Abstract
T cells are essential for the adaptive immune response to pathogens. However, dysfunctional T cell activity has been implicated in numerous diseases, including the failure of organ transplants, allergic reactions, asthma, autoimmune disorders, and coronary artery disease. T cell responses to pathogens require the induction of the primary activating receptor, the T cell receptor (TCR), along with other costimulatory and adhesion receptors. Signal transduction pathways activated downstream of these receptors drive T cell responses required for the immune response and disease progression. A key question in our understanding of the mechanism of T cell activation is how signalling pathways emanating from multiple receptors integrate together to alter T cell effector functions. One integration node for intracellular signalling is the membrane-associated adaptor protein linker for the activation of T cells or LAT. Upon stimulation of the TCR and other receptors, LAT is phosphorylated at several tyrosines residues on its cytoplasmic tail. This leads to the binding of SH2-domain containing proteins and their associated molecules and the formation of large multiprotein complexes. These dynamic and highly regulated signalling complexes facilitate the production of second messengers, activate downstream pathways, induce actin cytoskeleton polymerization, and stimulate the activity of multiple transcription factors. Thus, signalling pathways from several receptors feed into LAT, which then integrates this information and selectively induces pathways critical for T cell activation and the adaptive immune response.
In multiple and diverse ways, T cells control the human immune response. Pathogen invasion triggers the innate immune response, leading to inflammation and the activation of antigen presenting cells (APCs) such as macrophages, dendritic cells, and B cells1. The activated APCs express peptide antigen-bound major histocompatibility complexes (MHC) on their surface, which then activate antigen-specific T cell receptors (TCR) present on the surface of αβ T cells1. Activated T cells target specific pathogens by selectively differentiating into several unique functional subsets defined by their cytokine secretion, surface receptor expression, and transcription factor activation2.
TCR stimulation by the peptide-MHC complex begins the process of communicating environmental information from the exterior to the interior of the T cell to alter its functional status1. In the cytoplasm, the activation signal is amplified through a combination of post-translational protein modifications, multi-protein interactions, and the production of second messengers. The first step in this signal transduction cascade is the phosphorylation of immune receptor tyrosine-based activation motifs (ITAMs), located on the cytoplasmic tails of the CD3 chains of the TCR complex, by the Src family kinases Lck and Fyn3. Subsequently, Lck and Fyn phosphorylate and activate the Syk family kinase ZAP-70 when it is recruited to the phosphorylated ITAM motifs3. Active ZAP-70 then phosphorylates the downstream adaptor molecules SLP-76 and linker for activation of T cells (LAT), thus inducing the formation of the LAT and SLP-76 multi-protein complexes (Figure 1)1. Many of the SH2-domain containing molecules that interact with phosphorylated LAT also recruit other effectors. Structurally, these complexes form through the use of modular interaction domains to form a large, multi-protein complex that organizes and expedites signalling. Functionally, these interactions bring effector molecules close to both the membrane and other proteins where they are able to interact with their targets. The formation of a LAT-nucleated multi-protein complex leads to changes in cytoskeletal arrangement and gene expression, induces the production of second messengers, and elicits cellular responses specific to the environmental signal1. This results in the activation and coordination of the adaptive immune response that clears the body of the pathogen and leads to the development of a memory repertoire.
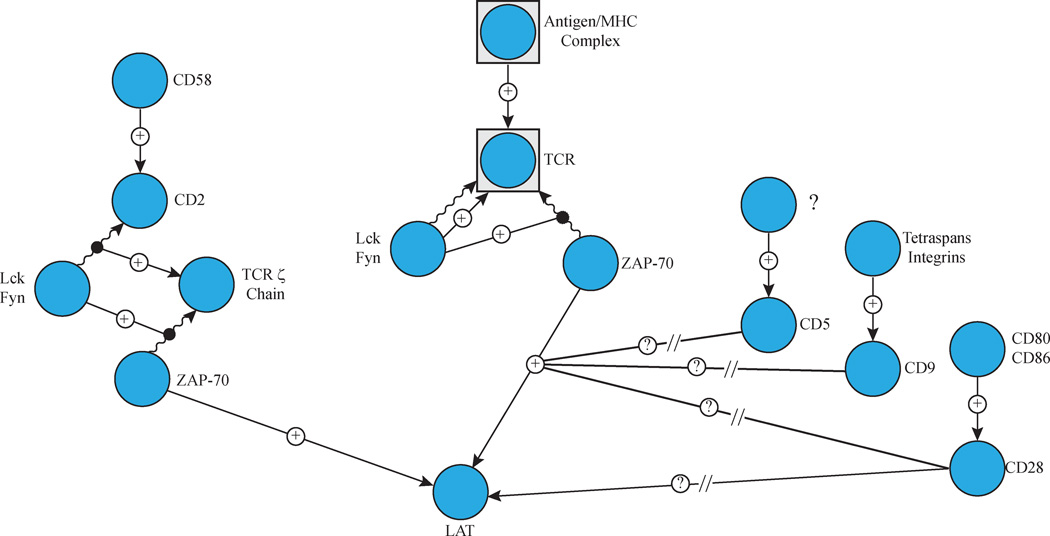
Figure 1. Receptor Mediated Induction of LAT Phosphorylation.
