI) database. The CORI database is a national endoscopic database that contains data for nearly two million endoscopic procedures conducted by over 400 endoscopists at more than 80 community, academic, and Veterans Affairs (VA) sites across the United States (representing ~1% of all endoscopies performed nationally in Medicare patients) (12). CORI data are collected in a structured, prospective fashion (13, 14). Furthermore, these data have been shown to be representative of national endoscopic practice patterns in Medicare patients in the United States (12).
Data Collection
The database was used to identify all ambulatory patients diagnosed with a finding of GU(s) between 2001 and 2005. Hospitalized patients were excluded because the most common indication for EGD in such patients is upper GI bleeding (15), and endoscopists may be reluctant to biopsy an ulcer in the setting of bleeding, markedly lowering the threshold for surveillance EGD. Surveillance EGD was defined as any EGD performed within 3 months of the index examination. Exclusion criteria included: (i) history of gastric surgery; (ii) history of gastric malignancy; or (iii) EGD performed within the previous 12 months. These criteria were selected to exclude patients at increased-risk of gastric cancer (who might warrant intensive surveillance). Data were extracted on: (i) patient age, gender, and race/ethnicity; (ii) practice setting (community/HMO, academic, or VA/military); (iii) geographic region; (iv) index ulcer size (<1 or ≥1 cm); (v) whether the index ulcer was located in the antrum; and (vi) whether the index ulcer was biopsied.
Statistical Analysis
Results were stratified by: (i) patient age, gender, and race/ethnicity; (ii) index ulcer size and location; (iii) practice setting; and (iv) geographic region. Surveillance rates between groups were compared statistically using Pearson’s chi-square test statistic. Multivariate logistic regression was performed to identify independent predictors of surveillance EGD utilization. Adjusted odds ratios and 95% confidence intervals are presented. An a priori determined P-value of ≤0.05 was considered statistically significant. All analyses were performed using SAS version 9.1 software (SAS Institute, Inc., Cary, NC).
RESULTS
A total of 11,749 patients in the CORI database who underwent EGD between 2001 and 2005 were found to have one or more GUs on index EGD. For a variety of reasons, 5,636 patients were excluded (Fig. 1), resulting in 6,113 patients with GUs who were included in the study. Demographic characteristics of the cohort are shown in Table 1. A specific indication was reported for 86% of index EGDs, with the vast majority of these (84%) being performed for upper gastrointestinal symptoms (51%) and suspected gastrointestinal blood loss (33%). Patients diagnosed with a GU were more likely to be females (56%), greater than 60 years old (57%), and of non-Hispanic white ethnicity (80%). The vast majority of procedures (68%) were performed in a community setting (Table 2). Furthermore, biopsy was performed at nearly 70% of index EGDs, a result that is concordant with previously reported data (16). More than half of GUs were noted to be <1 cm in size, and nearly 75% of patients had an ulcer in the antrum. Multiplicity of ulcers and pathology results could not be accurately determined from the database.
Figure 1.
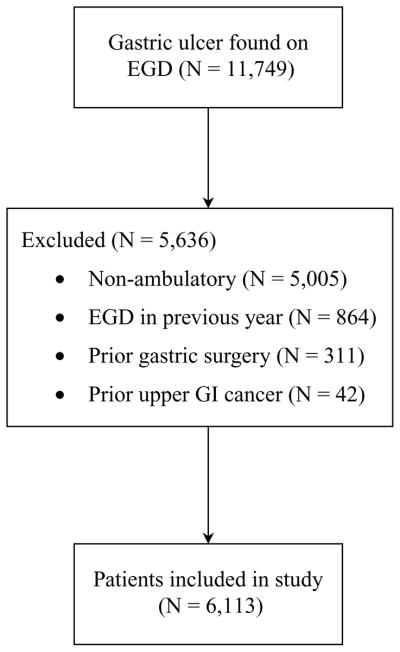
Flowchart of selection process for inclusion in study.
Table 1.
