Abstract
Toxoplasma and Plasmodium parasites exact a significant toll on public health. Host immunity required for efficient control of infection by these Apicomplexans involves the induction of potent T cell responses, which sometimes results in immunopathological damage. Thus, protective immune responses must be balanced by regulatory networks that limit immunopathology. We review several key cellular and molecular immunoregulatory networks operational during Toxoplasma and Plasmodium infections. Accumulating data show that despite differences in how the immune response controls these parasites, many host immunoregulatory pathways and cellular networks are common to both. Thus, understanding the cellular and molecular circuits that prevent or regulate immunopathological responses against one parasite is likely to inform our understanding of the host response to the other parasite.
Keywords: Plasmodium, Toxoplasma, immunopathology, IL-10, IL-27, TGF-β
Protective immunity and immunopathology after Plasmodium or Toxoplasma infection
Plasmodium and Toxoplasma represent two of the most prevalent and successful parasites. Reasons for this include the complex life cycle of each parasite and a limited understanding of the interplay between the parasites and host immune response. Although these organisms infect different tissues and cause distinct patterns of disease (Boxes 1 and 2), one feature common to both parasites is that some disease manifestations are directly linked to the highly inflammatory nature of the host immune response (Box 3). Moreover, hosts that lack key immunoregulatory molecules, cell types, or pathways cannot control parasite growth and succumb to lethal immunopathology [1–3]. Thus, several manifestations of malaria and toxoplasmosis are likely to be a consequence of the highly inflammatory nature of the innate and T cell mediated immune responses triggered during the acute phases of infection that develop to limit parasite replication.
Box 1. Induction of cell mediated immunity after Plasmodium infection.
Plasmodium infection begins with mosquito deposition of sporozoites in the mammalian dermis. Motile sporozoites enter the circulation, passively transit to the liver, and initiate an asymptomatic period of differentiation in hepatocytes. Plasmodium merozoites are released from hepatocytes and subsequently infect host erythrocytes. The blood stage of Plasmodium infection is responsible for all clinical symptoms of malaria. During this phase, asexual replication of merozoites in erythrocytes stimulates potent, highly inflammatory immune responses [76]. Early activation of host immunity is associated with accumulation of parasite-infected erythrocytes in the spleen. There, innate immune cells including inflammatory monocytes, macrophages, DCs, NK cells, and γδ T cells release several proinflammatory cytokines and pyrogens, including LT-α, TNF-α, IL-1, IFN-γ, and IL-6 (See Figure 1 in main text) [77]. IL-12-mediated induction of highly activated, parasite-specific CD4 T cells expressing IFN-γ (Th1) is also central to protection against blood stage Plasmodium infection [78–81].
Box 2. Induction of cell mediated immunity after Toxoplasma gondii infection.
Human infection with T. gondii results from the ingestion of oocysts from the environment, the ingestion of tissue cysts from infected animals, or through vertical transmission of parasites from infected mothers to their fetus [82]. Once digested, parasites rupture from the cyst, infect intestinal cells where they transform into tachyzoites, and trigger the recruitment of numerous leukocytes including monocytes and DCs [52]. The parasite can also infect phagocytes and use them to initiate their dissemination to a wide variety of tissues including immune-privileged sites such as the brain or retina [83]. In the tissue, the parasite converts from the tachyzoite form to the slowly replicating bradyzoite form that resides within tissue cysts. Bradyzoites periodically reactivate to rapidly replicating tachyzoites, and an immune response must be mounted to control the reactivated infection [82]. Resistance to T. gondii in both the gut and CNS involves innate immune activation coupled with the development of highly polarized T cell responses necessary to limit parasite survival and persistence [84]. Initial recognition of parasites by APCs triggers the expression of chemokines and inflammatory cytokines including IL-12, IL-6, and TNF-α. Recent studies have shown that CD8+ DCs are the critical source of IL-12 during T. gondii infection [85]. IL-12 polarizes CD4 helper cells towards the Th1 lineage [86] and along with other inflammatory cytokines, such as IL-18 and IL-1, can further amplify inflammation by stimulating the release of IFN-γ by NK cells [87,88].
Box 3. Inflammation and immunopathology during toxoplasmosis and malaria.
T. gondii and Plasmodium parasites activate innate phagocytic cells via interactions between parasite-expressed pathogen-associated molecular patterns (PAMPs) and pathogen recognition receptors (PRRs) on monocytes, macrophages, and DCs. Appropriately activated phagocytes respond by secreting proinflammatory cytokines (e.g., IL-12, TNF-α, IL-6, and IL-1), engulfing parasites or parasite-infected host cells and migrating to regional draining lymph nodes or the spleen [88–92]. Local cytokine production also activates other innate immune cells, such as NK cells and γδ T cells [93,94]. In secondary lymphoid tissues, proinflammatory cytokine expression continues, and antigens from the parasites are processed and presented to CD4 and CD8 T cells, which triggers T cell activation and proliferation [81,95,96]. Specific cytokines, notably IL-12, induce activated and proliferating CD4 T cells to differentiate into potent Th1 effector cells that secrete copious amounts of IFN-γ [86,88,97,98]. High levels of IFN-γ potentiate macrophage activation by driving the production of TNF-α, reactive oxygen species (NO−, O2−), and chemokine expression [99,100]. However, sustained high levels of IFN-γ are also associated with acute illness via the further induction and release of TNF-α, IL-6, and other pyrogenic factors responsible for fever, suppression of hematopoiesis, and anemia [27,101]. Th1 cells and proinflammatory cytokines are additionally associated with activation of brain endothelial cells and subsequent recruitment and activation of pathological CD8 T cells in rodent models of ECM [37,56,77,102]. During toxoplasmosis, local proinflammatory cytokine release can trigger apoptosis or necrosis of normal tissue. Systemically, proinflammatory cytokines trigger fever and alter hematopoiesis. Immunopathology is clinically evident during acute toxoplasmosis when parasites infect the gut and in cases of toxoplasmic retinochoroiditis, where unresolved inflammation threatens vision [103–105].
Clinical and experimental study of these two Apicomplexan parasites has provided critical insight into basic cellular and molecular circuits that regulate immunopathogenesis. We highlight the parallel pathways of immunoregulation that are operational after Plasmodium and Toxoplasma gondii infections. We focus on inhibitory receptors, regulatory cytokines, and functionally distinct immune cell subsets (Figure 1). Insight gained from the study of one parasite infection is likely to shed light on mechanisms of immunoregulation during infection by the other, as well as reveal fundamental insight into the biology of immunoregulation. A better understanding of the molecular and cellular factors that regulate immunopathogenesis should aid in the identification of novel opportunities to intervene and improve health outcomes after these or other microbial infections.