TCR and CD2 induction results in the receptor localization and/or activation of the Src family kinases Lck and Fyn. The kinases then phosphorylate ITAM motifs on the TCR. This leads to the recruitment and activation of ZAP-70 and this kinase subsequently phosphorylates LAT. Activation of CD5, CD9 and CD28 enhance TCR-mediated LAT phosphorylation via an unknown mechanism. CD28 induction alone is also capable of inducing LAT phosphorylation by an uncharacterized mechanism.
Besides the TCR, several other co-stimulatory and adhesion receptors also induce the activation of LAT. Ligation of human CD2 by its cognate ligand CD58 results in the phosphorylation of LAT and formation of LAT-mediated signalling clusters (Figure 1)4–7. CD2-induced LAT phosphorylation requires the activity of Lck and ZAP-70 and the activation of LAT is required for downstream functions of CD26. In addition, CD5, CD9, and CD28 enhance TCR-mediated LAT phosphorylation (Figure 1)7. Induction of CD28 alone has also been suggested to induce LAT phosphorylation (Figure 1)8. The mechanism for the enhancement of LAT phosphorylation by CD5, CD9 and CD28 is unknown (Figure 1). Ultimately, signals from antigen, adhesion, and co-stimulatory receptors merge at LAT to drive downstream signalling critical for T cell effector functions. The correct spatial and temporal regulation of receptor-driven signalling complexes is vital for the ability of LAT to integrate signals from multiple receptors and then precisely control the activation of multiple downstream events. Thus, LAT serves as a critical and required integrator of activation signals that drive the adaptive immune response.
THE STRUCTURE OF THE LAT COMPLEX
SH2-domain containing proteins bind phosphorylated LAT
LAT has a very short external region, a transmembrane domain, and a long tail containing several tyrosines that are conserved among rats, mice, and humans9. LAT is required for T cell development; LAT −/− mice have an absolute block in T cell development at the DN3 stage of thymocytic development where functional TCR-mediated signaling is required for further development10. A recent study has examined the role of LAT in mature T cells using a tamoxifen sensitive floxed LAT allele. Deletion of LAT in mature CD8+ T cells resulted in impaired microtubule organizing center polarization and granule reorientation, resulting in unstable target cell interactions and reduced granule mediated killing11. Phosphorylated tyrosines 132, 171, 191, and 226 (mouse Y136, Y175, Y195 and Y235) on human LAT are required for normal T cell signalling and function. Human LAT is also phosphorylated LAT tyrosine 127 (mouse Y132), but the physiological function of the phosphoryaltion of this site is unknown12. Mice expressing a mutant form of LAT where all four distal tyrosines are mutated to phenylalanine have abnormal T cell development, similar to LAT−/− mice13, 14. Phosphorylated tyrosines 171, 191, and 226 bind to the SH2 domains of the Grb2 family of adaptor proteins and must be present for optimal signalling12, 15, 16. Mice with the three distal tyrosines mutated have very few αβ T cells and develop a lymphoproliferative disease involving γδ T cells14. Human tyrosine 132 phosphorylation is required for PLCγ1 association with LAT, and requires the phosphorylation of at least one of the distal three tyrosine residues in order to become phosphorylated12, 16. Mice expressing a tyrosine to phenylalanine point mutation at LAT Y136 show a severe but incomplete block in T cell development early in life, and their T cells have severe signalling defects17, 18. In addition, later in life they develop signs of autoimmune disease and extraneous lymphocyte proliferation slightly later, potentially due to dysfunctional Tregs17–19. The phosphorylation of LAT Y132 is differentially regulated compared to other LAT tyrosines. The phosphorylation of LAT Y132 is slower than LAT Y191 and LAT Y132 requires the presence of at least one other LAT tyrosine for its in vivo phosphorylation12, 20.
