Abstract
The N-methyl-d-aspartate (NMDA) glutamate receptor antagonist ketamine may have rapid, albeit transient, antidepressant properties. This study in patients with treatment-resistant major depression (TRD) aimed to (1) replicate the acute efficacy of single-dose intravenous (i.v.) ketamine; (2) test the efficacy of the glutamate-modulating agent riluzole in preventing post-ketamine relapse ; and (3) examine whether pretreatment with lamotrigine would attenuate ketamine’s psychotomimetic effects and enhance its antidepressant activity. Twenty-six medication-free patients received open-label i.v. ketamine (0.5 mg/kg over 40 min). Two hours prior to infusion, patients were randomized to lamotrigine (300 mg) or placebo. Seventeen patients (65%) met response criterion (≥50% reduction from baseline on the Montgomery–Asberg Depression Rating Scale) 24 h following ketamine. Lamotrigine failed to attenuate the mild, transient side-effects associated with ketamine and did not enhance its antidepressant effects. Fourteen patients (54%) met response criterion 72 h following ketamine and proceeded to participate in a 32-d, randomized, double-blind, placebo-controlled, flexible-dose continuation trial of riluzole (100–200 mg/d). The main outcome measure was time-to-relapse. An interim analysis found no significant differences in time-to-relapse between riluzole and placebo groups [log-rank χ2 = 0.17, d.f. = 1, p = 0.68], with 80% of patients relapsing on riluzole vs. 50% on placebo. The trial was thus stopped for futility. This pilot study showed that a sub-anaesthetic dose of i.v. ketamine is well-tolerated in TRD, and may have rapid and sustained antidepressant properties. Riluzole did not prevent relapse in the first month following ketamine. Further investigation of relapse prevention strategies post-ketamine is necessary.
Keywords: Ketamine, lamotrigine, major depression, riluzole, treatment resistance
Introduction
Safe and effective therapies with rapid onset of action for treatment-resistant major depression (TRD) are needed (Machado-Viera et al. 2008). The glutamatergic system may offer a rational, disease-modifying target for drug development for severe mood disorders (Sanacora et al. 2008). Ketamine is a non-competitive, high-affinity N-methyl-d-aspartate (NMDA) glutamate receptor antagonist used as an anaesthetic and analgesic agent for many years, and investigated as a probe of NMDA receptor function relevant to psychotic, cognitive, and alcohol use disorders (Deakin et al. 2008; Honey et al. 2008; Krystal et al. 2005; Lahti et al. 1995; Petrakis et al. 2004). A growing body of translational research on ketamine has demonstrated antidepressant-like effects in forced swimming, learned helplessness, passive avoidance, and tail suspension tests (Chaturvedi et al. 2001; Garcia et al. 2008; Maeng et al. 2008; Mantovani et al. 2003; Yilmaz et al. 2002). Two published clinical trials in medication-free hospitalized patients with major depressive disorder have demonstrated the efficacy of a single sub-anaesthestic dose of intravenous (i.v.) ketamine (0.5 mg/kg over 40 min) using identical placebo-controlled, double-blind, cross-over designs (Berman et al. 2000; Zarate et al. 2006). In both studies, detectable antidepressant responses occurred within several hours, and ~50% of patients met response criteria 72 h following i.v. ketamine. Several patients sustained their response for more than 1 wk – a finding unexplained by ketamine’s short elimination half-life (2–3 h). Transient psychotomimetic side-effects normalized within 2 h of infusion in all patients (Berman et al. 2000; Zarate et al. 2006).
Continuation therapy following acute remission of depressive symptoms is the standard of practice due to high risk of relapse, particularly for pharmacotherapy-resistant patients (Rush et al. 2006a ; Sackeim et al. 1990, 2001). For example, relapse rates for depressed patients in remission following electroconvulsive therapy (ECT) are extremely high despite vigorous continuation pharmacotherapy (Sackeim et al. 2001) or continuation ECT (Kellner et al. 2006), with the greatest risk of relapse occurring within the first month.
The major objective of the present study was to test a novel pharmacological strategy for relapse prevention after acute ketamine administration with a randomized, double-blind, placebo-controlled continuation trial. Riluzole, a glutamate-modulating agent with neuroprotective properties initially approved for amyotrophic lateral sclerosis (ALS), was chosen. First, we hypothesized that a mechanistic synergy between ketamine and riluzole might confer protection against relapse. While ketamine’s primary site of action is at the phencyclidine site within the ionotropic channel of the NMDA receptor, ketamine also increases presynaptic release of glutamate (Maeng et al. 2008; Moghaddam et al. 1997), resulting in enhanced glutamate throughput via ionotropic α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) receptors. An immediate increase in AMPA-to-NMDA receptor function may be critical to ketamine’s rapid antidepressant activity (Maeng et al. 2008; Maeng & Zarate, 2008). Although not a direct NMDA receptor antagonist, riluzole also has multiple effects on the ionotropic glutamate receptor system, including enhancement of synaptic AMPA receptor expression (Du et al. 2007) and blockade of NMDA receptor activation (Kalia et al. 2008; Pittenger et al. 2008). Ketamine administered to rats at a dose that induces antidepressant-like effects increased levels of brain-derived neurotrophic factor (BDNF) in the hippocampus (Garcia et al. 2008). Chronic riluzole administration has also been associated with enhancement of BDNF and other neurotrophic growth factors (Fumagalli et al. 2006; Katoh-Semba et al. 2002; Mizuta et al. 2001), and was found to increase levels of hippocampal N-acetylaspartate (Mathew et al. 2008a), a neuronal marker linked to BDNF expression (Egan et al. 2003; Stern et al. 2008). Second, three open-label studies have shown that riluzole was potentially effective as monotherapy or adjunctive therapy in TRD or bipolar depression (Sanacora et al. 2007; Zarate et al. 2004, 2005) and was as well-tolerated as in ALS patients (Miller et al. 2007). Third, riluzole has a rapid dosage titration ; the therapeutic effect is achieved with a dose of 100 mg/d on the first day.
Additional aims were to replicate previous reports of the rapid and sustained antidepressant effects of i.v. ketamine, to investigate ketamine’s efficacy in the outpatient setting, and to test a method for optimizing the safe and effective delivery of i.v. ketamine. In a study of healthy volunteers (Anand et al. 2000), lamotrigine (300 mg) given 2 h prior to i.v. ketamine increased the immediate mood-elevating effects of ketamine while attenuating its acute psychotomimetic and cognitive effects (Anand et al. 2000). We employed the same method in the present study.