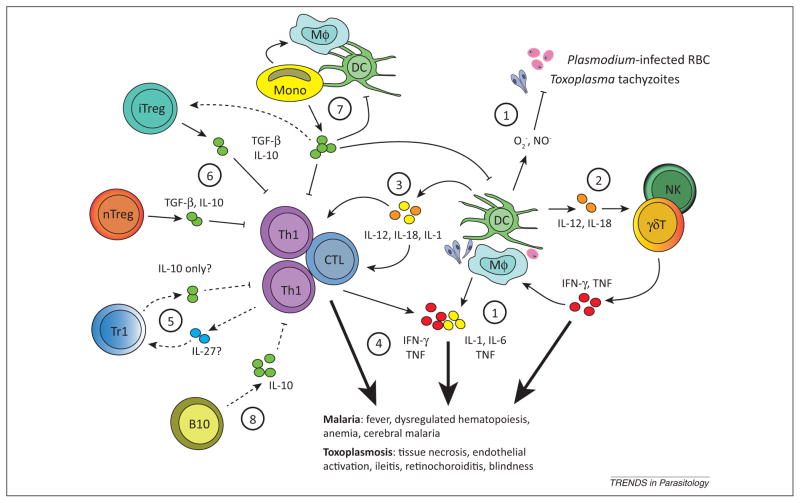
Figure 1.
Common regulatory networks limit Toxoplasma and Plasmodium immunopathogenesis. (1) Recognition of parasites or parasite-infected cells by macrophages (Mφ) and dendritic cells (DCs) triggers the production of antiparasitic reactive oxygen species and synthesis of inflammatory cytokines [88–92]. These inflammatory agents can act directly on parenchymal cells to induce necrotic or apoptotic pathways causing tissue damage, or (2) indirectly through the activation of innate natural killer (NK) or γδ T cells (γδT) [93,94]. (3) Cytokines such as IL-12, IL-1, and IL-18 also play a critical role in shaping the inflammatory nature of adaptive T cell subsets, which include IFN-γ secreting T helper cells (Th1) and cytotoxic cells (CTL) [85,93]. (4) Effector T cells express several inhibitory receptors including programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) that act to limit their activation, proliferation, and cytokine expression, which helps maintain immune homeostasis and limit immunopathology after infection [5,6,11,13]. Generally, and despite inhibitory receptor expression, during acute intestinal or ocular toxoplasmosis and malaria, highly activated T cells appear to directly contribute to pathology [37,46,98,104]. Pathological inflammatory reactions are themselves counterbalanced by several secreted regulatory cytokines and functionally distinct immune cell subsets. Regulatory cytokines such as IL-10 and TGF-β can be secreted by multiple cell types, including B cells, T cells, monocytes (Mono), Mφ, and DCs [2,17,18,24,26,29,51,67]. (5) Polarized IFN-γ-secreting Th1 cells also have the capacity to secrete IL-10 after their differentiation into Treg-like (Tr1) cells. Tr1 differentiation may involve IL-27 [23]. (6) Natural, thymus-derived Tregs (nTregs) suppress the activity of inflammatory CTL and Th1 cells through direct contact or secretion of IL-10 and TGF-β [12,46,106]. The precise role of nTreg, inducible Treg (iTreg), and Tr1 in limiting immunopathology during malaria and toxoplasmosis is not well understood. (7) Monocytes that infiltrate Toxoplasma-infected tissues or the spleen of Plasmodium-infected hosts exhibit dual roles in pathology and protection, by either secreting inflammatory cytokines (e.g., IL-12, TNF-α, IL-1, IL-6) or regulatory cytokines (IL-10 or TGF-β), and possibly by driving the differentiation of Tregs [52–56]. The cues that determine whether monocytes exhibit an inflammatory or regulatory function are unknown. Additional questions include the specific role of IL-27 and the cellular source(s) of this cytokine, as well as (8) the contribution of IL-10-secreting regulatory B cells (B10). Well-described pathways are shown as unbroken lines, and unknown or speculative pathways are shown as broken lines.
Immunoregulation by cell surface inhibitory receptors
After acute microbial infection or vaccination, professional antigen presenting cells (APCs) capture and present antigen to naïve T cells in secondary lymphoid tissues. Appropriately activated T cells undergo clonal expansion and transiently express an array of inhibitory receptors including cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1). Inhibitory receptors counterbalance exuberant T cell activation and are essential for preventing immunopathologies. The ligands for inhibitory receptors and the mechanisms by which these receptors suppress T cell activity vary. For example, CTLA-4 out-competes CD28 (an activating receptor) for binding B7 family member ligands (CD80 or CD86) expressed by APCs. Thus, sequential expression of CD28 followed by CTLA-4 results in a shift from activating to inhibitory signals in effector T cells. PD-1 is a second inhibitory receptor, its ligation by CD80, programmed death ligand 1 (PD-L1), or PD-L2 on APCs, or PD-L1 on non-hematopoietic cells attenuates T cell receptor signaling. Inhibitory receptors are generally downregulated during the transition from effector to memory T cells, whereas sustained expression can lead to their functional impairment or ‘exhaustion’ [4]. Functional T cell exhaustion has been reported during both prolonged Plasmodium [5] and chronic T. gondii [6] infection in mice (reviewed in [7]).
Multiple reports highlight the critical roles for CTLA-4 and PD-1 in preventing immunopathology during acute Plasmodium and Toxoplasma infection. In Plasmodium berghei-infected mice, blockade of CTLA-4 enhances central nervous system (CNS) and liver immunopathology in experimental cerebral malaria (ECM)-susceptible C57BL/6 mice [8], and blockade of CTLA-4 and PD-1 triggers the development of interferon-gamma (IFN-γ; see Glossary) and T cell mediated, ECM-like disease in normally resistant BALB/c mice [9–11]. Furthermore, CTLA-4 expression by regulatory CD4 T cells (Tregs) can also limit effector T cell activation and pathology in ECM-susceptible mice [12]. During experimental ocular toxoplasmosis, intravitreal delivery of Toxoplasma tachyzoites triggered IFN-γ-dependent upregulation of major histocompatibility complex (MHC) class II and PD-L1 on infiltrating hematopoietic and resident retinal cells. Importantly, infiltrating CD4 T cells from Toxoplasma-infected retinas expressed high levels of PD-1, and retinal cells suppressed CD4 T cell activation in a PD-L1-dependent manner [13]. These reports illustrate the important roles that CTLA-4 and PD-1 play in T cell activation and immunopathology in toxoplasmosis and malaria. Future work should address the factors that control the expression of CTLA-4, PD-1, and other inhibitory receptors during parasitic infections. It is also of interest to determine which factors influence the cellular, temporal, or spatial patterns of inhibitory receptor–ligand expression, and ultimately whether this information can be exploited to regulate T cell mediated inflammation.