THE ROLE OF THE LAT MULTI-PROTEIN COMPLEX IN SIGNALING
The recruitment and activation of PLCγ1 and the Gads/SLP-76 complex
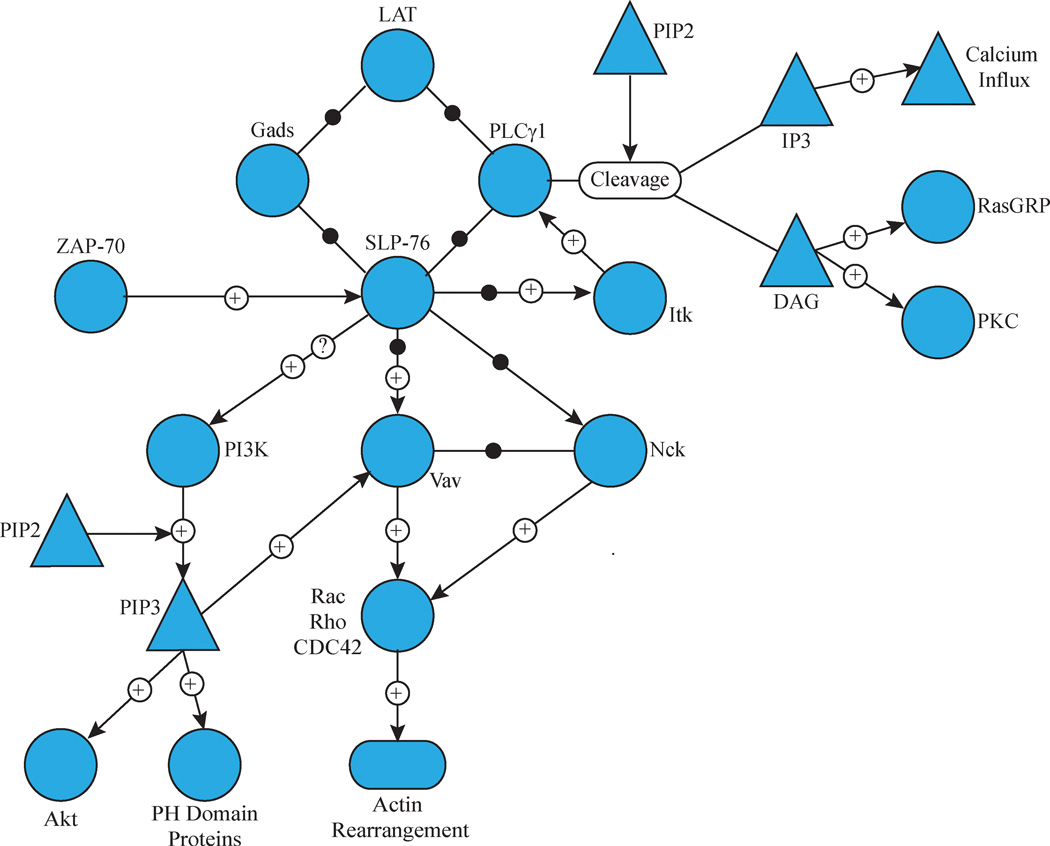
The importance of LAT’s four distal tyrosine residues for recruitment of effector molecules and subsequent T cell function highlights how the structure of the LAT complex is critical in organizing signalling events. The activation of PLCγ1 is an excellent example of how the structure of the LAT complex organizes T cell signalling. PLCγ1 is phosphorylated downstream of TCR stimulation, and this activating event is dependent on both ZAP-70 and the Tec family kinase Itk20, 21. ZAP-70 phosphorylates LAT Y132, which then binds PLCγ1 via its SH2 domain and recruits it to the large LAT-nucleated signalling complex (Figure 2)21. Simultaneously, Gads, a member of the Grb2 family of adaptor proteins, binds to phosphorylated LAT Y171 and Y191 via its central SH2 domain16, 22. All Grb2 family members consist of a central SH2 domain with flanking SH3 domains connected by flexible linkers23. The primary SH3 domain ligand for Gads is SLP-76, which is a 76 KDa adaptor protein that is brought to the LAT complex via its interaction with Gads and PLCγ124. SLP-76 uses its multiple domains to recruit effector molecules to the LAT complex and to stabilize the interaction of PLCγ1 with LAT (Figure 2)24, 25. Despite having no intrinsic enzymatic activity, SLP-76 recruitment to the LAT complex is essential for T cell function: mice with conditional SLP-76 deficiencies have no PLCγ1 phosphorylation or Ca2+ flux, and have defective proliferation26. SLP-76 also contains three tyrosines, tyrosines 113, 128 and 145, that are rapidly and transiently phosphorylated upon TCR stimulation24. SLP-76 knockout mice expressing a tyrosine to phenylalanine mutated version of SLP-76 produce mature T cells in drastically reduced numbers, and Jurkat E6.1 cells with the corresponding mutations are unable to proliferate or signal efficiently 24. Itk binds to one of these phosphorylated tyrosine residues, a step that is required for Itk activation and its subsequent phosphorylation of PLCγ1 (Figure 2)21. SLP-76 is also phosphorylated on tyrosine 173 and this event is needed to facilitate the phosphorylation of PLCγ1 by Itk27. Itk has both kinase and adaptor functions: it is the kinase for PLCγ1, and it facilitates the recruitment of Vav1 to the LAT complex21, 28. Phosphorylated tyrosines on SLP-76 also directly interact with Vav1 and the adaptor protein Nck24. The association of Vav1 and Nck with SLP-76 and each otheris required for normal actin polymerization (Figure 2)29. SLP-76 and LAT are required for the induction of PI3 kinase activity upon TCR stimulation, although how the PI3 kinase regulatory and catalytic subunits interact with LAT and/or SLP-76 is unknown (Figure 2)30. The induction of PI3K increases the production of phosphoinositide 3,4,5 phosphate (PIP3) that induces the recruitment and activation of pleckstrin homology (PH) domain-containing proteins such as Vav1, PLCγ1 and Akt31, 32.
Figure 2. Recruitment of Complexes Containing PLCγ1, Gads and SLP-76 to LAT.
LAT phosphorylation results in the binding of PLCγ1 to LAT Y132 and Gads to LAT Y171 or Y191. Gads also binds to SLP-76, which stabilizes the interaction of PLCγ1 with LAT. PLCγ1 cleaves PIP2 into IP3, which induces calcium influx, and DAG, which activates RasGRP and PKC isoforms. SLP-76 is phosphorylated by ZAP-70 and subsequently binds ITK, which phosphorylates and activates PLCγ1, and Vav and Nck, which are critical for actin polymerization. SLP-76 is also required for the induction of PI3 kinase, resulting in the induction of numerous downstream pathways.