Method
Study overview
This two-phase study was conducted between December 2006 and July 2008 at the Mount Sinai School of Medicine (MSSM), an academic medical centre. Phase 1 consisted of: (1) 2-wk psychotropic medication washout period (4 wk for fluoxetine); (2) 24-h admission to the General Clinical Research Center (GCRC) for randomized, double-blind pretreatment with a single dose of lamotrigine (300 mg p.o.) or placebo, followed by open-label i.v. ketamine (0.5 mg/kg over 40 min) and serial assessments; and (3) for 24-h responders (see below), 48-h and 72-h post-ketamine outpatient visits. Patients who continued to meet response criteria at 72-h post-ketamine were eligible for phase 2, a 32-d, randomized, double-blind, flexible-dose continuation trial of riluzole (100–200 mg/d) or placebo. The study was approved by the MSSM Institutional Review Board, in accordance with the principles of the Declaration of Helsinki. Patients provided written informed consent prior to participation.
Study participants
Patients (aged 21–70 yr) were either receiving psychiatric care at screening or were previously under the care of a psychiatrist. Diagnoses were made using the Structured Clinical Interview for DSM-IV – Patient Edition (First et al. 2001), performed by an experienced research clinician with an independent interview by a psychiatrist. A diagnosis of major depressive disorder, chronic and/or recurrent, was required, of at least moderate severity, determined by screening and pre-ketamine baseline scores of ≥32 on the Inventory of Depressive Symptomatology – Clinician Rated (IDS-C30 ; Rush et al. 1996). Patients needed to have demonstrated insufficient response to ≥2 adequate antidepressant trials in the current episode, according to Antidepressant Treatment History Form (ATHF) criteria (Sackeim, 2001). Patients were excluded if they had current psychotic symptoms; had lifetime histories of bipolar disorder, schizophrenia, or schizoaffective disorder; had current anorexia or bulimia nervosa; had alcohol or drug abuse within the past 6 months; or had any unstable medical or neurological illness that increased the risks of ketamine administration. Physical examination, vital signs, weight, ECG, standard blood tests (including liver function tests) and urinalysis confirmed absence of unstable medical illnesses. Urine toxicology and hCG tests confirmed absence of recent illicit substances use and pregnancy, respectively. Participants who reported lifetime use of ketamine, PCP, or riluzole were excluded.
Randomization and masking procedures
Patients who met enrolment criteria for phase 1 were randomly allocated to lamotrigine or placebo by a permuted block procedure consisting of blocks of two or four patients. The randomization list was created by a biostatistician with no patient contact. Based on this list, the MSSM pharmacy distributed to the GCRC for each patient three capsules (100 mg lamotrigine each or placebo) identical in size, weight, appearance, and taste. A separate randomization list for allocation to riluzole or placebo was created in a similar fashion for phase 2 participants. All study investigators, staff, and patients were masked to pretreatment (lamotrigine/placebo) and continuation group (riluzole/placebo) assignment.
Study design
Patients arrived at the GCRC at 07:30 hours after an overnight fast. Repeat urine tests (toxicology and, if applicable, pregnancy) were performed upon arrival. All procedures were performed in a private, quiet room with muted lighting. Following baseline ratings, 2 h prior to i.v. ketamine infusion, patients received 300 mg lamotrigine or placebo by mouth. Lamotrigine levels have been demonstrated to peak 1–4 h following oral administration (Hamilton et al. 1993). Repeat ratings were performed 1 h prior to ketamine infusion. An indwelling catheter was then placed in the antecubital vein of the non-dominant arm, and pulse (P), non-invasive blood pressure (BP), digital pulseoximetry, and ECG monitoring were instituted. Nasal cannula oxygen was administered with side-stream capnometry monitoring. Physiological monitoring data were recorded on a standard anaesthesia record beginning 5 min prior to infusion.
Racemic ketamine hydrochloride (Bedford Laboratories, USA) 0.5 mg/kg diluted in normal saline was administered over 40 min by i.v. infusion pump beginning 2 h after lamotrigine/placebo ingestion. An anaesthesiologist was present throughout the infusion. Following a 4-h post-infusion monitoring period, patients were given a meal and remained overnight at the GCRC with regular assessment of clinical status and vital signs by nursing staff.
Patients completed an outcome expectancy rating on a 5-point Likert scale (1 = no improvement, 5 = full recovery) at baseline. Mood ratings were conducted at baseline (pre- and post-lamotrigine/placebo pretreatment), at 40, 120, 180 and 240 min following the start of the i.v. infusion, and at 24-, 48- and 72-h post-ketamine, and included the Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979) and item 1 of the Young Mania Rating Scale (YMRS; Young et al. 1978). Patients completed the Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR16; Rush et al. 2003) at these same time- points. For repeated MADRS and QIDS-SR assessments on the day of infusion, scores for items related to sleep and appetite were carried forward from baseline. Psychotomimetic side-effects were evaluated at regular intervals for 4 h from the start of infusion with the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962) positive symptoms subscale (four items: conceptual disorganization, hallucinations, suspiciousness, and unusual thought content), the Clinician-Administered Dissociative States Scale (CADSS; Bremner et al. 1998), and a visual analog scale (VAS) for subjective ‘high’ (Petrakis et al. 2004). Side-effects throughout the study were recorded with the Systematic Assessment for Treatment Emergent Effects (SAFTEE-SI; NIMH, 1986). Raters were individuals with graduate degrees and extensive training who achieved high inter-rater reliability for the MADRS [intra-class correlation coefficient (ICC) = 0.96] and BPRS-positive symptoms subscale (ICC = 0.97).
Response, defined as ≥50% reduction in MADRS score at 24 h relative to the previous day’s baseline, with minimal reported/apparent sad mood (≤2) on items 1 and 2, was the initial primary efficacy measure, and determined eligibility for continued study participation. Remission was defined as MADRS ≤9. Participants were asked to guess their pretreatment arm assignment at the 24-h assessment. After a physical examination, patients were then discharged home with car service. Non-responders were exited and treated openly by a study psychiatrist. Initial responders were instructed to abstain from taking any psychotropic medications, were reassessed at 48 h at the outpatient clinic, and, if they continued to meet response criterion, reassessed 72 h post-infusion. Relapsers at the 48-h and 72-h visits were exited and treated openly.
