Abstract
Background. Despite the strong association between secondary dengue virus (DENV) infections and dengue hemorrhagic fever (DHF), the majority of secondary infections are subclinical or mild. The determinants of clinical severity remain unclear, though studies indicate a titer-dependent and time-dependent role of cross-protective anti-DENV antibodies.
Methods. Data from 2 sequential prospective cohort studies were analyzed for subclinical and symptomatic DENV infections in schoolchildren in Kamphaeng Phet, Thailand (1998–2002 and 2004–2007). Children experiencing ≥1 DENV infection were selected as the population for analysis (contributing 2169 person-years of follow-up).
Results. In total, 1696 children had ≥1 DENV infection detected during their enrollment; 268 experienced 2 or more infections. A shorter time interval between infections was associated with subclinical infection in children seronegative for DENV at enrollment, for whom a second-detected DENV infection is more likely to reflect a true second infection (average of 2.6 years between infections for DHF, 1.9 for DF, and 1.6 for subclinical infections).
Conclusions. These findings support a pathogenesis model where cross-reactive antibodies wane from higher-titer, protective levels to lower-titer, detrimental levels. This is one of the first studies of human subjects to suggest a window of cross-protection following DENV infection since Sabin's challenge studies in the 1940s.
Keywords: dengue, epidemiology, antibodies, immunity, pathogenesis, prospective cohort study
Dengue viruses (DENV) are a major cause of disease and death throughout tropical and subtropical regions of the globe [1]. In the 1960s, studies of the dramatic increases in dengue hemorrhagic fever (DHF) in Southeast Asia established a link with secondary DENV infections [2–3], which has since been confirmed in other epidemics and other locales [4–7]. Secondary DENV infection remains the strongest known risk factor for DHF, with a relative risk estimated to be as high as 50–100 compared to primary DENV infection [5, 8].
Despite this strong association, secondary infection appears neither sufficient nor necessary for causing DHF. DHF can be observed in primary DENV infections [9], suggesting that unknown host and/or viral factors are sufficient to drive the occurrence of DHF outside of the setting of preexisting antibodies. Second, only a small fraction of secondary infections progress to DHF; the majority of secondary infections are in fact subclinical [8, 10]. The factors that predispose one individual to develop DHF over another with secondary infection remain unclear. Last, tertiary and quaternary infections with DENV are typically thought to be milder [11], suggesting that cross-protection may be sufficient by the third infection to mitigate against clinical illness.
The dominant mechanism proposed for the pathogenesis of DHF is that of antibody-dependent enhancement (ADE) [2]. With ADE, preexisting neutralizing antibodies from a prior DENV infection are hypothesized to enhance a subsequent infection with a different DENV serotype, resulting in higher viral load, greater immune activation, and ultimately the profound plasma leakage characteristic of DHF [12]. The ability to neutralize or enhance heterologous DENV viruses has been shown to vary by antibody titer [13]. In vitro studies have demonstrated a titer dependence of ADE with peaks in both the percentage of infected cells and viral output per infected cell at low-to-intermediate concentrations of antibody [14]. The same prospective study used for the present analysis also provided evidence of a possible titer-dependent role of cross-reactive antibodies; higher levels of preexisting (cross reactive) antibodies to DENV-3 were associated with milder illness upon subsequent infection with DENV-3 [15].
An analogy to this titer-dependent function of cross-reactive antibodies may be found in human infants, who are born with high levels of maternal antibody and are seemingly protected from DHF [16]. As maternal antibody wanes, infants are postulated to pass into an “intermediate phase” at approximately 6–8 months where they experience an elevated risk of DHF. As maternal antibody wanes further and disappears, infants again pass into a phase with a low risk of DHF. This suggests that high maternal antibody titers are sufficient to provide some protection from DENV infection, but that as antibodies wane to low-intermediate levels, cross-reactive antibodies may facilitate the occurrence of DHF.
There is also experimental evidence of a temporal window of cross-protection with DENV from human challenge studies conducted by Albert Sabin [17]. Specifically, he found that up until 2 months post-primary inoculation, subjects were protected from illness when inoculated with a second heterologous DENV and were unable to infect mosquitoes (ie, were protected from infection). Two to three months post-primary infection, exposure to a heterologous DENV serotype resulted in transient fever and mild malaise, and the ability to infect mosquitoes was reestablished. Nine months post-primary infection, heterologous exposure resulted in 2–3 days of fever and a rash.