Recruitment and function of Grb2 containing complexes
In addition to PLCγ1 and Gads/SLP-76 complexes, LAT also interacts with Grb2. The adaptor protein Grb2 binds to phosphorylated LAT tyrosines with its central SH2 domain, and the proline rich regions of various effector molecules via its N- and C-terminal SH3 domains (Figure 3)12, 16, 22. For example, SOS1 and SOS2 are large, multi-domain proteins brought to the LAT complex via the constitutive interaction of their proline-rich C-termini with the SH3 domains of Grb233, 34. These Ras guanine nucleotide exchange factors (GEF) are widely expressed and have approximately 70% sequence identity, with the largest area of difference occurring in the C-terminal proline rich regions (Figure 3)34. The current model of Ras signalling in T cell activation involves a digital signal relying on the GEF activity of both SOS1 and the DAG-activated guanine nucleotide exchange factor RasGRP1. Upon initial T cell stimulation, Ras activation is mediated by this Golgi-associated protein’s GEF activity. This leads to the accumulation of GTP-bound Ras at the membrane, where the SOS1 REM domain can bind it and establish a positive feedback loop for Ras activation35. Activated Ras leads to induction of the MAP kinases Erk1/Erk2, which are essential for transmission of the signal from the TCR to the nucleus, and the induction of AP-1 transcription factors controlling T cell growth, differentiation, and development36. SOS1 and SOS2 appear to have unique roles in Ras signalling, as mice lacking SOS1 die before birth, while SOS2 deficient mice have normal growth, development, and reproduction37. In addition, SOS1 –but not SOS2— is required for thymocyte development 38. The precise role of SOS2 in T cells is unclear.
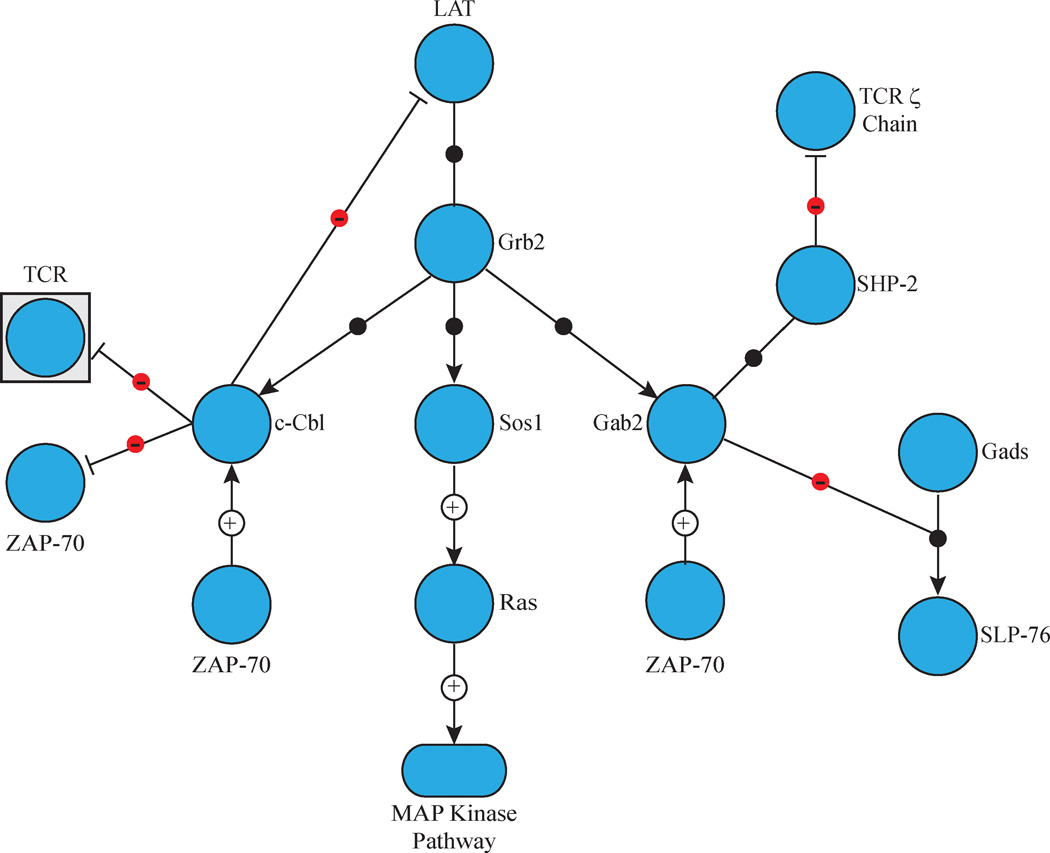
Figure 3. Recruitment of Grb2-Mediated Complexes to LAT.
LAT phosphorylation induces the binding of Grb2 to LAT Y171, Y191 and Y226. Grb2 simultaneously recruits several SH3 domain ligands to LAT. These include Sos1 and Sos2, which activated the MAP kinase pathway, c-Cbl, which facilitates the ubiquitination of multiple signalling proteins, and Gab2, which recruits and activates the phosphatase SHP-2 and inhibits the Gads/SLP-76 complex.