For continuation trial eligibility, patients had to demonstrate durable response or remission for 72 h, with a maximum allowable MADRS score of 15 at the 24-, 48- and 72-h assessments. At the 72-h visit, following blood draw for liver function tests and physical examination, patients were randomized to one of two continuation pharmacotherapy groups, receiving either two capsules of riluzole 50 mg each (100 mg/d) or matching pill placebo under double-blind conditions. Concomitant psychotropic medications were proscribed. Twice-weekly study visits were conducted at the outpatient clinic at 3- to 4-d intervals for up to 32 d. Efficacy (MADRS, QIDS-SR16) and safety assessments (vital signs, weight, SAFTEE-SI) were performed at each visit, and medication compliance was monitored by pill count. Clinical ratings during phase 2 were conducted by the same rater as in phase 1. A blinded study psychiatrist adjusted riluzole/placebo dose based on tolerability and Clinical Global Impression – Improvement (CGI-I; Guy, 1976) ratings. Dosing was initiated at 100 mg/d (50 mg b.i.d). At any visit, if CGI-I showed worsening (score >4), dosage was flexibly increased to a maximum of 200 mg/d, in increments of 50 mg/visit.
Time-to-relapse was the main outcome measure, denoted by: (1) MADRS score of ≥20 for two consecutive visits ; (2) minimum absolute increase of MADRS ≥10 points for two consecutive visits relative to phase 2 baseline ; and (3) meeting modified DSM-IV-TR diagnostic criteria for a major depressive episode (Rush et al. 2006b). At relapse visit or at the end of trial, repeat laboratory testing was performed. All participants were offered follow-up care or were referred back to their psychiatrist upon study completion.
Statistical analyses
Clinical and demographic features of randomized groups and comparisons of phase 2 participants with non-participants were performed using t tests for continuous measures and Fisher’s exact tests for dichotomous variables. Phase 1 efficacy data for MADRS and QIDS-SR were assessed by analysis of covariance (ANCOVA), with baseline score as a covariate, and pretreatment group (lamotrigine, placebo) as the independent variable. Time-to-relapse in phase 2 was compared across the riluzole and placebo groups with the Kaplan–Meier method and log-rank test. Safety analyses were considered post-hoc and exploratory. For day-of-infusion psychotomimetic effects, repeated-measures ANCOVA was performed, with pretreatment drug (two levels) as between-subject factor, time as repeated-measures factor, and the 60-min rating as a covariate. Follow-up ANCOVA analyses were conducted at specified time-points of interest. Randomized treatment groups in both study phases were compared in rates of each side-effect assessed by SAFTEE-SI, without formal statistical testing.
Twenty-five patients per continuation pharmacotherapy group were required to provide 80% power (experiment-wise α of 0.05) to detect a significant advantage in relapse time for riluzole vs. placebo, assuming 90% relapse for placebo and 50% relapse for riluzole. We estimated 100 patients would comprise the intent-to-treat sample in phase 1 (50 per pretreatment group), based on previous ketamine response durability data (Zarate et al. 2006). This sample size yielded >80% power to detect a 20% response rate difference between pretreatment groups at 24 h (two-tailed α = 0.05, ICC = 0.40). Size of actual treatment effects was calculated with Cohen’s d. All statistical tests were two-tailed, with significance set at p < 0.05. Results are reported as means ± s.d. unless otherwise noted.
Results
Participant characteristics
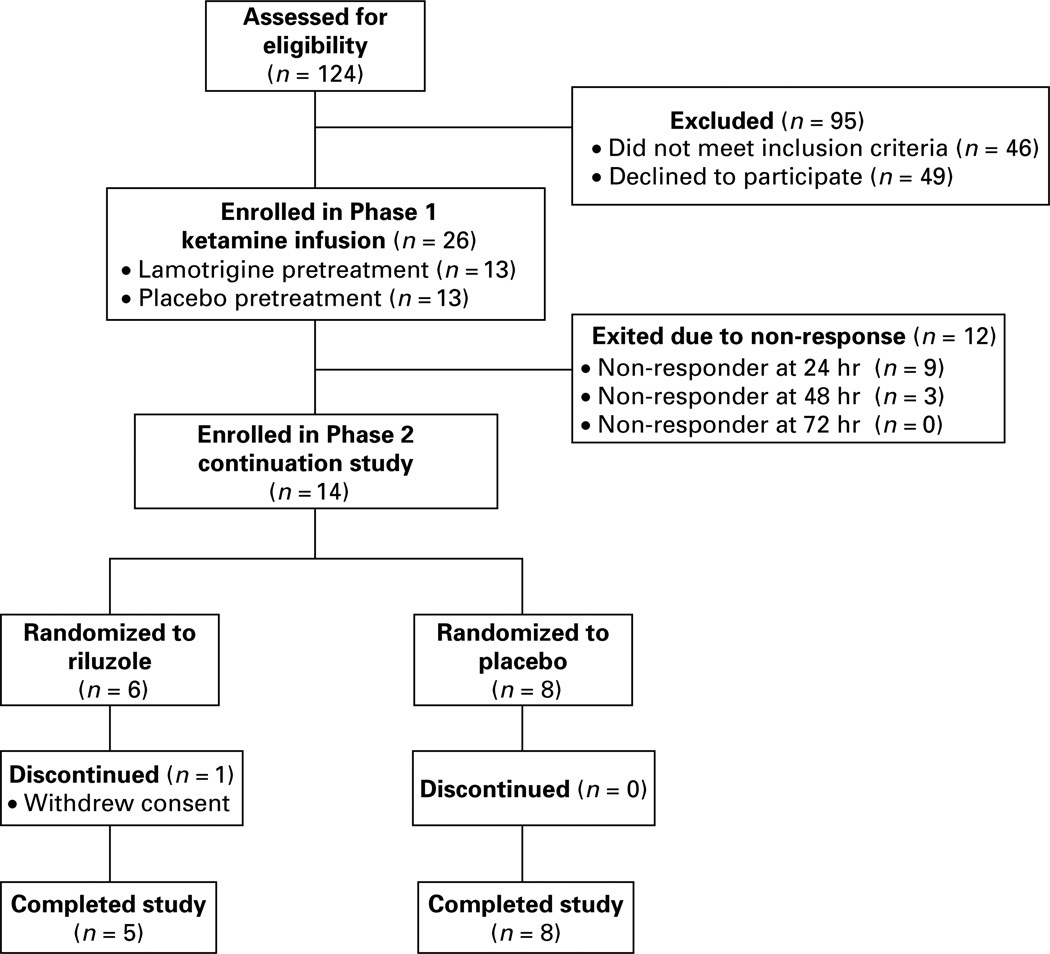
Eligibility assessments were performed for 124 potential participants (Fig. 1). The intent-to-treat sample (n = 26) was recruited from media/internet advertising (n = 14), psychiatrist referral (n = 8), or self-referral from the New York Mood Disorders Support Group (n = 4). Table 1 presents their demographic and clinical characteristics. Patients had marked depressive severity, chronicity, and anxiety comorbidity, and were highly pharmacotherapy-resistant. Four patients had previous treatment with ECT. One patient had received treatment with vagus nerve stimulation (the device was turned off during the study) and had previously participated in a trial of deep brain stimulation. There were no statistically significant baseline differences between lamotrigine and placebo pretreatment groups.