A large DENV-1 epidemic passed through Cuba in 1977–1978 followed by 2 epidemics of DENV-2, in 1981 and in 1997 [18, 19], providing 2 important epidemiological observations on temporal trends in disease risk. First, individuals that experienced their primary DENV-1 infection in 1977–1978 and their secondary DENV-2 infection in 1997 (20 years between infections) had a risk of DHF that was 3–4 times greater than individuals who experienced their secondary DENV-2 infection in 1981 (with 4 years between infections) [9]. Second, they observed a decline in the proportion of the immune response that was heterotypic over time and proposed that waning of cross-reactive antibodies over time may contribute to the increased risk of DHF with time between infections [20].
Based on these observations, a model of disease risk in secondary DENV infection is proposed wherein individuals pass through windows of protection and increased risk of illness based on the time between infections and the magnitude of their persistent cross-reactive antibody response to the previous infection. We hypothesized that exposures occurring within a shorter interval of time following a primary infection would be more likely to be subclinical (illustrated schematically in Figure 1A), whereas for third or fourth DENV infections, accumulation of specific immunity to multiple DENV viruses would confer cross-protective immunity without a strong influence of time between infections (illustrated in Figure 1B). We tested these hypotheses using data from 2 sequential prospective cohort studies for symptomatic and subclinical DENV infections in Kamphaeng Phet, Thailand.
Figure 1.
Illustration of hypothesized shifts in risk of dengue virus (DENV) illness and cross-reactive antibodies over time (theoretical, not based upon data). A, Schematic of shifts in risk of illness following primary DENV infection. B, Schematic of shifts in risk of illness following secondary DENV infection.
METHODS
Study Population
Data were derived from school-based, prospective cohort studies of DENV infections in children in Northern Thailand. The studies were conducted from 1998 to 2002 (Kamphaeng Phet study 1, or “KPS1”) and from 2004 to 2007 (“KPS2”). The study designs and methods for the 2 studies were similar and have been described elsewhere [10, 21]. In early 1998 and 2004, approximately 2000 children were recruited from primary schools in the community; 12 schools participated for KPS1 and 11 schools for KPS2. KPS1 and KPS2 generally took place at different schools and in different villages, and no child was enrolled in both studies. Children aged 5–16 years were eligible for enrollment in KPS1 and children aged 4–16 years for KPS2.
Active Surveillance for Incident Dengue Cases
Active, fever-based surveillance for dengue illnesses was conducted from June 1 to November 1 (KPS1) or December 1 (KPS2) each year. During the active surveillance period, potential illnesses in enrolled children were identified based on school absence, visit to a school nurse or clinic, or hospital admission, triggering prompt evaluation of the child by a village health worker. Acute blood samples were obtained from children that had had subjective fever within 7 days or oral temperature ≥38°C; 14-day convalescent blood samples were collected.
Acute and convalescent blood specimens from incident febrile illnesses were tested using immunoglobulin M (IgM) and immunoglobulin G (IgG) enzyme immunoassays for DENV and Japanese encephalitis virus (JEV). Acute DENV infections were defined serologically as a DENV-specific IgM level ≥40 units and with DENV-IgM > JEV-IgM. The infecting DENV serotype was identified from acute blood specimens using serotype-specific reverse-transcriptase polymerase chain reaction (RT-PCR) or virus isolation.
Symptomatic infections were defined as a febrile illness with virologic or serologic evidence of acute DENV infection. Charts of hospitalized children were independently reviewed and classified as DHF and assigned a severity grade following 1997 World Health Organization (WHO) criteria [22]. If a child experienced a febrile DENV illness but did not meet the criteria for DHF, they were characterized as having dengue fever (DF). The sensitive nature of the active fever surveillance system meant that the symptomatic infections captured in the cohort studies covered a wide range of clinical severities, from a single day of transient fever to dengue shock syndrome (DSS).
Routine Blood Specimens and Detection of Seroconversions
Routine blood specimens were drawn from all enrollees 4 times a year for KPS1 (January, June, August, and November) and 2 times a year for KPS2 (January and June). All routine specimens were tested for hemagglutination inhibition (HI) antibodies against all 4 DENV serotypes and JEV using the standard method of Clark and Casals [23]. Subclinical seroconversions were defined according to WHO criteria as a 4-fold or greater rise in HI titers for any of the 4 DENV serotypes between 2 consecutive routine serum samples in the absence of a concurrent 4-fold rise in JEV HI titers, or in the presence of a 4-fold rise in JEV HI titers but with higher HI titers for any DENV serotype than for JEV, and in the absence of a confirmed acute symptomatic DENV infection in that individual for the active surveillance period in that year.