Patient Demographics (N = 6,113)
| Number | % | |
|---|---|---|
| Gender | ||
| Female | 3,404 | 55.7 |
| Male | 2,709 | 44.3 |
| Age group (years) | ||
| <40 | 512 | 8.4 |
| 40–49 | 765 | 12.5 |
| 50–59 | 1,346 | 22.0 |
| 60–69 | 1,401 | 22.9 |
| 70–79 | 1,399 | 22.9 |
| ≥80 | 690 | 11.3 |
| Race/ethnicity | ||
| White non-Hispanic | 4,875 | 79.8 |
| Black non-Hispanic | 422 | 6.9 |
| Hispanic | 500 | 8.2 |
| Asian/Pacific Islander | 148 | 2.4 |
| Native American | 82 | 1.3 |
| Multi-racial | 13 | 0.2 |
| Not available | 73 | 1.2 |
Table 2.
Practice and Index Endoscopy Features (N = 6,113)
| Number | % | |
|---|---|---|
| Practice setting | ||
| Community/HMO | 4,166 | 68.2 |
| Academic | 1,105 | 18.1 |
| VA/military | 842 | 13.8 |
| Geographic region* | ||
| North Central | 508 | 8.3 |
| North East | 1,328 | 21.7 |
| North West | 867 | 14.2 |
| South Central | 603 | 9.9 |
| South East | 868 | 14.2 |
| South West | 1,939 | 31.7 |
| Index ulcer size | ||
| <1 cm | 3,137 | 51.3 |
| ≥1 cm | 1,561 | 25.5 |
| Not available | 1,415 | 23.2 |
| Antral ulcer | ||
| Yes | 4,490 | 73.5 |
| No | 1,623 | 26.6 |
| Ulcer biopsy taken | ||
| Yes | 4,266 | 69.8 |
| No | 1,847 | 30.2 |
North Central = IN, MN, ND, NE; North East = MA, NJ, NY, OH, PA, VT; North West = OR, WA; South Central = MS, OK, TN, TX; South East = FL, GA, KY, NC, VA; South West = AZ, CA, CO, NM, NV.
A total of 1,510 patients (24.7%, 95% CI 23.6–25.8%) underwent surveillance EGD within 3 months of the index EGD (Tables 3 and 4), and approximately 25% of patients who underwent surveillance were found to have a GU on surveillance examination (Table 5). GU surveillance was explicitly reported as an indication in 78% of these procedures. Patients with an index ulcer >1 cm in size were significantly more likely to also have a GU documented on surveillance EGD (37.1% vs 18.1%, P < 0.0001). When the definition of surveillance EGD was extended to any EGD performed within 6 months of the index examination, the proportion of patients undergoing surveillance increased to almost 33%. The proportion of patients who were ultimately found to have gastric cancer could not be determined from the database.
Table 3.
Proportion of Patients Undergoing Surveillance EGD (Stratified by Patient Demographic Characteristics) (N = 1,510)
| Number | % | P-value | |
|---|---|---|---|
| Total | 1,510 | 24.7 | |
| Gender* | |||
| Female | 788 | 23.2 | 0.002 |
| Male | 722 | 26.7 | |
| Age group (years) | |||
| <40 | 78 | 15.2 | <0.0001 |
| 40–49 | 167 | 21.8 | |
| 50–59 | 338 | 25.1 | |
| 60–69 | 371 | 26.5 | |
| 70–79 | 387 | 27.7 | |
| ≥80 | 169 | 24.5 | |
| Race/ethnicity | |||
| White non-Hispanic | 1,229 | 25.2 | 0.01 |
| Black non-Hispanic | 106 | 25.1 | |
| Hispanic | 89 | 17.8 | |
| Asian/Pacific Islander | 38 | 25.7 | |
| Native American | 25 | 30.5 | |
| Multi-racial | 2 | 15.4 | |
| Not available | 21 | 28.8 | |
These results include VA sites. When VA sites were excluded, gender was no longer associated with surveillance utilization (P = 0.67).
Table 4.