Fig. 1.
Flow diagram of patient enrolment.
Table 1.
Baseline demographic and clinical features (n = 26)
| Feature | |
|---|---|
| Age (yr) | 48.2 ± 11.8 |
| Sex (n, % female) | 10 (39) |
| Ethnicity (n, % minority) | 8 (31) |
| Body mass index (kg/m2) | 29.1 ± 5.9 |
| Received psychiatric disability (n, % yes) | 12 (46) |
| Education (yr) | 15.2 ± 2.7 |
| IQa | 114.9 ± 10.2 |
| Number of adequate antidepressants trials (median, range)b | 6.0 ± 4.1 (5, 2–18) |
| Past lamotrigine exposure (n, % yes) | 11(42) |
| Age at first major depressive episode (yr) | 18.5 ± 12.2 |
| Duration of index episode (yr) | 24.3 ± 16.3 |
| Ultrachronicc (n, %) | 24 (92) |
| Previous episodes (n) | 1.9 ± 1.7 |
| Comorbid anxiety disorder (n, % yes) | 20 (77) |
| Family history of alcohol use disorder (n, % yes) | 10 (39) |
| Past alcohol use disorder (n, % yes) | 6 (23) |
| Past substance use disorder (n, % yes) | 5 (19) |
| Family history of major depression (n, % yes) | 13 (50) |
| Baseline IDS-C30 | 47.0 ± 7.7 |
| Baseline MADRS | 36.9 ± 5.4 |
| Baseline QIDS-SR16 | 18.6 ± 3.9 |
| Baseline Clinical Global Impression – Severity | 5.3 ± 0.8 |
| Expectancy rating patient outcome (1–5) | 2.9 ± 1.0 |
IDS-C30, Inventory of Depressive Symptoms-Clinician Rated; MADRS, Montgomery–Asberg Depression Rating Scale ; QIDS-SR16, Quick Inventory of Depressive Symptomatology-Self-Report.
Values are mean ± s.d. unless otherwise indicated.
Weschler Abbreviated Scale of Intelligence (WASI).
Antidepressant Treatment History Form, minimum score of 3 (threshold for classification as medication resistant).
index major depressive episode ≥42 months (Gilmer et al. 2008).
Phase 1 efficacy outcomes
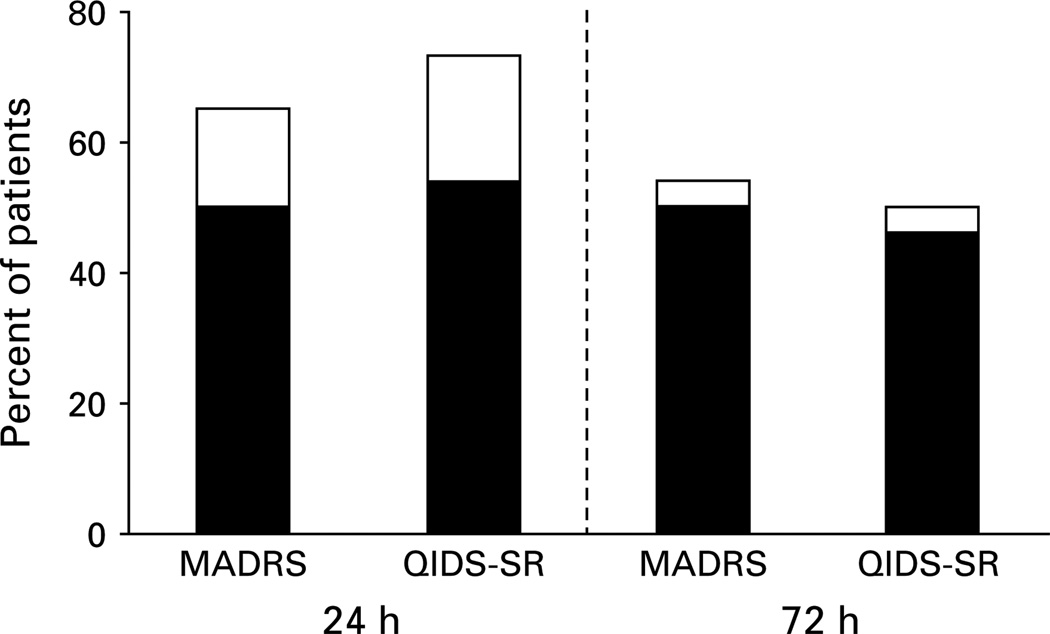
At the initial efficacy endpoint of 24 h, the overall reduction in MADRS score was 22.1 ± 13.0 (t = −8.63, d.f. = 25, p < 0.001), with an average improvement of 60 ± 32%, representing a very large effect size (d = 2.11, 95% CI 1.25–2.97). At the 24-h rating, 17/26 patients (65%) met MADRS response criteria and 13 patients (50%) met remission criteria (Fig. 2). At the 48-h rating, 14 patients (54%) maintained their response and 13 patients (50%) met remission criteria ; the same response and remission rates were observed at 72-h. Very large reductions in QIDS-SR16 scores were also found at 24 h (11.4 ± 6.2, t = 9.30, d.f. = 25, p < 0.001, d = 2.22, 95% CI 1.42–2.23), an average improvement of 62% ± 28. Response and remission rates at 24-h and 72-h assessed by QIDS-SR16 were consistent with MADRS (Fig. 2).
Fig. 2.
Phase 1 response (□) and remission (■) rates. Response and remission rates 24-h and 72-h following a single infusion of intravenous ketamine (0.5 mg/kg over 40 min) in treatment-resistant major depression (n = 26), determined by MADRS and QIDS-SR16.
Onset of response generally occurred in the initial 4 h (240 min) following i.v. ketamine. Twelve patients (46%) met MADRS response criteria by 120 min, and 16 patients (62%) met response criteria at 240 min. Response at 240 min strongly predicted 24-h outcome: two responders at 240 min failed to maintain response at 24 h, while three non-responders at 240 min subsequently met response criteria at 24 h. Exploratory analyses of individual MADRS items at 24 h revealed that all 10 items improved from baseline, with lassitude showing the largest effect size (d = 1.98, 95% CI 1.25–2.71).