Immunoregulation by secreted factors
Interleukin-10 (IL-10)
IL-10 is a pleotropic cytokine that acts to suppress the activity of several immune cell types, including APCs, B cells, and T cells (Table 1). Initially characterized as a cytokine expressed by Type 2 T helper cells (Th2), it is now clear that most lymphocyte subsets [natural killer (NK) cells, CD8 T cells, and Th1, Th17, and Tregs] and several myeloid-derived cells [including macrophages, dendritic cells (DCs), and neutrophils] express IL-10 [14]. The finding that IL-10-deficient mice develop spontaneous immunopathology due to T cell responses directed against endogenous gut flora revealed the essential role for IL-10 in maintaining immune homeostasis [15]. Subsequently, IL-10 was shown to prevent immunopathology after infection with several pathogens, including Toxoplasma and Plasmodium [2,3,16–21].
Table 1.
Regulatory cytokines that limit immunopathology during toxoplasmosis and malaria
| Cytokine | Key cellular sources | Cellular targets | Predominant effect in vivo | Refs |
|---|---|---|---|---|
| IL-10 | T cells, macrophages, DCs, B cells | DCs, B cells, macrophages, epithelial cells, endothelial cells | Suppress activation and antigen presentation by DCs, B cells, and macrophages | [2,17,18,24,26,29,51,67] |
| TGF-β | T cells, B cells, NK cells, macrophages, epithelial cells, endothelial cells, platelets, neurons | T cells, DCs, macrophages, B cells | Induction of iTreg and Th17 differentiation, trigger effector T cell apoptosis | [26,32,33,40,63,107–109] |
| IL-27 | Unknown | CD4 T cells, DCs, macrophages | Induction of Tr1 cell differentiation | [23,40,46–49,110] |
The earliest and clearest example of the importance of IL-10 in limiting immunopathogenesis after microbial infection was shown by infection with Toxoplasma in IL-10-deficient mice. Compared with wild type (WT) mice, IL-10-deficient mice had greater Th1 responsiveness, elevated IFN-γ levels, and superior control of Toxoplasma growth. Despite better parasite control, these mice had more severe disease and died from fulminant immunopathology. Importantly, depletion of CD4 T cells rescued Toxoplasma-infected IL-10-deficient mice demonstrating that CD4 T cells are responsible for immunopathological responses [2]. Although an initial report suggested that follicular B cells were an important source of IL-10 in Toxoplasma-infected mice [22], subsequent work demonstrated that IFN-γ-expressing Th1 effector cells were the major source of IL-10 during infection and that IL-10 acted by antagonizing the expression co-stimulatory molecules and IL-12 by APCs [18,19]. This was an important shift in the view of immunoregulation by IL-10, because the long-standing model suggested that effector T cells responsible for immunopathology or parasite clearance did not express regulatory cytokines. Identifying additional cellular sources of IL-10 and determining whether IL-10 expression by Th1 cells is regulated by APCs or other cytokines remain areas of intense investigation.
In mice acutely infected with Plasmodium chabaudi, Th1 effectors also appear to be a critical source of IL-10 that limits immunopathology [20,21]. Studies using specific ablation of IL-10 expression in B cells, myeloid cells, or T cells in combination with adoptive transfer of WT or IL-10-deficient Tregs elegantly showed that highly activated Th1 effectors, but not Tregs, were essential sources of IL-10 that minimized weight loss, hypothermia, and anemia in mice [23]. Notably, the absence of IL-10 did not improve parasite clearance in these animals, suggesting that the effects of IL-10 do not include suppression of protective immunity against P. chabaudi. By contrast, IL-10 in Plasmodium yoelii-infected mice acted to both limit immunopathology and to impede the induction and maintenance of highly activated T cells necessary for efficient parasite clearance [17]. In that report, the majority of IL-10 was produced by an infection-induced population of Foxp3-negative Tregs, termed Tr1 cells, which are distinct from Foxp3-expressing natural and peripherally induced Tregs. Thus, depending on the Plasmodium species and rodent host, both the source of IL-10 and its impact on immunopathogenesis and parasite control can be fairly distinct.
Consistent with data from experimental rodent model studies, data from studies on humans infected with Plasmodium falciparum indicate that IL-10 may have variable roles. For example, significant correlations between increased serum IL-10 levels and reduced risk of developing severe malarial anemia have been established [24], whereas other studies have either failed to identify links [25] or demonstrated that high IL-10 levels correlate with higher parasitemia or worse clinical outcomes [26–28]. Recent data also showed that sustained circulating levels of IL-10 in P. falciparum- or Plasmodium vivax-infected individuals correlated with reduced frequencies of mature DCs. Decreased numbers of DCs were due to DC apoptosis, and in vitro blockade of IL-10 inhibited DC apoptosis [29]. IL-10-mediated DC apoptosis represents a novel pathway of immunoregulation during Plasmodium infection; earlier data from experimental Plasmodium infection suggested that reduced DC survival was associated with direct interactions between DCs and infected erythrocytes [30]. Thus, these new data show that the loss of circulating mature DCs can largely be a consequence of the immune response directed against the parasite, rather than a direct effect of the parasite on the survival or maintenance of mature DCs.
Transforming growth factor-β (TGF-β)
TGF-β is expressed by and acts upon a diverse range of cells and tissues (Table 1). In the immune system, TGF-β primarily acts to suppress effector T cells and promote Treg maintenance and differentiation [31,32]. In this context, TGF-β has proven essential for regulating T cell mediated inflammation and maintaining immune homeostasis and tolerance. Given its critical role in regulating inflammatory Th1 cells and cytotoxic CD8 T cells, it is not surprising that TGF-β is linked to regulating the immunopathogenesis of both Plasmodium and Toxoplasma infections.
TGF-β was initially implicated as a regulator of Plasmodium infection-induced inflammation in studies comparing P. berghei infection in ECM-resistant and -susceptible strains of mice [33]. In these studies, analyses of cytokine profiles revealed that ECM-susceptible mice had markedly reduced expression of TGF-β, suggesting that it might be critical for maintaining the balance between T cell mediated protection and immunopathology. Circulating TGF-β is also a key correlate of improved outcomes during severe malarial anemia and cerebral malaria (CM) in P. falciparum-infected humans [34,35]. Although the precise pathways are unknown, development of CM is associated with proinflammatory cytokine storms that include release of lymphotoxin-α (LT-α), IL-6, and tumor necrosis factor-α (TNF-α), as well as the expansion of cytotoxic CD8 T cells and inflammatory Th1 cells [36–38]. Thus, suppression of effector T cell activation is probably one way that TGF-β regulates and/or reduces the severity of CM. A recent report demonstrated that TGF-β limits effector T cell survival via suppression of the antiapoptotic molecule Bcl-2 [39]. Future studies aimed at determining the precise contribution of TGF-β to regulating T cell mediated immunopathology during malaria could reveal potential avenues for therapeutic intervention during severe Plasmodium infections.