The LAT complex also plays a role in the down-regulation of signalling
The LAT complex also plays a role in the negative regulation of TCR signalling. Mature CD4+ T cells from mice with induced LAT deficiencies show signs of out-of-control lymphocyte proliferation39. This negative regulatory role is possible in several ways. First, LAT can recruit negative regulators to the multi-protein complex, such as the phosphatase SHP-1. Additionally, the E3 ubiquitin ligase c-Cbl and the inhibitory adaptor molecule Gab2 associate with LAT via Grb2 (Figure 3). Similarly to SOS1, the association of Grb2 with c-Cbl or Gab2 occurs via the interaction of the proteins’ proline rich regions with Grb2’s SH3 domains40, 41. c-Cbl mediates the ubiquitination and down regulation of stimulated TCRs, an event that occurs at the site of contact between the APC and activated T cell, and is known to ubiquitinate other proteins involved in the TCR signalling cascade, including LAT, PLCγ1, and Vav1 (Figure 3)40, 42, 43. Gab2 is a highly conserved adaptor protein that is able to bind multiple negative regulators in addition to Grb2 family members44. Following TCR stimulation, Gab2 is recruited to the LAT complex via its association with Grb2, a step required for its phosphorylation41. Phosphorylated Gab2 then binds the protein tyrosine phosphatase SHP-2, resulting in decreased NFAT and NFkB transcription44. Gab2 is also able to bind to Gads and this complex may compete with the Gads/SLP-76 complex for binding to LAT (Figure 3)41. By targeting LAT, this is an effective way for negative regulatory proteins to control an entire signalling node.
THE LAT COMPLEX OLIGOMERIZES INTO MICROCLUSTERS
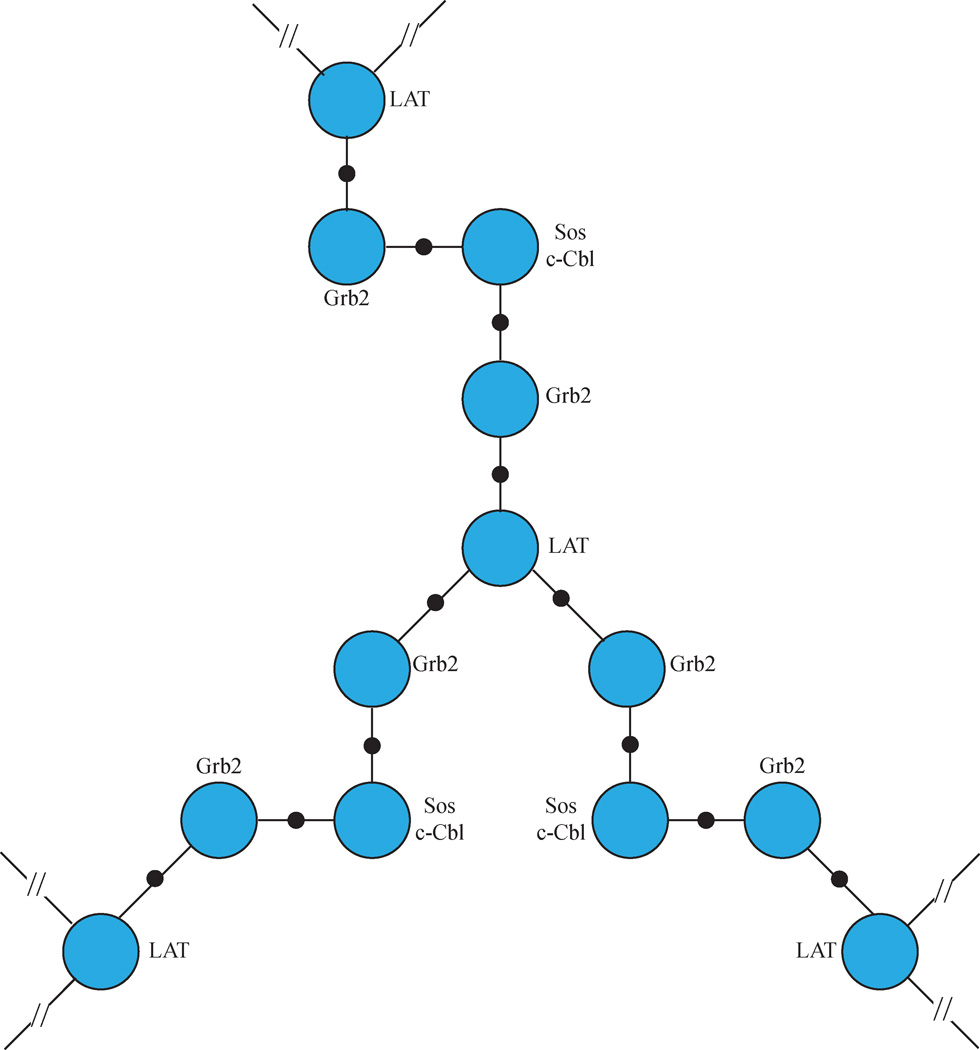
In addition to its role in downstream signalling, Grb2 also has an essential function in the oligomerization of multiple LAT molecules into microclusters. The clustering of important signalling molecules is a phenomenon that is thought to be vital for the regulation of essential signalling events45. Clusters of TCR and costimulatory molecules, such as CD28 and CD2, form quickly following stimulation4, 5, 46, 47. LAT has also been shown to be nucleated into microclusters containing its binding partners following TCR and CD2 stimulation, and these microclusters intersect with the TCR clusters following stimulation by an actin-dependent, lipid raft-independent mechanism5, 46–49. The phosphorylation of LAT at the three distal tyrosine residues following TCR stimulation, and subsequent binding of Grb2, has been shown to be essential for LAT cluster formation (Figure 4)33, 47, 49. This requirement is likely due to the oligomerization of multiple LAT molecules, Grb2, and Grb2 SH3 domain-binding partners (Sidebar 1)33. One LAT molecule is capable of binding up to three Grb2 molecules at a time andtwo Grb2 molecules bind one SOS1 or c-Cbl proline rich domain (Figure 4)33. The ability of LAT and Grb2 to interact with multiple molecules facilitates the formation of LAT signaling clusters 33, 50. Although thought to be vital for signal transduction, the precise role of LAT clusters in T cell signal transduction has eluded investigators for a decade. Houtman and colleagues observed a slight decrease in calcium flux following a low level of TCR stimulation in cells unable to cluster LAT33, but other biological functions for LAT clusters have not been identified. A major experimental focus in the future will be to uncover the role that the oligomerization of LAT plays in its ability to integrate receptor signals and properly activate multiple downstream signalling pathways.