Lamotrigine and placebo pretreatment groups did not differ in any efficacy measure at the 24-, 48-, or 72-h rating (smallest p = 0.38). We did not detect differences in MADRS scores between groups 240-min post-ketamine (lamotrigine 16.3 ± 12.9 vs. placebo 20.7 ± 13.2; F = 0.84, d.f. = 1, 23, p = 0.36).
Phase 1 psychotomimetic side-effects
No patients reported frank positive psychotic symptoms or symptoms consistent with formal thought disorder. Twenty-three of 26 (89%) patients had negligible changes in BPRS positive symptom subscale scores over the 240-min post-ketamine period, evidenced by decreased (n = 6), no change (n = 13), or a 1-point peak increase (n = 4) in baseline scores (three patients had peak increases ranging from 3–5 points]. There were no differences between lamotrigine and placebo groups in peak change on BPRS positive symptoms subscale (F = 0.20, d.f. = 1, 23, p = 0.66). CADSS scores were clustered into low (0–2), medium (3–10), high (11–25), or very high (≥26), restricting analyses to the 40-min post-ketamine time-point (Petrakis et al. 2004). Lamotrigine and placebo groups did not differ in mean CADSS scores at 40 min (F = 0.63, d.f. = 1, 24, p = 0.80), and did not differ in the proportion of patients with high (11–25) or very high (≥26) CADSS scores [placebo 4/13 (31%) vs. lamotrigine 3/13 (23%); Fisher’s exact test p > 0.99]. The lamotrigine group reported significantly elevated ‘high’ on the VAS only at 40 min; the placebo group did not (lamotrigine 5.15 ± 3.95, placebo 2.00 ± 2.79; p = 0.02, 95% CI 0.71–5.92). For YMRS item 1, there was a significant main effect of time (F = 3.31, d.f. = 4, 20, p = 0.03), with no main effect of pretreatment group (F = 2.53, d.f. = 1, 23, p = 0.13), or group × time effects (F = 0.62, d.f. = 4, 20, p = 0.66).
Phase 2 continuation trial of riluzole
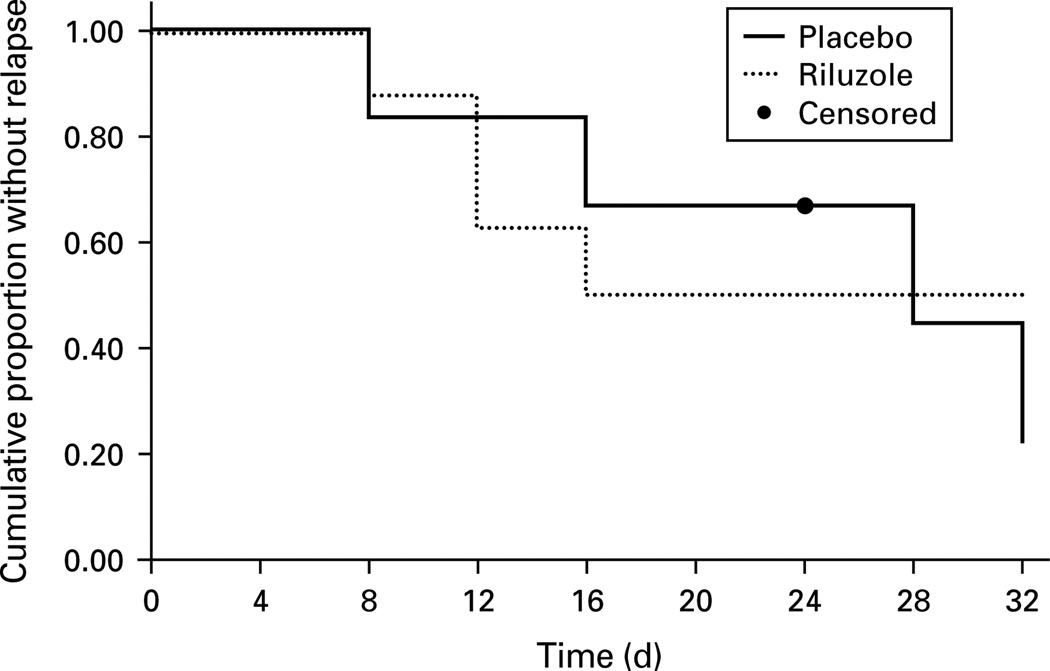
Continuation trial participants (n = 14) did not differ from non-participants (n = 12) in any clinical or demographic variable in Table 1, except for years of education (14.0 ± 2.4 yr vs. 16.5 ± 2.5 yr ; t = 2.6, d.f. = 24, p = 0.02). Riluzole and placebo groups were comparable in age, sex, treatment resistance, pretreatment randomization, phase 1 baseline MADRS score, phase 1 response/remission rates, and phase 2 baseline MADRS scores (Supplementary Table S1, online). One patient dropped out before completing 32 d without meeting relapse criteria. This patient withdrew consent after the 24-d visit for personal reasons (was randomized to riluzole and met remission criteria at exit). The probability of relapse was evaluated using survival analysis (Fig. 3). Eight of 13 (62%) study completers relapsed. There were no continuation group differences in time-to-relapse (log-rank χ2 = 0.17, d.f. = 1, p = 0.68). Mean time-to-relapse for the riluzole group was 24.4 d (95% CI 15.9–33.0), while mean time-to-relapse for the placebo group was 22.0 d (95% CI 14.9–29.1). Among trial completers, 4/5 (80%) relapsed on riluzole vs. 4/8 (50%) on placebo. Relapsers did not differ from non-relapsers in any characteristic in Table 1. One of six (17%) patients in the riluzole group met MADRS response criteria at day 32 compared to 4/8 (50%) patients in the placebo group. MADRS scores at study exit (LOCF) did not differ between groups (riluzole 20.2 ± 15.8, placebo 16.5 ± 11.1; t = −0.51, d.f. = 12, p = 0.62). Medication dosage at study exit did not differ significantly between groups (riluzole 116.7 ± 25.8 mg, placebo 137.5 ± 23.1 mg; t = 1.59, d.f. = 12, p = 0.14).
Fig. 3.
Kaplan–Meier survival plot. Cumulative proportion of patients remaining relapse-free during continuation trial (phase 2). Log-rank test compared time-to-relapse for riluzole and placebo groups (, p = 0.68.).