Proportion of Patients Undergoing Surveillance EGD (Stratified by Practice and Index Endoscopy Features) (N = 1,510)
| Number | % | P-value | |
|---|---|---|---|
| Total | 1,510 | 24.7% | |
| Practice setting | |||
| Community/HMO | 970 | 23.3 | <0.0001 |
| Academic | 225 | 20.4 | |
| VA/military | 315 | 37.4% | |
| Geographic region* | |||
| North Central | 131 | 35.9 | <0.0001 |
| North East | 229 | 22.3 | |
| North West | 129 | 23.6 | |
| South Central | 34 | 16.0 | |
| South East | 189 | 24.6 | |
| South West | 258 | 20.7 | |
| Index ulcer size | |||
| <1 cm | 689 | 22.0 | <0.0001 |
| ≥1 cm | 512 | 32.8 | |
| Not available | 309 | 21.8 | |
| Antral ulcer | |||
| Yes | 1,104 | 24.6 | 0.73 |
| No | 406 | 25.0 | |
| Ulcer biopsy taken | |||
| Yes | 1,083 | 25.4 | 0.06 |
| No | 427 | 23.1 | |
Community practices only (N = 4,166)
Table 5.
Proportion of Patients with Gastric Ulcer on Surveillance EGD (Stratified by Index Ulcer Size)
| Number | % | |
|---|---|---|
| Total | 383 | 25.4 |
| Index ulcer <1 cm | 125 | 18.1 |
| Index ulcer ≥1 cm | 190 | 37.1* |
| Index ulcer size missing | 68 | 22.0 |
Chi-square P < 0.0001 (68 patients with missing data not included in this analysis).
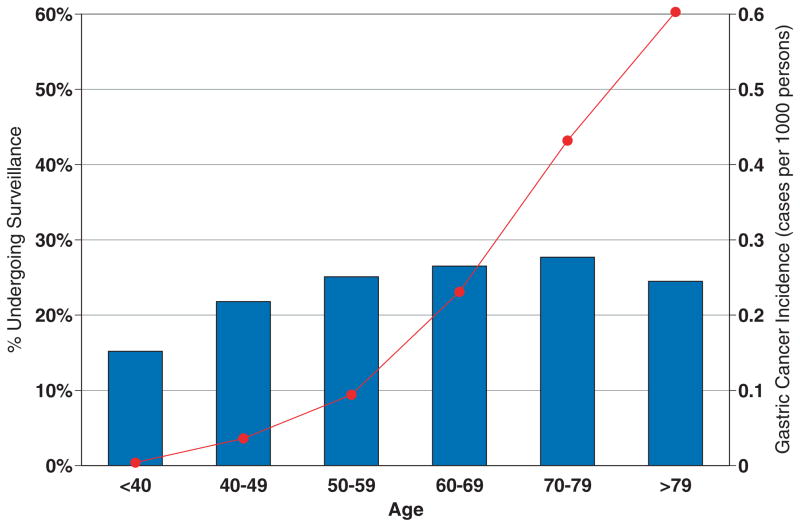
In bivariate analysis, age (range 15.2–27.7%, P < 0.0001), male gender (26.7% vs 23.2%., P = 0.002), race/ethnicity (range 15.4–30.5%, P = 0.01), practice setting (range 20.4–37.4%, P < 0.0001), geographic region (range 16.0–35.9%, P < 0.0001), and index ulcer size >1 cm (32.8% vs 22.0%, P < 0.0001) were each found to be significantly associated with performance of surveillance EGD within 3 months of the index examination (Tables 3 and 4). When VA sites (with predominantly male patients) were excluded, the aforementioned gender association was no longer significant (22.4% vs 22.9%, P = 0.67). Furthermore, patients with antral and nonantral ulcers underwent surveillance at similar rates (24.6% vs 25.0%, P = 0.73). In multivariate analysis, only age, practice setting, geographic region, and GU size were found to be important predictors of surveillance EGD utilization (Table 6). Notably, in contrast to the age-related incidence of gastric cancer, surveillance utilization remained remarkably stable across age groups, particularly among patient over 40 years of age (Fig. 2).
Table 6.