Characterization of First-detected and Second-detected Infections
In this analysis, DENV infections are designated “first-detected” and “second-detected” infections, irrespective of enrollment antibody profile. The ability to characterize the clinical severity of these infections was dependent on when an infection occurred. Infections occurring during the active surveillance period were classified as subclinical, DF, or DHF. Outside of the active surveillance period, subclinical and nonhospitalized illnesses were solely detected as seroconversions. Therefore, second-detected infections occurring outside of the active surveillance window were excluded from this analysis. Time to second infection was calculated as 1-year intervals from the first infection since date of infection was not known for subclinical infections and a finer temporal resolution was not possible.
Defining Baseline DENV Immunity and Other Immunological Parameters
Using routine HI data, children were classified upon enrollment as HI-negative (enrollment HI antibody titers ≤10 for all 4 DENV serotypes), HI-monotypic (HI >10 for 1 serotype), and HI-multitypic (HIs >10 for 2 or more serotypes). Total seropositivity was defined as the summed number of DENV serotypes with HI titer >20. Rise in HI titers was calculated as the summed increase in HI titers for all 4 DENV serotypes comparing the HI titers in routine blood specimens pre- and post-first-detected infection. Decay rate was calculated as the percent decline in summed HI titers from after the first-detected infection to just prior to the second-detected infection divided by the years between infections.
Statistical Analyses
For univariate analyses, Wilcoxon rank ( nonparametric) tests were used to compare the time to infection between groups, using SAS' NPAR1WAY. Exact χ2 testing was performed for categorical variables.
For multivariate analysis, a conditional logistic regression estimated the odds of symptomatic second infection, given that a second infection was detected. We evaluated the association between time between infections and severity of symptomatic second infection, controlling for HI enrollment profile and conditioning on subdistrict of residence, age, and study period (KPS1 or KPS2). Age in years at first infection was split into equally divided tertiles. Immunological factors (summed HI titers, seropositivity, and decay rate) were dichotomized as higher or lower than the mean for each variable. All immunological factors, and their interactions, were initially incorporated into the model and were removed using backward elimination until only significant variables remained in the model. Both models used the EXACT statement in proc logistic and conditional regression was specific using the STRATA statement.
Analyses were performed using SAS (SAS Institute, Cary, North Carolina), SPSS (SPSS Inc., Chicago, Illinois), and R software (R Foundation for Statistical Computing, Vienna, Austria).
Human Subjects Research Approval
The study protocol for KPS1 was approved by the Office of the Army Surgeon General, University of the Massachusetts Medical School, and the Ministry of Public Health, Thailand. The protocol for KPS2 was additionally approved by the University of California–Davis and San Diego State University.
RESULTS
The baseline characteristics of enrolled children have been presented elsewhere [10, 21]. In total, 1696 individuals experienced at least one DENV infection during the 2 cohort studies combined; 268 experienced a second infection during their enrollment; and 123 of second infections occurred during the active surveillance period and were eligible for inclusion in the analysis. The mean ages of first- and second-detected infections were 9.7 and 10.9, respectively. The distribution of ages and HI positivity at enrollment did not differ significantly by whether the second infection occurred within the active surveillance period (data not shown). School of attendance and epidemic year did differ, reflecting observed temporal and spatial clustering of infections in the region [21]. All 4 dengue serotypes were detected in both studies, with DENV-2 and DENV-3 dominant in KPS1 (48% and 32% of PCR positive cases, respectively) and DENV-1 and DENV-4 dominant in KPS2 (46% and 39%; data not shown).
Time From First to Second-detected Infection and Clinical Severity
Second infections detected during active surveillance for all 9 years of the studies consisted of a total of 89 subclinical infections, 27 cases of DF, and 7 cases of DHF. Figure 2 outlines the numbers of children remaining at risk for a second infection by each year interval from the first infection. In unstratified analysis, there was no significant difference in time between infections by clinical severity (Table 1). There was a significant trend among children that were HI-negative at enrollment: subclinical individuals had the shortest mean time between infections (1.41 years), then DF cases (1.92 years), then DHF (2.60 years; P = .010). This pattern was not observed among those with some DENV-HI immunity at enrollment.