Correlates of outcome
Exploratory analyses revealed that 24-h responders were significantly older (52.4 ± 10.9 yr) than non-responders (40.3 ± 9.6 yr) (t = −2.79, d.f. = 24, p = 0.01), and that age was positively correlated with percentage improvement in 24-h MADRS (r = 0.38, p = 0.03). Phase 2 participants were older (51.4 ± 11.5 yr) than non-participants (44.6 ± 11.6 yr), although not significantly so (p > 0.05). No other demographic or clinical variable was associated with phase 1 outcome or relapse in phase 2, including expectancy ratings and psychotomimetic side-effects.
Adverse events
There were no serious adverse events, and no treatment-emergent mania or suicidality. As expected, transient increases in mean BP and P were observed during the ketamine infusion (peak increase in systolic BP 19.8 ± 10.1 mmHg, diastolic BP 13.4 ± 7.7 mmHg; P 10.9 ± 11.9 bpm), generally returning to baseline levels by 40–80 min. There were no clinically meaningful changes in respiratory rate, arterial oxygen saturation, or ECG. The most commonly reported side-effects during ketamine infusion and up to 240-min post-infusion were blurred vision, diminished mental/sharpness, dizziness/faintness, drowsiness/sleepiness, feeling strange/unreal, headache, numbness/tingling, ringing in the ears/trouble hearing, and slurred speech. In phase 2, there were no differences in the emergence of moderate-to-severe adverse events between riluzole and placebo groups (Supplementary Table S2, online), and no meaningful changes from baseline in BP, P, weight, or liver function tests.
Integrity of masking
Of patients guessing pretreatment group assignment in phase 1 (n = 23), 14 (60%) guessed correctly, suggesting adequate masking (Fisher’s exact test, p > 0.99). In phase 2, 5/12 patients (42%) correctly guessed their treatment assignment (Fisher’s exact test, p > 0.99), while the rater correctly guessed assignment for 8/12 patients (67%, Fisher’s exact test, p = 0.68.)
Discussion
The main findings of this study were the following: (1) 65% of TRD patients receiving open-label i.v. ketamine at a sub-anaesthetic dose of 0.5 mg/kg met 24-h response criteria, and 54% showed sustained benefit for an additional 48 h in the outpatient setting, corroborating previous reports (Berman et al. 2000; Zarate et al. 2006). (2) Riluzole (100–200 mg/d) did not protect against post-ketamine relapse in the first month, prompting early trial termination. (3) Ketamine, administered with or without lamotrigine pretreatment, was well tolerated. Dissociative symptoms were transient and generally mild or moderate in severity.
Open-label trial of ketamine
The time-course of antidepressant onset following a single 40-min infusion of ketamine suggests a rapid and potentially durable benefit in a majority of patients. In studies to date in major depression, mood benefit was detected as early as 2–4 h post-infusion, after the resolution of acute psychotomimetic side-effects (Berman et al. 2000; Zarate et al. 2006). In the present study, the majority of patients continued to meet response criteria 24–72 h following infusion, a time period extending well beyond ketamine’s elimination half-life. The time-course of antidepressant onset has suggested a ‘rebound hypothesis’, in which neural compensatory effects are enacted following acute NMDA receptor blockade (Javitt, 2004). Non-NMDA receptor targets such as the AMPA glutamate receptor may be critical to ketamine’s rapid onset of action (Maeng et al. 2008; Mathew et al. 2008b). Compounds with AMPA receptor-potentiating properties have shown antidepressant-like effects in rodents (Black, 2005; Knapp et al. 2002; Li et al. 2001; Nakamura & Tanaka, 2001) and AMPA receptor subunit knockout mice demonstrated features of major depressive disorder (Chourbaji et al. 2008).
A recent pharmaco-magnetic resonance imaging study in healthy male volunteers found that i.v. ketamine (administered by bolus and maintenance infusion of 0.25 mg/kg.h) induced a rapid deactivation of ventromedial prefrontal cortex (including orbitofrontal cortex and subgenual cingulate), and increased activation in dorsolateral prefrontal, middle, and posterior cingulate and temporal cortical regions (Deakin et al. 2008). Lamotrigine pretreatment prevented many of the regional blood oxygenation level-dependent (BOLD) signal changes, and consistent with Anand et al. (2000), attenuated dissociative symptoms. The acute pattern of ventromedial deactivation and dorsolateral and posterior cingulate activation is similar to the topographic normalization of brain function following chronic deep brain stimulation in refractory depression (Mayberg et al. 2005). Pharmaco-MRI studies are necessary to further develop neural hypotheses of i.v. ketamine’s mechanism of action in TRD.
Riluzole continuation trial
We predicted that riluzole, because of its pharmacological similarities to ketamine, would confer protection against relapse during the 32-d continuation trial. Studies in chronic pain disorders have found that the acute analgesic response to a single low-dose i.v. ketamine infusion (0.1 mg/kg) strongly correlated with the subsequent antinociceptive response to oral dextromethorphan, a low-affinity, non-competitive NMDA receptor antagonist (Cohen et al. 2004, 2006). In our TRD sample, the inability of riluzole to maintain the acute antidepressant response to i.v. ketamine could be explained by numerous factors, including differences in mechanism, route of administration, and therapeutic dose. Dose–response studies are required to identify the optimal dose of riluzole for the acute treatment of depressive symptoms and for the prevention of relapse.
The durability of the placebo group’s response suggests that benefit following i.v. ketamine administration may persist for >4 wk in some patients. The durability of response was especially striking given the magnitude of pharmacotherapy resistance in our sample, and the fact that the entire study was conducted on an outpatient basis, except for the initial 24-h period. In the Zarate et al. (2006) in-patient study, six patients (35%) maintained their response for >1 wk, and two patients maintained response for >2 wk. Our continuation trial results are largely consistent with a pooled analysis that showed persistence of placebo response in patients who had an acute response to antidepressant pharmacotherapy and were subsequently switched to placebo (Geddes et al. 2003). Given the unblinded nature of i.v. ketamine administration in phase 1, the confounding impact of initial placebo response on phase 2 outcome must be considered. A recent report, for example, showed that initial placebo responders in double-blind, long-term efficacy studies have marked persistence of placebo response if continued on placebo for an additional 12 wk or longer (Khan et al. 2008).