During Toxoplasma infection of the CNS, neuronal cells directly respond to IL-6-driven inflammation by expressing the regulatory cytokines TGF-β and IL-27. When IL-6 signaling was specifically ablated in neurons by deleting a component of the IL-6 receptor (gp130), mice failed to express either TGF-β or IL-27 at the site of infection and died as a result of an encephalitis associated with Th1 and Th17 cell accumulation in the CNS [39,40]. Because gp130 is also a component of a functional IL-27 receptor, the role of IL-27 in this study remains a query. An essential immunoregulatory role for TGF-β has also been described during intestinal Toxoplasma infection. Resident gut intraepithelial CD8 T cells were the major source of TGF-β, the expression of which was essential for reducing Toxoplasma-induced inflammation and mucosal immunopathology, and for maintaining homeostasis in the intestine [41].
These examples highlight the similar and important functions of TGF-β in regulating immunopathogenesis after Plasmodium or Toxoplasma infection. Moreover, these studies reveal that the cellular sources of TGF-β during these infections can be diverse. In addition to understanding how TGF-β expression is regulated, it is important to identify the cellular targets of TGF-β as well as define how the inflammatory environment influences the activity and effects of TGF-β. For example, studies have shown that in the presence of IL-6, TGF-β triggers inflammatory Th17 differentiation instead of suppressing effector T cell activity or promoting Treg differentiation [42]. Thus, TGF-β can function via complicated circuits to integrate environmental cues to both suppress T cell activity and potentiate inflammation.
Interleukin-27
IL-27 is a member of a family of cytokines that includes IL-6 and IL-12, two factors associated with inducing proinflammatory immune responses during infection with either Plasmodium or Toxoplasma. Early studies demonstrated that IL-27 could skew the differentiation of naïveCD4T cells towards a Th1 functional profile via the induction of the key Th1 transcription factor, T-bet [43]. Thus, IL-27 was originally thought to function solely as a proinflammatory cytokine. More recently, IL-27 has been linked to functional suppression of effector CD4 T cells, including Th1, Th2, and Th17 cell populations [44]. The suppression of Th2 and Th17 cell activity is linked to the ability of IL-27 to antagonize IL-2 production [45], a cytokine necessary for proliferation and survival of antigen-specific T cells during an immune response.
The specific mechanisms by which IL-27 suppresses Th1 responses are also becoming clear; in mice, Th1-driven intestinal immunopathology that develops in the absence of IL-27 appears linked to reductions in unique Treg populations [46]. Oral high-dose Toxoplasma infections were associated with expansions of phenotypically and functionally distinct Tregs that also expressed molecules associated with effector Th1 cell trafficking and function, including CXCR3 and T-bet. After adoptive transfer, these T-bet+ CXCR3+ Treg populations appeared to act most efficiently in the gut, and their suppression of Th1-associated immunopathology was functionally linked to their ability to produce IL-10 [46]. Thus, IL-27 can induce highly specialized Tregs that limit Toxoplasma-induced immunopathology in a tissue-specific manner. Several important questions remain regarding IL-27-mediated immunoregulation during intestinal Toxoplasma infection, including the cellular source(s) of this regulatory cytokine and whether Tregs require signals from cytokines other than IL-27 to acquire and exert their suppressive activity.
Plasmodium infection has also been linked to the induction and activity of IL-27. Mice lacking a functional IL-27 receptor were highly sensitive to Plasmodium-induced immunopathology [46,47]. Despite the ability of these mice to efficiently control parasite replication, IL-27 receptor deficiency resulted in dysregulated Th1 responses, systemic inflammation, immunopathology, and disruption of normal liver physiology. The mechanisms underlying the ability of IL-27 to regulate Th1 inflammatory T cells during Plasmodium infection are just now becoming clear. In contrast to its ability to induce specific populations of Tregs in the Toxoplasma-infected gut, IL-27 signaling in effector T cells during blood stage Plasmodium infection appears to suppress inflammatory Th1 cell responsiveness to specific chemokines and cytokines [48,49]. A recent report showed that IL-27 signaling in highly polarized Th1 cells led to marked reductions in the expression of the CCR5 chemokine receptor. The authors contend that dysregulation of CCR5-dependent T cell chemotaxis contributes to the ability of IL-27 to suppress inflammatory Th1 T cell migration to the spleen during Plasmodium infections [49]. Moreover, a subsequent study examining pathogenic Th1 cells induced by P. berghei infection found that IL-27 limited T cell responsiveness to IL-12 [48], which is a critical positive regulator of Th1 cell differentiation and survival as discussed above.
Through a variety of experimental models, these results highlight the importance of IL-27 in regulating Th1-mediated immunopathogenesis during infections with either Toxoplasma or Plasmodium and influencing the outcome of disease. In addition to inducing specific populations of Tregs or limiting responsiveness to cytokines or chemo-kines, IL-27 is also a well-known trigger of IL-10 expression by T cells [50,51]. For example, the generation of IL-10-expressing effector T cells during P. chabaudi infection requires IL-27 [23]. By contrast, a recent report showed that IL-27 is also important for IL-10-independent protection against immunopathology in P. berghei-infected mice [47]. The heterogeneous patterns of expression and responsiveness to IL-27 in vivo underscore the complexity of immunoregulation by this cytokine.
Immunoregulation by myeloid cells
Bone marrow-derived myeloid precursors differentiate into several functionally distinct innate immune cells including neutrophils, eosinophils, basophils, and monocytes. Significant interest in understanding the function and regulation of monocytes has grown from early reports showing that these cells are essential for control of both Toxoplasma and Plasmodium [52–54]. After infection, monocytes traffic from the blood to inflamed tissues where they can further differentiate into phagocytic macrophages and DCs. Thus, monocytes contribute to resistance by clearing pathogens through phagocytosis, releasing proinflammatory cytokines, and providing a pool of APCs to aid in promoting T and B cell responses.
In addition to these protective inflammatory responses, infiltrating monocytes and bone marrow-derived myeloid cells play immunoregulatory roles during both toxoplasmosis and malaria. For instance, intestinal infection by specific strains of Toxoplasma is associated with an influx of inflammatory monocytes that adopt regulatory properties characterized by IL-10 and prostaglandin E2 (PGE2) expression. Unexpectedly, IL-10 and PGE2 were expressed in response to infection with commensal bacteria but not other inflammatory cues such as Toxoplasma lysates, and in the absence of these secreted regulatory factors mice develop neutrophil-dependent intestinal pathology [55]. Thus, monocytes respond to diverse signals to regulate Toxoplasma-induced immunopathology.
