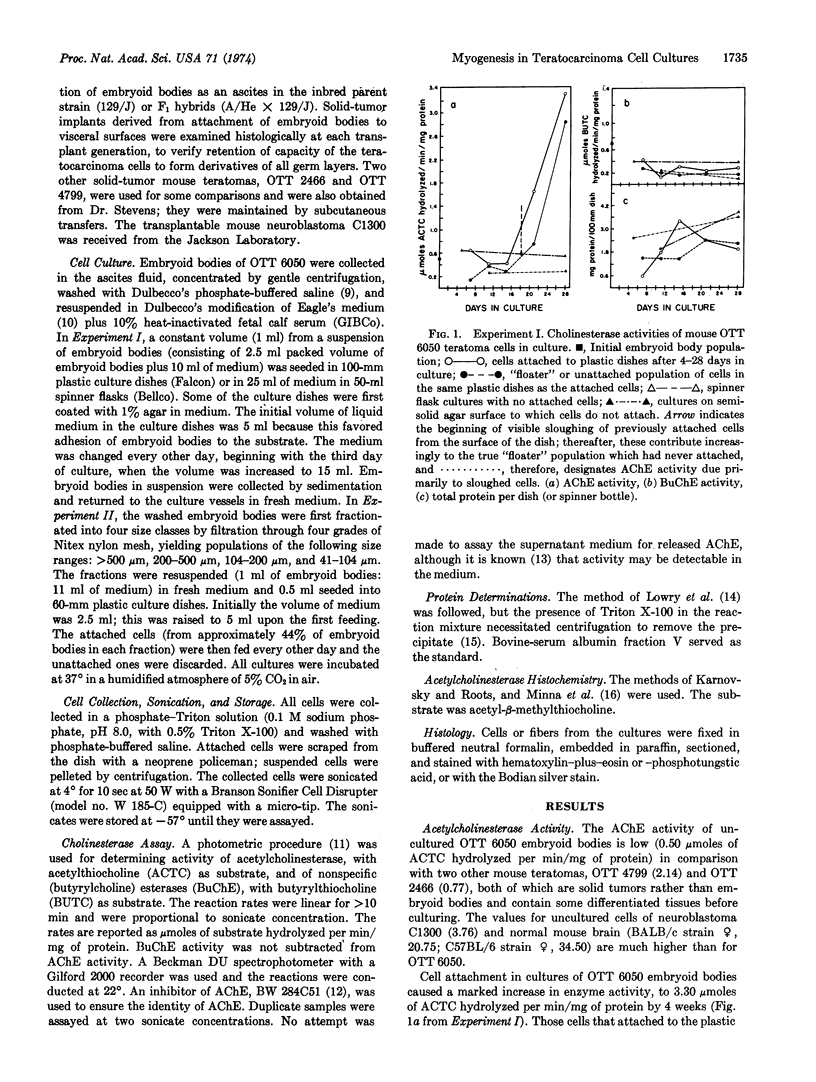
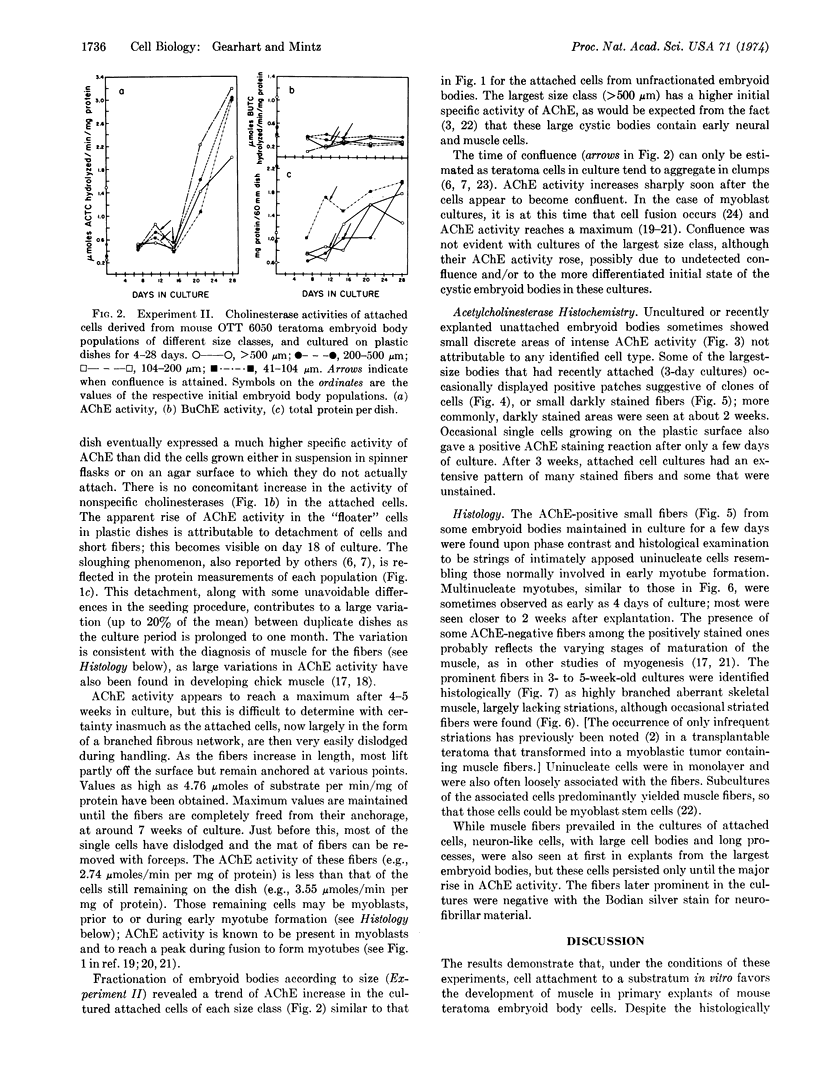
Abstract
Transplantable mouse teratomas are known to contain multipotential teratocarcinoma stem cells analogous to early embryo cells and capable of giving rise to a wide variety of specialized cell types in vivo when they attach to a substratum; if they are grown instead in suspension in the body cavity, they form multicellular embryoid bodies which proliferate with little or no cell specialization. Thus, changes initiated at the cell surface may play some role in promoting early cell differentiation. In order to establish an in vitro system for experimental investigation of this hypothesis, embryoid body cells were explanted under conditions of cell attachment versus suspension and maintained in primary culture. Because cell differentiation in previous reports was relatively limited in vitro, the two cellular populations were first compared for genesis of a quantifiable macromolecular phenotype, acetylcholinesterase (AChE) activity, which characterizes several of the cell types most commonly formed in the attached tumors in vivo. The attached cells produced markedly increased levels of AChE activity within a few weeks, while cells in suspension retained basal levels. AChE was histochemically visualized and was found to occur chiefly in cells undergoing myogenesis, especially during myotube formation. Aberrant muscle fibers formed and became predominant in the cultures. When embryoid bodies were first fractionated by increasing size, which reflects their progressive differentiation, the smallest ones, with relatively more multipotential cells and no apparent muscle cells, also showed AChE increase in attached cultures. The results are consistent with the view that attachment of the cell surface to a substratum may play a critical role in initiating some cellular developmental commitments, as well as in sustaining differentiation of cells whose specialization has already been determined. Further experimental modifications of this primary culture system of teratocarcinoma cells should be useful in analyzing cell-substratum relations and cell-surface changes in early mammalian development.