Impact of lamotrigine pretreatment
Contrary to our expectations based on a report in healthy volunteers (Anand et al. 2000), we did not observe attenuated psychotomimetic side-effects in patients randomized to lamotrigine prior to receiving i.v. ketamine, nor did we find a superior antidepressant response in patients randomized to lamotrigine pretreatment. A key difference between our study and the Anand et al. (2000) study was that the latter used a bolus-constant infusion paradigm with a higher ketamine dose that produced steady peak ketamine plasma levels across the testing period, while we used a slow ketamine infusion protocol which probably resulted in briefer exposure to peak ketamine plasma levels (Krystal et al. 1998). Differences in subject characteristics (healthy vs. TRD), and uncertainty that the loading dose of lamotrigine (300 mg) would produce the desired pharmacodynamic effect may also explain contradictions with previous reports (Anand et al. 2000; Deakin et al. 2008). In addition, processes associated with mood elevation in healthy volunteers, detected by the YMRS (item 1), probably differ from reductions in depressive symptoms captured by depression rating scales in TRD.
The study has several important strengths. (1) It represents the largest and most medication-resistant sample of depressed patients treated with i.v. ketamine to date. (2) To our knowledge, it is the first study to test response durability and safety on an outpatient basis. (3) It tested a novel pharmacological strategy (lamotrigine pretreatment) to optimize ketamine’s delivery in TRD. (4) The continuation trial, while brief in duration, marks the first placebo-controlled investigation of riluzole in mood disorders.
Study limitations
Several methodological limitations are noted. First, the open-label administration of i.v. ketamine limits definitive interpretation of phase 1 results. We opted not to use i.v. saline as a comparator agent because of limitations in preserving masking due to the negligible side-effects and lack of efficacy observed in this condition (Berman et al. 2000; Zarate et al. 2006). Future investigations of i.v. ketamine in TRD should consider use of an active control comparator arm (e.g. i.v. midazolam, amphetamine, etc.). Second, the sample size was probably too small to detect moderators and mediators of response, although the preliminary suggestion of an age association with acute response, with a positive relationship found between age and 24-h response, is intriguing. We did not observe a significant relationship between treatment resistance and acute responsiveness to ketamine, consistent with a recent report exploring moderators of acute ECT outcome (Rasmussen et al. 2007). Other factors requiring further study in larger samples include class of previous psychotropic medication and family or personal history of alcohol dependence (Petrakis et al. 2004).
Third, regarding the continuation trial, we believe the marked treatment resistance of the sample justified the use of riluzole, despite the lack of placebo-controlled efficacy data supporting its use in TRD. While continuation therapy with i.v. ketamine merits investigation, increased subject burden compared to oral continuation therapy may limit its application to the most refractory patients. We also considered continuation pharmacotherapy with other glutamatergic agents (e.g. lamotrigine, memantine) but these options are limited by their slow titration schedules. However, a recent case report of a patient with severe depression treated with i.v. ketamine (0.5 mg/kg) found that subsequent adjunctive treatment with memantine (dosed at 15 mg/d) prevented relapse for 6 months (Kollmar et al. 2008), suggesting that this strategy should be further explored.
Regarding lamotrigine pretreatment, a minimum sample size of 40 per pretreatment group would have been necessary to provide 80% power to detect a difference of 20% in efficacy at 24 h. Thus, the negative findings for lamotrigine pretreatment of ketamine may possibly be attributed to Type II error. We also did not obtain blood levels of lamotrigine. This limitation is somewhat mitigated by patients being psychotropic medication-free for at least 2 wk prior to infusion; however, pharmacokinetic differences in drug metabolism could be relevant. Our study design did not allow us to compare the lamotrigine/ketamine combination treatment relative to lamotrigine alone; a 2 × 2 fully factorial design using an i.v. control group would be required. Moreover, a single dose of lamotrigine may have different effects than chronic administration and may have been insufficient to test the utility of this approach.
Finally, there are only limited data regarding the validity of depression rating scales used more frequently than weekly. It is possible that the acute antidepressant response to i.v. ketamine was underestimated due to carrying forward the sleep and appetite items of the MADRS on the day of infusion. However, given the open-label nature of the ketamine administration, we wished to present the most conservative estimate of the acute treatment effect. Further, the primary outcome for the initial phase of the trial was the 24-h MADRS score, which included all 10 MADRS items. Instruments such as the QIDS-SRD14 (modified for daily use) might offer greater sensitivity to detect antidepressant effects occurring during time-periods <1 wk than the MADRS (Lenderking et al. 2008). It is noteworthy that in our sample the QIDS-SR16 revealed a greater 24-h response rate than did the MADRS.
Conclusions
These pilot data show that i.v. ketamine is well tolerated in TRD, and may have rapid antidepressant properties. Lamotrigine pretreatment did not influence the acute efficacy or tolerability of i.v. ketamine. Given the inevitable relapse of depressive symptoms, further controlled investigations of interventions that maintain the initial response, as well as active-control comparator trials of i.v. ketamine, are required.
Supplementary Material
Acknowledgements
Supported by NIMH grant K23-MH-069656; grant number MO1-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health; and NARSAD, Young Investigator Award. The authors thank the clinical and administrative staff of the Mount Sinai General Clinical Research Center, and Gizely N. Andrade, B.A., Neil Chadha, M.D., John Doucette, Ph.D., Amir Garakani, M.D., Heidi L. Fitterling, M.P.H, Michele Gonen, B.S., Chris A. Kelly, B.A., Andrew M. Perez, M.D., Rebecca B. Price, M.S., Harold A. Sackeim, Ph.D., Eric L.P. Smith, Ph.D., Mara Pohl, M.A. and Yanping Wang, Ph.D. for their contributions to this work. The authors gratefully acknowledge the following persons who served on the study’s External Advisory Committee: James Kocsis, M.D., Gerard Sanacora, M.D., Ph.D. and Jonathan Stewart, M.D. [ClinicalTrials.gov identifier NCT00419003.]
Statement of Interest
Dr Mathew and Dr Charney have been named as inventors on a use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr Charney and the Mount Sinai School of Medicine could benefit financially. Dr Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine were approved for this use. Over the past 12 months, Dr Mathew has received compensation from AstraZeneca and Jazz Pharmaceuticals, and has received research support from Alexza Pharmaceuticals, GlaxoSmithKline, and Novartis. Dr Charney has received consulting fees from Unilever UK Central Resources Ltd.
Footnotes
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/pnp).