Similarly, Plasmodium infections are associated with antimalarial, monocyte-driven inflammatory cascades [54]. These protective activities, which include phagocytosis of infected erythrocytes and secretion of cytokines, have been recently reviewed [56]. By contrast, Plasmodium blood stage infection has been linked to the accumulation of distinct subsets of myeloid cells that exhibit potent regulatory functions. In rodents infected with blood stage Plasmodium parasites, a striking inversion occurs in the ratio of proinflammatory DCs and regulatory DCs that express IL-10 themselves and also induce IL-10 expression in CD4 T cells [57]. Finally, in vitro studies have shown that interactions between circulating monocytes and P. falciparum-infected erythrocytes can trigger Treg differentiation. Notably, the in vitro induction of these Tregs was associated with the capacity of monocytes to secrete IL-10 and TGF-β [58,59]. These latter observations are consistent with data showing that splenic inflammatory monocytes express IL-10 during acute P. chabaudi infection in mice [54]. Further study is required to determine whether such cellular interactions and regulatory pathways occur in P. falciparum-infected individuals. Given the critical role of IL-10 in regulating immunity during Plasmodium infection, these data suggest that infiltrating monocytes and resident myeloid cells can also function within regulatory networks that act to prevent or limit immunopathology.
The precise origins and relationships between parasite-induced myeloid cells exhibiting inflammatory or regulatory functions are not entirely clear, and in each of the examples cited above the regulatory function of monocytes was dependent on local cytokine, chemokine, or tissue microenvironments. Thus, it will be necessary to not only determine developmental relationships between inflammatory and regulatory myeloid cells but to also understand how specific microenvironments shape their differentiation or function. Such information could reveal novel strategies for regulating pathological inflammatory responses during malaria or toxoplasmosis.
Immunoregulation by lymphoid cells
T regulatory cells
Multiple subsets of CD4 T cells exhibiting regulatory function have been described, including Foxp3+ natural Tregs (nTreg) that arise from the thymus, Foxp3+ activation-induced Tregs (iTreg) that develop in the periphery during immune activation, and Foxp3-negative Th1 cells expressing IL-10 (Tr1) that also develop after peripheral activation [60]. In addition to Foxp3 expression, Tregs are also typified by expression of the high-affinity IL-2 receptor, CD25. nTreg and iTreg function via release of soluble factors, such as IL-10 and TGF-β, and via direct cell–cell contact involving inhibitory molecules such as CTLA-4 or the glucocorticoid-induced TNF receptor (TNFR) family related gene (GITR) [60,61], whereas the suppressive function of Tr1 cells appears limited to IL-10 and TGF-β secretion. In addition to their critical role in maintaining immune homeostasis and preventing autoimmunity, Tregs have become an important contributor in controlling immunopathogenesis during microbial infections [62].
The appearance and expansion of Tregs has been described during infection with either Toxoplasma or Plasmodium. For instance, malaria has been linked to a higher ratio of circulating Tregs relative to effector T cells [63]; however, their relative contribution to parasite control and disease pathogenesis is not fully understood and remains controversial. Some studies have shown that Tregs paradoxically correlate with enhanced immune-mediated clearance of parasites and protection against pathological inflammatory responses [64,65]. By contrast, other studies have reported that Treg suppression of protective immunity correlated to higher parasite burdens [65]. Similarly, conflicting results have been observed in rodent models (primarily murine ECM models), in which mechanistic dissection of the role of Tregs is possible (reviewed in [66,67]). Thus, the biological relevance of Treg expansions in humans and other species remains an unanswered question.
Inconsistencies surrounding the role of Tregs in modulating immunity against Toxoplasma are also apparent. Multiple studies reported that the depletion of Tregs using the anti-CD25 monoclonal antibody (clone PC61) in rodents revealed limited roles for these cells during the chronic phases of infection [68]. Conversely, adoptive transfer of FoxP3+ Tregs was shown to potently limit Th1-driven immunopathology in mice acutely infected with Toxoplasma [46]. Yet, DCs and effector Th1 cells were shown to simultaneously impair Treg suppressive function and induce Treg differentiation into Th1-like effector cells that contribute to immunopathogenesis via secretion of IFN-γ [69]. Further adding to this debate, recent data also suggest a harmful role for Tregs via their ability to constrain protective immunity; rapid proliferative expansion of Th1 effector cells after Toxoplasma infection is reportedly linked to a transient decrease in Tregs [70]. Mechanistically, this effect was attributed to Treg deprivation of IL-2, because exogenous IL-2 could restore Treg numbers, which suppressed Th1 effector responses and resulted in loss of parasite control. Thus, a transient reduction in Tregs appears necessary for optimal Th1 effector responses against Toxoplasma [70]. This latter study demonstrates that the numerical expansion and suppressive activity of Tregs, if not restrained, can significantly limit protective immunity after acute infection. Furthermore, the treatment of chronically infected mice with IL-2 complexes leads to increased cyst burdens in the CNS, suggesting that increased numbers of Tregs can limit protection in the CNS [69,70].
Tregs appear to play a role in either limiting immunopathogenesis or controlling antiparasitic immunity during Toxoplasma and Plasmodium infections. Of key importance will be defining how or whether local secretion of regulatory cytokines in specific tissues and microenvironments influences disease outcomes. For example, it is of interest to determine the role of Tregs and the contribution of their suppressive effector molecules in the CNS of Toxoplasma- and Plasmodium-infected hosts. Toxoplasma can breach the gut and disseminate throughout the host, including the eyes and brain. Within these immune-privileged tissues, highly activated T cells and IFN-γ are required to limit parasite replication. Similarly, accumulation of Th1 cells in the microvasculature of the CNS after Plasmodium infection is linked to the development of ECM. Thus, it is essential to tightly regulate these polarized cellular reactions in these critical tissues to prevent immune-mediated pathology. Tregs could act in numerous ways to balance protection and pathology; it is possible that Tregs suppress antigen presentation in Toxoplasma-infected neural tissues, the vasculature of the CNS during CM, or limit the local activity ofeffector T cells. Dissecting these mechanisms in vivo will require the use of powerful technologies, including intravital microscopy, to identify and study cellular interactions that may prove critical for understanding the regulation of immunopathogenesis during Toxoplasma infections. Finally, it is also of interest to define the roles of nTregs and iTregs, and determine whether true parasite-specific iTregs are expanded after Toxoplasma or Plasmodium infection. Given the critical roles for Tregs to both limit immunopathology and constrain protective immunity after infection, modulation of the number, localization, or function of Tregs may hold promise as interventional immune-based strategies for toxoplasmosis or malaria.