Figure 4. Grb2/SH3 Domain Ligand Complexes Facilitate LAT Clustering.
Phosphorylated LAT is capable of simultaneously binding three Grb2 proteins. At the same time, Grb2 is dimerized by its SH3 domain ligands Sos1 and c-Cbl. The ability to bind multiple Grb2 molecules and the dimerization of Grb2 leads to the formation of a lattice of LAT that drives LAT clustering.
SIGNALING DOWNSTREAM OF LAT CLUSTERS
The phosphorylation of LAT and the subsequent formation of multi-protein signalling complexes lead to the induction of several downstream events. LAT activation leads to the stimulation of proteins, such as SLP-76 and Vav1, that drives changes in the actin cytoskeleton (Figure 2). The actin cytoskeleton is important for many aspects of T cell signalling. TCR, costimulatory, and adhesion receptor stimulation leads to a rapid response that includes morphological changes to the cell. Actin cytoskeletal polymerization is important for T cell interaction with the APC, a process that requires the formation of lamellipodia through the polymerization of filamentous actin58. Additionally, following stimulation by the APC, microclusters of TCRs, co-receptors, or signalling molecules form due to the rearrangement of protein islands in the plasma membrane: this step is dependent on the rearrangement of the cortical actin mesh that supports the plasma membrane1, 59. At the site of T cell-APC contact, actin is also important for the down-regulation of signalling, and moves activated TCR microclusters into an area where they are endocytosed60. Defects in actin polymerization lead to severe immunodeficiency, as seen in Wiskott-Aldrich syndrome (WAS). WAS is a genetic disease where patients lack the actin nucleation protein WASp, and most die by 10 years of age due to infections or autoimmune disease58.
The induction of LAT also controls proteins that drive second messenger production, spreading the signal from the cell surface through the cytoplasm. For example, LAT and SLP-76 are needed for the TCR-induced activation of PI3 kinase, which produces phosphoinositide 3,4,5 phosphate (PIP3). PIP3 interacts with molecules’ PH domains, recruiting signalling molecules to the membrane and keeping signalling pathways primed32. In this way, the phosphoinositide pathway is important for signalling events such as regulating Ca2+ flux, Rac activation, and Erk1/Erk2 phosphorylation (Figure 2)31. Similarly, phosphoinositide 4,5 phosphate (PIP2) is cleaved by PLCγ1 following TCR stimulation to form the second messengers IP3 and DAG (Figure 2)32. DAG is membrane-associated and recruits proteins to the plasma membrane and organelle membranes via their cysteine-rich regions, leading to Ras and NFκB transcription factor activation32. IP3 mediates store-operated calcium entry by binding to the IP3 receptor on the endoplasmic reticulum, which triggers the release of calcium stores into the cytoplasm. This subsequently leads to calcium release-activated calcium channels opening in the plasma membrane and results in a sustained increase in Ca2+ concentration32, 61. Sustained, increased Ca2+ concentration is required for cytokine gene expression, motility, and immunological synapse formation in T cells61.
Finally, signalling from LAT results in changes to gene expression by controlling the activation of important transcription factors such as NFκB, NFAT, and AP-1 (Figure 2 and Figure 3). The nearly ubiquitously expressed transcription factor NFκB regulates the expression of genes related to regulation of the immune response, production of cytokines, proliferation, and induction of apoptosis and cell survival pathways in T cells62. NFκB activation is induced when its bound inhibitors are phosphorylated and degraded in response to cell stimulation or other cell stresses, allowing NFκB to translocate to the nucleus62. Nuclear Factor of Activated T cells (NFAT) is induced by increased levels of calcium in the cell and regulates T cell activation, development, differentiation, and self-tolerance63. NFAT promotes or represses transcription of specific genes based on its binding with other transcription factors63. AP-1 transcription factors are the main partners of NFAT following TCR stimulation-induced Ras activation63. By controlling the transcription of genes for cytokines such as IL-2, AP-1 and its partners control T cell proliferation and differentiation62.
Conclusion
The ultimate effect of signalling cascades induced by the activation of the TCR, costimulatory and adhesion receptors is changes in cell morphology and gene expression, leading to specific outcomes for the T cell. These outcomes are critical for immune system homeostasis, the activation of the adaptive immune response and ultimately the clearance of pathogens. One large knowledge gap in our understanding of the molecular mechanism of T cell activation is where signals emanating from multiple receptors integrate together to alter T cell function. LAT is a key integration node for signalling pathways emanating from multiple receptors. Multiple receptors, including the TCR, CD2 and CD28 can all directly induce the activation of LAT. Similarly, CD5, CD9 or CD28 can further enhance TCR-induced LAT phosphorylation. The induction of LAT phosphorylation leads to the formation of dynamic signalling complexes containing PLCγ1, Grb2, Gads, SLP-76, and their ligands, which then drive downstream signalling to second messenger production, actin polymerization, and transcription factor activation. We have a basic knowledge of which receptors activate LAT, the mechanism of its phosphorylation, and which ligands interact at specific sites on LAT. However, we are just beginning to understand how the composition of specific LAT complexes is temporally and spatially regulated by the TCR and other receptors, and how LAT integrates this information to selectively drive specific downstream pathways. Examining the complexity of how LAT is activated and how LAT-mediated complexes are dynamically regulated will be an active area of research for the coming years.