Independent Predictors of Surveillance EGD Utilization (Logistic Regression Analysis) (N = 6,113)
| Odds Ratio | 95% CI | |
|---|---|---|
| Age group | ||
| <40 years | 1.00 (reference) | |
| 40–49 years | 1.48 | 1.10–2.00 |
| 50–59 years | 1.65 | 1.25–2.18 |
| 60–69 years | 1.81 | 1.38–2.38 |
| 70–79 years | 1.95 | 1.48–2.56 |
| ≥80 years | 1.63 | 1.20–2.20 |
| Practice setting | ||
| Community/HMO | 1.00 (reference) | |
| Academic | 0.86 | 0.73–1.02 |
| VA/Military | 2.49 | 2.08–2.99 |
| Geographic region | ||
| North Central | 1.00 (reference) | |
| North East | 0.52 | 0.41–0.65 |
| North West | 0.50 | 0.39–0.64 |
| South Central | 0.32 | 0.24–0.43 |
| South East | 0.56 | 0.44–0.72 |
| South West | 0.47 | 0.37–0.58 |
| Index ulcer size | ||
| <1 cm | 1.00 (reference) | |
| ≥1 cm | 1.88 | 1.64–2.16 |
| Not available | 1.11 | 0.95–1.30 |
| Ulcer biopsy taken | ||
| No | 1.00 (reference) | |
| Yes | 1.22 | 1.07–1.40 |
Figure 2.
Surveillance utilization (%, blue) and gastric cancer incidence (cases per 1,000, red) (SEER 17, http://seer.cancer.gov/faststats/, accessed October 25, 2007).
DISCUSSION
GUs are a common finding at upper EGD (1–3). Though the vast majority of benign-appearing GUs are indeed benign (4, 5), approximately 5% of endoscopically benign-appearing GUs harbor malignancy (6–8). Multiple ulcer biopsies at index EGD are highly sensitive for detecting malignancy (8, 9), and these data are acknowledged by ASGE and ACG practice guidelines and by the ASGE/ACG Task Force on Quality of Endoscopy (10, 17, 18). Furthermore, no major U.S. professional gastroenterology society recommends surveillance of GUs. However, a recent Canadian survey suggested that nearly two-thirds of endoscopists choose to routinely perform surveillance EGD to document GU healing (11). Using the CORI database, our study demonstrates that U.S. endoscopists perform surveillance EGD within 3 months in approximately 25% of patients diagnosed with a GU. When the time interval was extended from 3 to 6 months, the proportion of patients undergoing surveillance EGD increased to almost 33%. It should be noted that these numbers likely represent minimum estimates, as patients may undergo surveillance EGD at a non-CORI site. In multivariate analysis, the surveillance rate varied significantly across age groups, practice setting, and geographic region. Most notably, patients under age 40 (15%), those with small index ulcers (22%), those with antral ulcers (25%), and those within the VA system (37%) appear to undergo surveillance at higher than expected rates, which suggests overuse of surveillance EGD. Of note, our comparison of age-specific GU surveillance rates to age-specific gastric cancer incidence (SEER registry) is somewhat limited by the fact that our study included only ambulatory patients (as opposed to the SEER registry, which includes all-comers).
The management of GUs has been a controversial topic in gastroenterology for well over two decades. The first study to challenge the necessity of surveillance EGD was published 25 years ago by Graham et al. (9). This study demonstrated that obtaining at least seven biopsy specimens of the index ulcer was sufficient to exclude underlying malignancy. However, subsequent studies raised concerns that malignancy could be missed despite index biopsies and that missing early gastric cancer could lead to poorer long-term outcomes (7, 19). Indeed, a 1991 study of endoscopic surveillance of GUs was carried out under the assumption that such surveillance was a “mandatory” practice (20). This study found that surveillance EGD was also imperfect in excluding gastric cancer, with up to one-third of gastric cancer cases being missed despite endoscopic surveillance. A subsequent study in 1993 by Pruitt et al. concluded that surveillance EGD was unnecessary provided that sufficient biopsy specimens were obtained at the index examination (8). In 1997, a U.S. study from a single tertiary referral center utilized an endoscopic database to determine the sensitivity and specificity of endoscopists’ visual impression in predicting the pathology of GUs. As a secondary aim, this study reported surveillance rates, finding these to be very low (11%), though the authors concluded that this was likely related to incomplete follow-up of the patient population. The generalizability of results from a tertiary referral center is also questionable. It was not until 1999 that a study was performed specifically to assess how gastroenterologists manage benign-appearing GUs. In this survey-based study, nearly 60% of Canadian gastroenterologists reported performing surveillance EGD routinely in patients with benign-appearing GUs (11). To our knowledge, our study is the first since this 1999 study to assess how gastroenterologists manage benign-appearing GUs. Furthermore, our study is the first since the 1997 study to report actual rather than self-reported practice patterns specifically on this topic and is also the first ever study reporting practice patterns across the United States. Like the Canadian study, our study suggests continued overuse of surveillance EGD in patients with benign-appearing GUs. Potential reasons for this finding include lack of knowledge or disagreement with guidelines, medico-legal concerns, and financial incentives. As gastroenterologists in the United States face increasing demand for endoscopic services and experts project that this demand will continue to increase, decreasing overuse of EGD will assume increasing importance (21–24).