B regulatory cells
Another regulatory immune cell subset that has been the focus of attention recently is the B regulatory (B10) cell, so-called because of its propensity to regulate effector CD4 T cell activity through the secretion of IL-10. B10 cells are phenotypically defined as CD19+CD1dhiCD5+. CD1d is a non-classical MHC class I molecule necessary for presentation of lipid antigens, and CD5 has been functionally linked to antagonizing both T cell and B cell receptor signaling [71,72]. Originally defined as being critical regulators of Th1-driven inflammation in autoimmune disease [73], B10 cells are now a focus of attention as important regulators of inflammation during infection. Recent data show that B10 cells numerically expand and modulate immunity during parasitic infections, including babesiosis and schistosomiasis [74,75]. Follicular (B-2) B cell derived IL-10 was reported as a potent inhibitor of host immunity during Toxoplasma infection [22], although the precise ontogeny of B10 cell development is unknown. Even though no formal reports of B10 cell expansion during either Plasmodium or Toxoplasma infection exist, given the critical mutual counterbalance between inflammatory Th1 cells and IL-10-mediated immunoregulation reported for these two Apicomplexan infections, it would not be surprising if B10 cells play a functional role.
Concluding remarks
During Toxoplasma and Plasmodium infections, several overlapping immunoregulatory pathways maintain the balance between health and disease. Indeed, the potent immune responses that serve to limit parasite persistence within the host can also be responsible for local or systemic pathologies associated with these infections. Multiple regulatory factors can independently and coordinately act to limit immunopathology during the acute stages of toxoplasmosis and malaria. The striking regulatory potential of monocytes is now appreciated, and the developmental relationships, similarities, and functional distinctions between naturally occurring and peripherally induced Tregs is growing clearer. Secreted factors, such as IL-10, TGF-β, and IL-27, are known potent regulators of immunity, and data from experimental models and human clinical studies highlight the critical and complex role that these cytokines play in regulating immunity against Toxoplasma and Plasmodium. Finally, several cell surface-expressed inhibitory receptors are critical for limiting T cell mediated immunopathology during ocular toxoplasmosis and ECM.
Although much information exists regarding the ability of these cellular and secreted factors to regulate immunopathology after infection, many questions remain (Box 4). Regulatory cytokines and inhibitory receptor ligands are expressed by and act on a diverse range of cell types, underscoring the complexity of these regulatory circuits. TGF-β can both promote and prevent immunopathology, depending on whether IL-6 and specific T cell subsets are present, and IL-27 has the capacity to either potentiate or inhibit Th1 responses. Whether or how Tregs modulate inflammatory effector T cells in local tissue environments, such as the eye or brain, during toxoplasmosis, remains unknown. Understanding how these cytokines are temporally regulated after infection, identifying the specific cellular sources, and determining whether the anatomy of interactions between inflammatory and regulatory cell subsets determines disease outcomes remain important and unanswered questions.
Box 4. Outstanding questions.
Much information has been learned about these regulatory cells, circuits, and pathways from experimental models, but which features of immunoregulation are operational during clinical malaria or acute ocular or intestinal toxoplasmosis?
What are the key cellular sources for secreted regulatory factors such as IL-27, IL-10, and TGF-β, and do the key cellular sources of regulatory cytokines temporally shift as infection progresses?
How does the tissue microenvironment influence the expression of regulatory cytokines and inhibitory receptor ligands, or the manner in which they exert suppressive effects on target cells?
Are parasite-specific Tregs induced during toxoplasmosis or malaria, and what are the critical signals driving their activation, differentiation, or proliferation?
Do monocytes exhibit specific regulatory functions before differentiating into macrophages or DCs in Toxoplasma-infected tissues or the spleen of Plasmodium-infected hosts, and what are the signals that stimulate the acquisition of suppressive function by infiltrating monocytes?
Given that both IL-6 and TGF-β are coexpressed after infection with either Toxoplasma or Plasmodium, why is there so little evidence for an immunopathological role for Th17 cells during toxoplasmosis or malaria?
Rodent models of Toxoplasma and Plasmodium infection have been extensively used to dissect host–pathogen interactions and pathways of immunoregulation during acute and chronic protozoan infections. The power and utility of these models relate to the numerous Toxoplasma genotypes and rodent-specific species of Plasmodium parasites available for study, as well as the vast number of strains and genotypes of susceptible and resistant laboratory rodents. The use of multiple independent models has revealed important information about immunoregulatory pathways during these infections. Depending on the model system, the contribution of a given immunoregulatory factor can be different. For example, the immunoregulatory biology of IL-27 may be fairly distinct when expressed in the Toxoplasma-infected intestine and the spleen of Plasmodium-infected mice. Although unique experimental systems can reveal seemingly disparate mechanisms of action for these regulatory cytokines and cellular subsets, the mass of accumulating data will ultimately translate into a more comprehensive understanding of the biology of immunoregulation. A more complete understanding of the contribution of regulatory pathways to both limiting immunopathology and impeding potent immunity against Toxoplasma and Plasmodium infections will help shape future treatment and therapy against these and other infections.
Acknowledgments
The authors acknowledge members of their laboratories for their helpful discussions and Dr. Kristina Wasson-Blader for editorial assistance. We also offer apologies to the many investigators whose contributions we were unable to discuss owing to space limitations. Work in the Butler laboratory is supported by grants from the National Institutes of Health (NIH, AI099070) and the American Heart Association (13BGIA17140002). Work in the Blader laboratory is supported by grants from the NIH (AI069986 and EY021259).
Glossary
- Bradyzoites
slow dividing form of Toxoplasma that encysts in tissues of mammalian hosts
- CCR5
cell surface expressed receptor for chemokines CCL4 and CCL5
- Chemokine
secreted factor that mediates chemotaxis of cells expressing appropriate receptors
- CXCR3
cell surface receptor for chemokines CXCL9, CXCL10, and CXCL11
- Cytotoxic T cells
T cell subsets characterized by expression of the CD8 coreceptor, restricted to association with antigen/MHC class I complexes, and critical for protective immunity against intracellular pathogens via induction of apoptosis in pathogen-infected cell targets
- Dendritic cells (DCs)
key antigen presenting phagocytic cells responsible for activating naïve T cells and initiating cellular adaptive immunity
- Effector T cells
recently activated T cells that have acquired the capacity to traffic to sites of infection and exhibit potent antimicrobial activity via cytokine secretion or cytolysis of infected cells
- Foxp3
transcription factor essential for the differentiation and maintenance of natural and subsets of peripherally induced T regulatory cells
- γδ T cells
a small subset of T cells that expresses a T cell receptor composed of γ and δ chains. γδ T cells are found in mucosal tissues and contribute to innate and adaptive responses
- Helper T cells
T cell subsets characterized by expression of the CD4 coreceptor and restricted to association with antigen/MHC class II complexes. These cells are critical for the activation of macrophages, orchestration of antibody-secreting B cell responses, and regulation of immunity via their differentiation into one of several functionally distinct subsets (e.g., Th1, Th2, Th17, Treg, Tr1, etc.)