Sidebar 1: SH3 domain interactions.
Grb2 is a ubiquitously expressed and evolutionarily conserved adaptor protein51. Although extensively studied, the exact mechanism for Grb2 binding to its full length SH3-domain partners is unclear. The interaction between Grb2 and SOS1 has been the subject of the most vigorous research. This has led to this interaction being considered a model SH3 domain-ligand interaction52.
Four possible Grb2 SH3-domain binding sites with the consensus amino acid sequence of PXXPXR have been identified in the proline rich C-terminal tail of SOS152, 53. Numerous studies have examined the relationship between Grb2 and this motif using short 10–15 amino acid long peptides and several different techniques, including isothermal titration calorimetry, surface plasmon resonance, and NMR. These studies indicated that the interaction of the whole Grb2 molecule with short peptides containing one PXXPXR motif has a low affinity, with reported dissociation constants (Kd) ranging from 2 µM to over 200 µM54–56. Interestingly, the interaction of full SOS1 with Grb2 appears be at least 100-fold stronger than these peptide-based studies would indicate, meaning that these studies may not be indicative of what is occurring in the cell33, 57. While informative, the use of short peptides and/or only one of the two Grb2 SH3 domains in these previous studies leaves the physiological relevance of the peptide-SH3 domain interaction ambiguous.
Contributor Information
Rebekah R. Bartelt, University of Iowa Department of Microbiology
Jon C.D. Houtman, Email: jonhoutman@uiowa.edu, University of Iowa Department of Microbiology.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228(1):9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121(6):937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. The Journal of cell biology. 2009;185(3):521–534. doi: 10.1083/jcb.200809136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martelli MP, Lin H, Zhang W, Samelson LE, Bierer BE. Signaling via LAT (linker for T-cell activation) and Syk/ZAP70 is required for ERK activation and NFAT transcriptional activation following CD2 stimulation. Blood. 2000;96(6):2181–2190. [PubMed] [Google Scholar]
- 7.Yashiro-Ohtani Y, Zhou XY, Toyo-Oka K, Tai XG, Park CS, et al. Non-CD28 costimulatory molecules present in T cell rafts induce T cell costimulation by enhancing the association of TCR with rafts. J Immunol. 2000;164(3):1251–1259. doi: 10.4049/jimmunol.164.3.1251. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchida M, Manthei ER, Knechtle SJ, Hamawy MM. CD28 ligation induces rapid tyrosine phosphorylation of the linker molecule LAT in the absence of Syk and ZAP-70 tyrosine phosphorylation. Eur J Immunol. 1999;29(7):2354–2359. doi: 10.1002/(SICI)1521-4141(199907)29:07<2354::AID-IMMU2354>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, et al. Essential role of LAT in T cell development. Immunity. 1999;10(3):323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 11.Ou-Yang CW, Zhu M, Fuller DM, Sullivan SA, Chuck MI, et al. Role of LAT in the granule-mediated cytotoxicity of CD8 T cells. Mol Cell Biol. 2012;32(14):2674–2684. doi: 10.1128/MCB.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol. 2003;170(1):325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 13.Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J Exp Med. 2001;194(2):135–142. doi: 10.1084/jem.194.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nat Immunol. 2003;4(10):999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem. 2001;276(31):29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275(30):23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 17.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296(5575):2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 18.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296(5575):2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 19.Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol. 2010;184(5):2476–2486. doi: 10.4049/jimmunol.0902876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houtman JC, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. J Immunol. 2005;175(4):2449–2458. doi: 10.4049/jimmunol.175.4.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104(16):6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houtman JC, Higashimoto Y, Dimasi N, Cho S, Yamaguchi H, et al. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry. 2004;43(14):4170–4178. doi: 10.1021/bi0357311. [DOI] [PubMed] [Google Scholar]
- 23.Yuzawa S, Yokochi M, Hatanaka H, Ogura K, Kataoka M, et al. Solution structure of Grb2 reveals extensive flexibility necessary for target recognition. J Mol Biol. 2001;306(3):527–537. doi: 10.1006/jmbi.2000.4396. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MS, Koretzky GA. Coordination of receptor signaling in multiple hematopoietic cell lineages by the adaptor protein SLP-76. Cold Spring Harbor perspectives in biology. 2010;2(4):a002501. doi: 10.1101/cshperspect.a002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9(2):67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu GF, Corbo E, Schmidt M, Smith-Garvin JE, Riese MJ, et al. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. Eur J Immunol. 2011;41(7):2064–2073. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sela M, Bogin Y, Beach D, Oellerich T, Lehne J, et al. Sequential phosphorylation of SLP-76 at tyrosine 173 is required for activation of T and mast cells. Embo J. 2011;30(15):3160–3172. doi: 10.1038/emboj.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, et al. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J. Immun. 2005;174:1385. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 29.Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, et al. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. Embo J. 2010;29(14):2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim EK, Jung SH, Lee JR. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J Immunol. 2011;186(5):2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Orcutt N, Houtman JC. PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Mol Immunol. 2009;46(11–12):2274–2283. doi: 10.1016/j.molimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Huang YH, Sauer K. Lipid signaling in T-cell development and function. Cold Spring Harbor perspectives in biology. 2010;2(11):a002428. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nature structural & molecular biology. 2006;13(9):798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 34.Yang SS, Van Aelst L, Bar-Sagi D. Differential interactions of human Sos1 and Sos2 with Grb2. J Biol Chem. 1995;270(31):18212–18215. doi: 10.1074/jbc.270.31.18212. [DOI] [PubMed] [Google Scholar]