References
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine : support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Archives of General Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berlin) 2005;179:154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Chaturvedi HK, Bapna JS, Chandra D. Effect of fluvoxamine and N-methyl-D-aspartate receptor antagonists on shock-induced depression in mice. Indian Journal of Physiology and Pharmacology. 2001;45:199–207. [PubMed] [Google Scholar]
- Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hörtnagl H, Riva MA, Sprengel R, Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. Journal of the Federation of American Societies for Experimental Biology. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Chang AS, Larkin T, Mao J. The intravenous ketamine test : a predictive response tool for oral dextromethorphan treatment in neuropathic pain. Anesthesia & Analgesia. 2004;99:1753–1759. doi: 10.1213/01.ANE.0000136953.11583.7B. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Verdolin MH, Chang AS, Kurihara C, Morlando BJ, Mao J. The intravenous ketamine test predicts subsequent response to an oral dextromethorphan treatment regimen in fibromyalgia patients. Journal of Pain. 2006;7:391–398. doi: 10.1016/j.jpain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine : a pharmaco-magnetic resonance imaging study. Archives of General Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization : relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dena M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version – Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- Fumagalli E, Bigini P, Barbera S, De Paola M, Mennini T. Riluzole, unlike the AMPA antagonist RPR119990, reduces motor impairment and partially prevents motoneuron death in the wobbler mouse, a model of neurodegenerative disease. Experimental Neurology. 2006;198:114–128. doi: 10.1016/j.expneurol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM. Relapse prevention with antidepressant drug treatment in depressive disorders : a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- Gilmer WS, Gollan JK, Wisniewski SR, Howland RH, Trivedi MH, Miyahara S, Fleck J, Thase ME, Alpert JE, Nierenberg AA, et al. Does the duration of index episode affect the treatment outcome of major depressive disorder? A STAR*D report. Journal of Clinical Psychiatry. 2008;29:e1–e11. doi: 10.4088/jcp.v69n0807. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU. Assessment Manual for Psychopharmacology. Washington, DC: Superintendent of documents, U.S. Government Printing Office, U.S. Department of Health, Education, and Welfare; 1976. Publication no. 76–338. [Google Scholar]
- Hamilton MJ, Cohen AF, Yuen AW, Harkin N, Land G, Weatherley BC, Peck AW. Carbamazepine and lamotrigine in healthy volunteers : relevance to early tolerance and clinical trial dosage. Epilepsia. 1993;34:166–173. doi: 10.1111/j.1528-1157.1993.tb02393.x. [DOI] [PubMed] [Google Scholar]
- Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Bullmore ET, Menon DK, Fletcher PC. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. Journal of Neuroscience. 2008;28:6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Molecular Psychiatry. 2004;9:984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurology. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, Kato K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. Journal of the Federation of American Societies for Experimental Biology. 2002;16:1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, Mueller M, Bernstein HJ, O’Connor K, Smith G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Archives of General Psychiatry. 2006;63:1337–1344. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Redding N, Brown WA. The persistence of the placebo response in antidepressant clinical trials. Journal of Psychiatric Research. 2008;42:791–796. doi: 10.1016/j.jpsychires.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, Crites G, Malatynska E. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. European Journal of Pharmacology. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Markovic K, Thürauf N, Schmitt H, Kornhuber J. Correspondence: ketamine followed by memantine for the treatment of major depression. Australian and New Zealand Journal of Psychiatry. 2008;42:170. doi: 10.1080/00048670701787628. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, MacDougall L, Abi-Saab W, D’Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine : implications for glutamatergic and dopaminergic model psychoses and cognitive function. Archives of General Psychiatry. 2005;62:985–995. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Archives of General Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lenderking WR, Hu M, Tennen H, Cappelleri JC, Petrie CD, Rush AJ. Daily process methodology for measuring earlier antidepressant response. Contemporary Clinical Trials. 2008 doi: 10.1016/j.cct.2008.05.012. Published online : 17 June 2008. [DOI] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Machado-Viera R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action : a new paradigm in the research and treatment of major depressive disorder. Journal of Clinical Psychiatry. 2008;22 doi: 10.4088/jcp.v69n0610. 31–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA. The role of glutamate in mood disorders : results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current Psychiatry Reports. 2008;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schoesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mantovani M, Pertile R, Calixto JB, Santos AR, Rodrigues AL. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: evidence for involvement of N-methyl-d-aspartate receptors and the L-arginine-nitric oxide pathway. Neuroscience Letters. 2003;343:1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and drug targets for severe mood disorders. Neuropsychopharmacology. 2008b;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Mao X, Smith ELP, Coplan JD, Charney DS, Shungu DC. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biological Psychiatry. 2008a;63:891–898. doi: 10.1016/j.biopsych.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophoic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD001447.pub2. Art. No.: CD001447. [DOI] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neuroscience Letters. 2001;310:117–120. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal of Neuroscience. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Tanaka Y. Antidepressant-like effects of aniracetam in aged rats and its mode of action. Psychopharmacology (Berlin) 2001;158:205–212. doi: 10.1007/s002130100849. [DOI] [PubMed] [Google Scholar]
- NIMH. Systematic Assessment for Treatment Emergent Effects (SAFTEE) Rockville, MD: National Institute of Mental Health; 1986. [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Report. 1962;10:799–812. [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. American Journal of Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22:761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Mueller M, Knapp RG, Husain MM, Rummans TA, Sampson SM, O’Connor K, Petrides G, Fink M, Kellner CH. Antidepressant medication treatment failure does not predict lower remission with ECT for major depressive disorder : a report from the consortium for research in electroconvulsive therapy. Journal of Clinical Psychiatry. 2007;68:1701–1706. doi: 10.4088/jcp.v68n1109. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS) : preliminary findings. Psychopharmacology Bulletin. 1996;22:985–990. [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, et al. ACNP Task Force. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006b;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [Erratum, p. 585] [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps : a STAR*D report. American Journal of Psychiatry. 2006a;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry. 2001;62(Suppl. 16):10–17. [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. Journal of the American Medical Association. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. Journal of Clinical Psychopharmacology. 1990;10:96–104. doi: 10.1097/00004714-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biological Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AJ, Savostyanova AA, Goldman A, Barnett AS, van der Veen JWC, Callicott JH, Mattay VS, Weinberger DR, Marenco S. Impact of the brain-derived neurotrophic factor val66met polymorphism on levels of hippocampal N-acetyl-aspartate assessed by magnetic resonance spectroscopic imaging at 3 tesla. Biological Psychiatry. 2008;64:856–862. doi: 10.1016/j.biopsych.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacology Biochemistry and Behavior. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Young RC, Schreiber MT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. An open-label trial of riluzole in patients with treatment-resistant major depression. American Journal of Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biological Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. Robust, rapid and relatively sustained antidepressant effects with a single-dose of an NMDA antagonist in treatment-resistant major depression: a double-blind placebo controlled study. Archives of General Psychiatry. 2006;63:856–863. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.