- Interferon-γ (IFN-γ)
inflammatory cytokine mainly expressed by T cells that promotes parasite clearance through activation of phagocytes and antibody isotype switching in B cells
- Interleukin-2 (IL-2)
an essential mitogen and T cell growth factor
- Interleukin-6 (IL-6)
inflammatory cytokine and pyrogen that promotes IL-17 expression by T cells, B cell activation and differentiation, and the antimicrobial properties of phagocytes
- Interleukin-10 (IL-10)
regulatory cytokine that directly suppresses professional APC activation, which indirectly suppresses the induction of potent T cell responses
- Interleukin-17 (IL-17)
inflammatory cytokine that promotes recruitment, activation, and differentiation of phagocytes and neutrophils
- Interleukin-27 (IL-27)
regulatory cytokine that suppresses the activation and proliferation of IL-17-secreting T cells and promotes the generation of IL-10-secreting T cells
- Macrophages
tissue resident phagocytes essential for control of Plasmodium and Toxoplasma via direct engulfment of parasite or parasite-infected host cells
- Merozoites
non-motile, asexually reproducing form of the Plasmodium parasite responsible for infection and destruction of host red blood cells
- Monocytes
circulating cells of the myeloid lineage that are rapidly recruited to sites of inflammation where they differentiate into macrophages or DCs
- Naturally occurring T regulatory cells (nTregs)
regulatory Foxp3+ CD4 T cell that develops in the thymus, exhibits specificity for self-antigens, and exerts suppressive function through both contact-dependent mechanisms and/or secretion of IL-10 and/or TGF-β
- Peripherally induced T regulatory cells (iTregs)
regulatory Foxp3+ CD4 T cell that develops in the periphery after infection or immune insult, exhibits specificity for either self- or non-self-antigens, and exerts suppressive function through both contact-dependent mechanisms and secretion of IL-10 and/or TGF-β
- Polarized T cell
a T cell that is activated and expresses molecules associated with a particular T helper cell subtype
- Pyrogens
proteins produced by phagocytes that induce fever
- Secondary lymphoid tissue
an essential immune tissue (draining lymph nodes and spleen) that serves as the site of naïve T cell activation and induction of adaptive immune responses
- Sporozoites
form of Plasmodium deposited during mosquito blood meal feeding. Plasmodium sporozoites infect hepatocytes to establish infection in mammalian hosts. Also an infectious form of Toxoplasma excreted by felids while encased in oocysts. Sporozoites are released from ingested oocysts and develop into bradyzoites or tachyzoites
- Tachyzoites
rapidly replicating form of Toxoplasma responsible for direct cell death, tissue pathology, and systemic dissemination in mammalian hosts
- T-bet
transcription factor functionally linked to the differentiation and activity of Th1 cells and inhibition of Th2 and Th17 cell differentiation
- Tr1
peripherally induced T regulatory cells, regulatory population of CD4 T cell that develops in the periphery after infection or immune insult, exhibits specificity for non-self-antigens and exerts suppressive function through secretion of IL-10 and/or TGF-β. Unlike iTregs, Tr1 cells do not express Foxp3
- Transforming growth factor-β (TGF-β)
pleiotropic anti-inflammatory cytokine that suppresses T helper cell activity and promotes the differentiation and maintenance of Tregs
- Tumor necrosis factor-α (TNF-α)
inflammatory cytokine and pyrogen expressed primarily by macrophages but also by T cells and B cells. TNF-α promotes apoptotic cell death and contributes to pathogen control through activation, proliferation, and differentiation of leukocytes
- Type 1 T helper cell (Th1) cells
functionally distinct subset of CD4 T helper cells associated with IFN-γ and TNF-α expression and resistance to intracellular pathogens
- Type 2 T helper (Th2) cells
functionally distinct subset of CD4 T helper cells associated with IL-4, IL-5, and IL-13 expression and the induction of allergic responses
- Type 17 T helper (Th17) cells
functionally distinct subset of CD4 T helper cells associated with IL-17 expression and resistance to bacterial and fungal pathogens
References
- 1.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 2.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 3.Wilson EH, et al. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 5.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadra R, et al. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1–PDL-1 blockade. Proc Natl Acad Sci USA. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gigley JP, et al. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28:377–384. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs T, et al. Murine malaria is exacerbated by CTLA-4 blockade. J Immunol. 2002;169:2323–2329. doi: 10.4049/jimmunol.169.5.2323. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs T, et al. CTLA-4-dependent mechanisms prevent T cell induced-liver pathology during the erythrocyte stage of Plasmodium berghei malaria. Eur J Immunol. 2004;34:972–980. doi: 10.1002/eji.200324477. [DOI] [PubMed] [Google Scholar]
- 10.Lepenies B, et al. CTLA-4 blockade differentially influences the outcome of non-lethal and lethal Plasmodium yoelii infections. Microbes Infect. 2007;9:687–694. doi: 10.1016/j.micinf.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Hafalla JC, et al. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS Pathog. 2012;8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haque A, et al. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathog. 2010;6:e1001221. doi: 10.1371/journal.ppat.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles E, et al. CD4 T-cell suppression by cells from Toxoplasma gondii-infected retinas is mediated by surface protein PD-L1. Infect Immun. 2010;78:3484–3492. doi: 10.1128/IAI.00117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 17.Couper KN, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankovic D, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roers A, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, et al. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor α and exacerbated by anti-transforming growth factor β antibodies. Infect Immun. 2003;71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linke A, et al. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 22.Mun HS, et al. Pathogenicity of Toxoplasma gondii through B-2 cell-mediated downregulation of host defense responses. Microbiol Immunol. 2003;47:533–542. doi: 10.1111/j.1348-0421.2003.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 23.Freitas do Rosario AP, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouma C, et al. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124:515–524. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson JN, et al. Analysis of IL10 haplotypic associations with severe malaria. Genes Immun. 2005;6:462–466. doi: 10.1038/sj.gene.6364227. [DOI] [PubMed] [Google Scholar]
- 26.Prakash D, et al. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006;194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 27.Thuma PE, et al. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis. 2011;203:211–219. doi: 10.1093/infdis/jiq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong’echa JM, et al. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–4680. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinzon-Charry A, et al. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med. 2013;210:1635. doi: 10.1084/jem.20121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundie RJ, et al. Blood-stage Plasmodium berghei infection leads to short-lived parasite-associated antigen presentation by dendritic cells. Eur J Immunol. 2010;40:1674–1681. doi: 10.1002/eji.200939265. [DOI] [PubMed] [Google Scholar]
- 31.Gorelik L, et al. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Kossodo S, Grau GE. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 34.Shikani HJ, et al. Cerebral malaria: we have come a long way. Am J Pathol. 2012;181:1484–1492. doi: 10.1016/j.ajpath.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins DJ, et al. Inverse relationship of plasma prostaglandin E2 and blood mononuclear cell cyclooxygenase-2 with disease severity in children with Plasmodium falciparum malaria. J Infect Dis. 2001;183:113–118. doi: 10.1086/317660. [DOI] [PubMed] [Google Scholar]