Several limitations and strengths of our study deserve mention. First, this was a retrospective study. Though we attempted to control for confounders in our analysis, the potential for unmeasured confounding and bias remains. In addition, CORI does not reliably record specific endoscopic details, such as ulcer location, number of ulcers, and pathology data, or clinical information about individual patients outside of the EGD report. We therefore were unable to report or account for these factors in our analysis. Finally, our study may have suffered from loss to follow-up. Patients who undergo index EGD at a center that participates in CORI are free to undergo additional endoscopic examinations at a non-CORI site. This may have resulted in incomplete capture of our patient population, meaning that the proportion of patients who undergo surveillance within 3 months may actually be greater than 25%. This phenomenon may partly explain the significantly higher rate of surveillance observed in VA centers in our study. VA patients may be less likely to follow-up at a non-CORI site for their surveillance EGD, resulting in more complete follow-up (and therefore higher surveillance rates) in this subgroup of patients. Alternatively, our study could have overestimated the proportion of patients undergoing surveillance due to the definition of surveillance EGD that was used (any EGD performed within 3 months of index EGD). This may have particularly affected the results for older patients, overestimating the surveillance rates in these patients. However, this would have weakened the observed age-related surveillance gradient, further strengthening our conclusion that younger patients undergo surveillance at disproportionately higher rates than warranted based on underlying gastric cancer incidence. Using only EGDs with a documented indication of “GU surveillance” (a less sensitive but more specific definition for “surveillance EGD” than that used in our primary analysis), the overall surveillance rate was 19.3%. One of the important strengths of our study is the use of actual rather than self-reported practice patterns. Furthermore, we used data collected from a wide variety of practice settings that is representative of EGD in Medicare patients in the United States today (12). Finally, our study was performed after the publication of ACG and ASGE guidelines supporting the use of multiple biopsies, allowing us to draw conclusions regarding the uptake of these guidelines into clinical practice (17, 18).
In summary, approximately 25% of ambulatory patients diagnosed with GUs undergo surveillance EGD within 3 months. This rate varies significantly by patient age, ulcer size, practice setting, and geographic location. However, young patients, those with small ulcers, those with antral ulcers, and those within the VA system continue to undergo surveillance at higher than expected rates, suggesting overuse of surveillance EGD. Future studies should use patient-specific risk factors for gastric cancer to define a tailored approach to excluding malignancy in patients with benign-appearing GUs.
STUDY HIGHLIGHTS.
What Is Current Knowledge
Benign-appearing gastric ulcers are unlikely to be malignant.
Guidelines recommend that all gastric ulcers be biopsied.
Guidelines do not specifically recommend surveillance EGD.
What Is New Here
U.S. endoscopists perform surveillance in approximately 25% of patients.
Surveillance rates do not increase appropriately as gastric cancer risk increases.
Surveillance EGD appears to be overused in the United States.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Sameer D. Saini, M.D., M.Sc.
Specific author contributions: Sameer D. Saini and Philip Schoenfeld were responsible for the conception and design of the study and the drafting of the manuscript. Nora Mattek performed the statistical analysis. All authors participated in the analysis and interpretation of the results and critical revision of the manuscript.
Financial support: This project was supported with the funding from NIDDK UO1 CA 89389-01 and R33-DK61778-01. In addition, the practice network (CORI) has received support from the following entities to support the infrastructure of the practice based network: AstraZeneca, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon.
Potential competing interests: Dr. Saini has no financial conflicts of interest relevant to this study, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. The commercial entities had no involvement in this research.