- 35.Das J, Ho M, Zikherman J, Govern C, Yang M, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136(2):337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2(1):20–27. [PubMed] [Google Scholar]
- 37.Qian X, Esteban L, Vass WC, Upadhyaya C, Papageorge AG, et al. The Sos1 and Sos2 Ras-specific exchange factors: differences in placental expression and signaling properties. Embo J. 2000;19(4):642–654. doi: 10.1093/emboj/19.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kortum RL, Sommers CL, Pinski JM, Alexander CP, Merrill RK, et al. Deconstructing ras signaling in the thymus. Mol Cell Biol. 2012;32(14):2748–2759. doi: 10.1128/MCB.00317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31(2):197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Balagopalan L, Barr VA, Samelson LE. Endocytic events in TCR signaling: focus on adapters in microclusters. Immunological reviews. 2009;232(1):84–98. doi: 10.1111/j.1600-065X.2009.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamasaki S, Nishida K, Sakuma M, Berry D, McGlade CJ, et al. Gads/Grb2-mediated association with LAT is critical for the inhibitory function of Gab2 in T cells. Mol Cell Biol. 2003;23(7):2515–2529. doi: 10.1128/MCB.23.7.2515-2529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balagopalan L, Ashwell BA, Bernot KM, Akpan IO, Quasba N, et al. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc Natl Acad Sci U S A. 2011;108(7):2885–2890. doi: 10.1073/pnas.1007098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balagopalan L, Barr VA, Sommers CL, Barda-Saad M, Goyal A, et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol. 2007;27(24):8622–8636. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki S, Nishida K, Hibi M, Sakuma M, Shiina R, et al. Docking protein Gab2 is phosphorylated by ZAP-70 and negatively regulates T cell receptor signaling by recruitment of inhibitory molecules. J Biol Chem. 2001;276(48):45175–45183. doi: 10.1074/jbc.M105384200. [DOI] [PubMed] [Google Scholar]
- 45.Groves JT, Kuriyan J. Molecular mechanisms in signal transduction at the membrane. Nature structural & molecular biology. 2010;17(6):659–665. doi: 10.1038/nsmb.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. The Journal of cell biology. 2002;158(7):1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, et al. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 2006;26(19):7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11(1):90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto-Tane A, Yokosuka T, Ishihara C, Sakuma M, Kobayashi W, Saito T. T-cell receptor microclusters critical for T-cell activation are formed independently of lipid raft clustering. Mol Cell Biol. 2010;30(14):3421–3429. doi: 10.1128/MCB.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nag A, Monine M, Perelson AS, Goldstein B. Modeling and simulation of aggregation of membrane protein LAT with molecular variability in the number of binding sites for cytosolic Grb2- SOS1-Grb2. PLoS One. 2012;7(3):e28758. doi: 10.1371/journal.pone.0028758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matuoka K, Shibata M, Yamakawa A, Takenawa T. Cloning of, ASH a ubiquitous protein composed of one Src homology region (SH) 2 and two SH3 domains, from human and rat cDNA libraries. Proc Natl Acad Sci U S A. 1992;89(19):9015–9019. doi: 10.1073/pnas.89.19.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon JA, Schreiber SL. Grb2 SH3 binding to peptides from Sos: evaluation of a general model for SH3-ligand interactions. Chem Biol. 1995;2(1):53–60. doi: 10.1016/1074-5521(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 53.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 54.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, et al. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 55.Lemmon MA, Ladbury JE, Mandiyan V, Zhou M, Schlessinger J. Independent binding of peptide ligands to the SH2 and SH3 domains of Grb2. J Biol Chem. 1994;269(50):31653–31658. [PubMed] [Google Scholar]
- 56.Cussac D, Frech M, Chardin P. Binding of the Grb2 SH2 domain to phosphotyrosine motifs does not change the affinity of its SH3 domains for Sos proline-rich motifs. Embo J. 1994;13(17):4011–4021. doi: 10.1002/j.1460-2075.1994.tb06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chook YM, Gish GD, Kay CM, Pai EF, Pawson T. The Grb2-mSos1 complex binds phosphopeptides with higher affinity than Grb2. J Biol Chem. 1996;271(48):30472–30478. doi: 10.1074/jbc.271.48.30472. [DOI] [PubMed] [Google Scholar]
- 58.Reicher B, Barda-Saad M. Multiple pathways leading from the T-cell antigen receptor to the actin cytoskeleton network. FEBS Lett. 2010;584(24):4858–4864. doi: 10.1016/j.febslet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7(2):131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 60.Beemiller P, Krummel MF. Mediation of T-cell activation by actin meshworks. Cold Spring Harbor perspectives in biology. 2010;2(9):a002444. doi: 10.1101/cshperspect.a002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunological reviews. 2009;231(1):210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 62.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]