Keywords: multipotential tumor cells, teratoma embryoid bodies, cell surface, cell determination, cell differentiation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Dubois P., Bennett D., Condamine H., Babinet C., Jacob F. Surface antigens common to mouse cleavage embryos and primitive teratocarcinoma cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2988–2992. doi: 10.1073/pnas.70.10.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Casola L., Grasso A. Neuroblastoma cells and 14-3-2, a brain specific protein. Cell Differ. 1973 Jul;2(3):157–161. doi: 10.1016/0045-6039(73)90016-x. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Evans M. J. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol. 1972 Aug;28(1):163–176. [PubMed] [Google Scholar]
- Fluck R. A., Strohman R. C. Acetylcholinesterase activity in developing skeletal muscle cells. Dev Biol. 1973 Aug;33(2):417–428. doi: 10.1016/0012-1606(73)90147-4. [DOI] [PubMed] [Google Scholar]
- GOODWIN B. C., SIZER I. W. EFFECTS OF SPINAL CORD AND SUBSTRATE ON ACETYLCHOLINESTERASE IN CHICK EMBRYONIC SKELETAL MUSCLE. Dev Biol. 1965 Feb;11:136–153. doi: 10.1016/0012-1606(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. Interference by detergents, chelating agents, and buffers with the Lowry protein determination. Anal Biochem. 1973 Apr;52(2):517–521. doi: 10.1016/0003-2697(73)90056-0. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Kahan B. W., Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. J Natl Cancer Inst. 1970 May;44(5):1015–1036. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Oh T. H., Johnson D. D., Kim S. U. Neurotrophic effect on isolated chick embryo muscle in culture. Science. 1972 Dec 22;178(4067):1298–1300. doi: 10.1126/science.178.4067.1298. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., DIXON F. J., Jr Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959 May-Jun;12(3):573–583. doi: 10.1002/1097-0142(195905/06)12:3<573::aid-cncr2820120316>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., Jr, DIXON F. J., Jr, VERNEY E. L. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960 Nov-Dec;9:583–602. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, VERNEY E. L. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer. 1961 Sep-Oct;14:1017–1029. doi: 10.1002/1097-0142(196109/10)14:5<1017::aid-cncr2820140516>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. D., Wishnow R. M., Sato G. H. In vitro growth and differetiation of clonal populations of multipotential mouse clls derived from a transplantable testicular teratocarcinoma. J Natl Cancer Inst. 1970 May;44(5):1001–1014. [PubMed] [Google Scholar]
- STEVENS L. C. Embryonic potency of embryoid bodies derived from a transplantable testicular teratoma of the mouse. Dev Biol. 1960 Jun;2:285–297. doi: 10.1016/0012-1606(60)90010-5. [DOI] [PubMed] [Google Scholar]
- STEVENS L. C. The biology of teratomas including evidence indicating their origin form primordial germ cells. Annee Biol. 1962 Nov-Dec;1:585–610. [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Jacob F., de Vitry F. Induced differentiation of a neuroblastoma. Dev Biol. 1971 Aug;25(4):514–546. doi: 10.1016/0012-1606(71)90004-2. [DOI] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., Harris A. J., Heinemann S. Induction of acetylcholine esterase activity in a mouse neuroblastoma. Nat New Biol. 1971 Sep 15;233(37):79–80. doi: 10.1038/newbio233079a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., Humphreys S., Heinemann S., Patrick J. Protein synthesis and secretion in a myogenic cell line. Dev Biol. 1973 Jul;33(1):18–37. doi: 10.1016/0012-1606(73)90161-9. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. The biology of teratomas. Adv Morphog. 1967;6:1–31. doi: 10.1016/b978-1-4831-9953-5.50005-6. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970 Mar;21(3):364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M., Brzin M., Kremzner L. T. Acetylcholinesterase activity in the myotube and muscle satellite cell of the fetal rabbit. An electron microscopic-cytochemical and biochemical study. J Histochem Cytochem. 1973 Jul;21(7):634–652. doi: 10.1177/21.7.634. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M., Brzin M., Slotwiner P. The appearance of acetylcholinesterase in the myotome of the embryonic rabbit. An electron microscope cytochemical and biochemical study. J Cell Biol. 1971 Dec;51(3):703–721. doi: 10.1083/jcb.51.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. W., Nieberg P. S., Walker C. R., Linkhart T. A., Fry D. M. Production and release of acetylcholinesterase by cultured chick embryo muscle. Dev Biol. 1973 Aug;33(2):285–299. doi: 10.1016/0012-1606(73)90138-3. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Schrier B. K., Farber J. L., Thompson E. J., Rosenberg R. N., Blume A. J., Nirenberg M. W. Markers for gene expression in cultured cells from the nervous system. J Biol Chem. 1972 May 25;247(10):3159–3169. [PubMed] [Google Scholar]
- Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971 May;66(1):33–48. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]