- 36.de Souza JB, et al. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137:755–772. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 37.Villegas-Mendez A, et al. IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012;189:968–979. doi: 10.4049/jimmunol.1200688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howland SW, et al. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med. 2013;5:984–999. doi: 10.1002/emmm.201202273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanjabi S, et al. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handel U, et al. Neuronal gp130 expression is crucial to prevent neuronal loss, hyperinflammation, and lethal course of murine Toxoplasma encephalitis. Am J Pathol. 2012;181:163–173. doi: 10.1016/j.ajpath.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Buzoni-Gatel D, et al. Murine ileitis after intracellular parasite infection is controlled by TGF-β-producing intraepithelial lymphocytes. Gastroenterology. 2001;120:914–924. doi: 10.1053/gast.2001.22432a. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Takeda A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 44.Hall AO, et al. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 45.Villarino AV, et al. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 46.Hall AO, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Findlay EG, et al. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol. 2010;185:2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 48.Villegas-Mendez A, et al. IL-27 receptor signalling restricts the formation of pathogenic, terminally differentiated Th1 cells during malaria infection by repressing IL-12 dependent signals. PLoS Pathog. 2013;9:e1003293. doi: 10.1371/journal.ppat.1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gwyer Findlay E, et al. IL-27 receptor signaling regulates CD4+ T cell chemotactic responses during infection. J Immunol. 2013;190:4553–4561. doi: 10.4049/jimmunol.1202916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 51.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 52.Dunay IR, et al. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robben PM, et al. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sponaas AM, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 55.Grainger JR, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chua CL, et al. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 2013;29:26–34. doi: 10.1016/j.pt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Wong KA, Rodriguez A. Plasmodium infection and endotoxic shock induce the expansion of regulatory dendritic cells. J Immunol. 2008;180:716–726. doi: 10.4049/jimmunol.180.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Svensson M, et al. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Scholzen A, et al. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009;5:e1000543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohkura N, et al. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Josefowicz SZ, et al. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowe JH, et al. Foxp3+ regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther M, et al. Upregulation of TGF-β, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Walther M, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minigo G, et al. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholzen A, et al. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. 2010;26:16–25. doi: 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol. 2012;42:549–555. doi: 10.1016/j.ijpara.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Couper KN, et al. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. J Immunol. 2009;182:3985–3994. doi: 10.4049/jimmunol.0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benson A, et al. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J Immunol. 2012;188:800–810. doi: 10.4049/jimmunol.1100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perez-Villar JJ, et al. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gary-Gouy H, et al. CD5-negative regulation of B cell receptor signaling pathways originates from tyrosine residue Y429 outside an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 2002;168:232–239. doi: 10.4049/jimmunol.168.1.232. [DOI] [PubMed] [Google Scholar]
- 73.Kalampokis I, et al. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong YI, et al. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS ONE. 2012;7:e46553. doi: 10.1371/journal.pone.0046553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang H, et al. Development of adult worms and granulomatous pathology are collectively regulated by T- and B-cells in mice infected with Schistosoma japonicum. PLoS ONE. 2013;8:e54432. doi: 10.1371/journal.pone.0054432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riley EM, et al. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 77.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 78.Langhorne J, et al. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–107. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- 79.Meding SJ, et al. Role of γ interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stephens R, et al. Malaria-specific transgenic CD4+ T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–1684. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 81.Spence PJ, Langhorne J. T cell control of malaria pathogenesis. Curr Opin Immunol. 2012;24:444–448. doi: 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Hill DE, et al. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 83.Bierly AL, et al. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol. 2008;181:8485–8491. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki Y, et al. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 85.Mashayekhi M, et al. CD8α+ dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gazzinelli RT, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 87.Hunter CA, et al. Production of γ interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor α. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scanga CA, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 89.Koblansky AA, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 91.Baccarella A, et al. Toll-like receptor 7 mediates early innate immune responses to malaria. Infect Immun. 2013 doi: 10.1128/IAI.00923-13. http://dx.doi.org/10.1128/IAI.00923-13. [DOI] [PMC free article] [PubMed]
- 92.Coban C, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 94.Goldszmid RS, et al. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dzierszinski F, et al. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect Immun. 2007;75:5200–5209. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Del Rio L, et al. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J Immunol. 2004;172:6954–6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- 98.Vossenkamper A, et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol. 2004;34:3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- 99.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan IA, et al. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 101.Lamikanra AA, et al. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 102.Amante FH, et al. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol. 2010;185:3632–3642. doi: 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 103.Liesenfeld O, et al. Association of CD4+ T cell-dependent, interferon-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garweg JG, Candolfi E. Immunopathology in ocular toxoplasmosis: facts and clues. Mem Inst Oswaldo Cruz. 2009;104:211–220. doi: 10.1590/s0074-02762009000200014. [DOI] [PubMed] [Google Scholar]
- 105.Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu JJ, et al. Natural regulatory T cells mediate the development of cerebral malaria by modifying the pro-inflammatory response. Parasitol Int. 2010;59:232–241. doi: 10.1016/j.parint.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Wahl SM. Transforming growth factor β: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Longenecker G, et al. Endocrine expression of the active form of TGF-β1 in the TGF-β1 null mice fails to ameliorate lethal phenotype. Cytokine. 2002;18:43–50. doi: 10.1006/cyto.2002.1025. [DOI] [PubMed] [Google Scholar]
- 109.Nagineni CN, et al. Transforming growth factor-β expression in human retinal pigment epithelial cells is enhanced by Toxoplasma gondii: a possible role in the immunopathogenesis of retinochoroiditis. Clin Exp Immunol. 2002;128:372–378. doi: 10.1046/j.1365-2249.2002.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]