References
- 1.Akdamar K, Ertan A, Agrawal NM, et al. Upper gastrointestinal endoscopy in normal asymptomatic volunteers. Gastrointest Endosc. 1986;32:78–80. doi: 10.1016/s0016-5107(86)71760-4. [DOI] [PubMed] [Google Scholar]
- 2.Aro P, Storskrubb T, Ronkainen J, et al. Peptic ulcer disease in a general adult population: The Kalixanda study: A random population-based study. Am J Epidemiol. 2006;163:1025–34. doi: 10.1093/aje/kwj129. [DOI] [PubMed] [Google Scholar]
- 3.Bernersen B, Johnsen R, Straume B, et al. Towards a true prevalence of peptic ulcer: The Sorreisa gastrointestinal disorder study. Gut. 1990;31:989–92. doi: 10.1136/gut.31.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo MT, Forne M, de Argila CM, et al. The prevalence of peptic ulcer not related to Helicobacter pylori or non-steroidal anti-inflammatory drug use is negligible in southern Europe. Helicobacter. 2004;9:249–54. doi: 10.1111/j.1083-4389.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa K, Sugiyama T, Kato M, et al. Non-Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur J Gastroenterol Hepatol. 2000;12:635–40. doi: 10.1097/00042737-200012060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Chan TO, Kuo YC, Chien RN, et al. Gastric adenocarcinoma simulating benign gastric ulcer. Changgeng Yi Xue Za Zhi. 1992;15:59–63. [PubMed] [Google Scholar]
- 7.Podolsky I, Storms PR, Richardson CT, et al. Gastric adenocarcinoma masquerading endoscopically as benign gastric ulcer. A five-year experience. Dig Dis Sci. 1988;33:1057–63. doi: 10.1007/BF01535778. [DOI] [PubMed] [Google Scholar]
- 8.Pruitt RE, Truss CD. Endoscopy, gastric ulcer, and gastric cancer. Follow-up endoscopy for all gastric ulcers? Dig Dis Sci. 1993;38:284–8. doi: 10.1007/BF01307545. [DOI] [PubMed] [Google Scholar]
- 9.Graham DY, Schwartz JT, Cain GD, et al. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology. 1982;82:228–31. [PubMed] [Google Scholar]
- 10.Cohen J, Safdi MA, Deal SE, et al. Quality indicators for esophagogastroduodenoscopy. Am J Gastroenterol. 2006;101:886–91. doi: 10.1111/j.1572-0241.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Breslin NP, Sutherland LR. Survey of current practices among members of CAG in the follow-up of patients diagnosed with gastric ulcer. Can J Gastroenterol. 1999;13:489–93. doi: 10.1155/1999/738907. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg A, Amorosi SL, Lacey MJ, et al. Patterns of endoscopy in the United States: Analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–96. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Outcomes Research Initiative. [Accessed July 30, 2007]; http://www.cori.org.
- 14.Lieberman DA, Holub J, Eisen G, et al. Utilization of colonoscopy in the United States: Results from a national consortium. Gastrointest Endosc. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Sperber AD, Fich A, Eidelman L, et al. Open access endoscopy for hospitalized patients. Am J Gastroenterol. 1997;92:1823–6. [PubMed] [Google Scholar]
- 16.Harewood GC, Holub JL, Lieberman DA. Biopsy specimen acquisition in patients with newly diagnosed peptic ulcer disease as determined from a national endoscopic database. Gastrointest Endosc. 2004;59:664–9. doi: 10.1016/s0016-5107(04)00179-8. [DOI] [PubMed] [Google Scholar]
- 17.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330–8. doi: 10.1111/j.1572-0241.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Eisen GM, Dominitz JA, Faigel DO, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2001;54:815–7. doi: 10.1016/s0016-5107(01)70083-1. [DOI] [PubMed] [Google Scholar]
- 19.Longo WE, Zucker KA, Zdon MJ, et al. Detection of early gastric cancer in an aggressive endoscopy unit. Am Surg. 1989;55:100–4. [PubMed] [Google Scholar]
- 20.Bytzer P. Endoscopic follow-up study of gastric ulcer to detect malignancy: Is it worthwhile? Scand J Gastroenterol. 1991;26:1193–9. doi: 10.3109/00365529108998613. [DOI] [PubMed] [Google Scholar]
- 21.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72–7. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 22.Robertson RH, Burkhardt JH, Powell MP, et al. Trends in colon cancer screening procedures in the US Medicare and Tricare populations: 1999–2001. Prev Med. 2006;42:460–2. doi: 10.1016/j.ypmed.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–9. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 24.Vijan S, Inadomi J, Hayward RA, et al. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507–15. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]