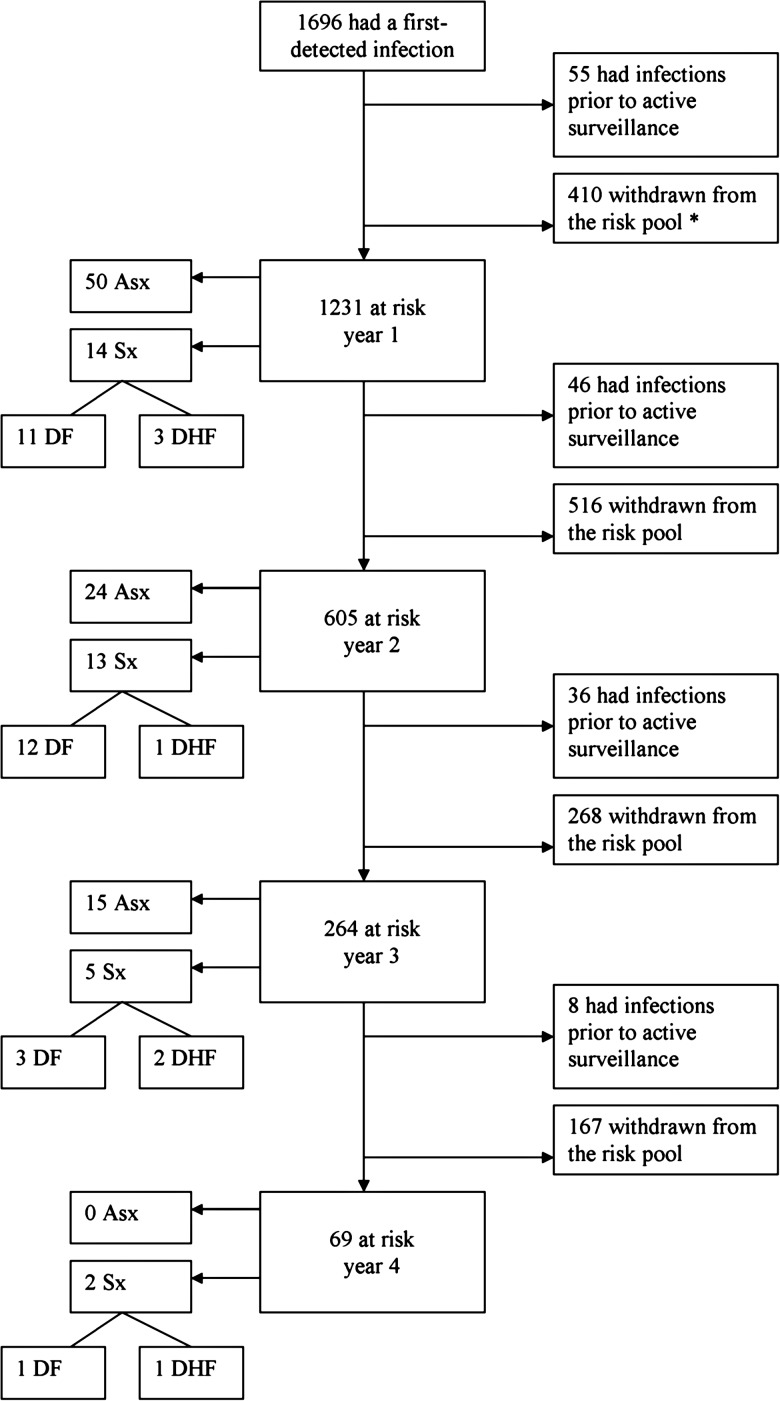
Figure 2.
Flowchart of the children that experienced at least one dengue virus (DENV) infection, detailing the numbers remaining at risk for a second infection each year, the numbers of cases, and their severities. * Withdrawals may represent (1) children that “graduated” out of the study by turning 16 years of age, (2) children that were no longer at risk for a second infection because the study ended (eg, if a child's first detected infection occurred in 2002, the study period [spanning 1998–2002 and 2004–2007] would have ended before a second infection could be detected), and (3) children that “dropped out” while still eligible for enrollment in an ongoing study. Abbreviations: Asx, Subclinical/asymptomatic; Sx, Symptomatic; DF, Dengue fever; DHF, Dengue hemorrhagic fever.
Table 1.
Mean and Median Time Between First and Second Detected Infections, by Subclinical Infection, DF or DHF
| 2nd-detected Infection | N | Mean Time to 2nd Infection in Years (SD) | Median Time to 2nd Infection in Years (range) | P* |
|---|---|---|---|---|
| Subclinical | 89 | 1.61 (0.76) | 1 (1–2) | .31 |
| DF | 27 | 1.78 (0.80) | 2 (1–2) | |
| DHF | 7 | 2.14 (1.21) | 2 (1–3) | |
| By enrollment HI Titer: | ||||
| HI- Negative | ||||
| Subclinical | 22 | 1.41 (0.67) | 1 (1–2) | .01 |
| DF | 12 | 1.92 (0.67) | 2 (1–2) | |
| DHF | 5 | 2.60 (1.14) | 3 (2–3) | |
| HI- Monotypic | ||||
| Subclinical | 9 | 2.11 (0.93) | 2 (1–3) | .29 |
| DF | 2 | 1.00 (0.00) | 1 (1–1) | |
| DHF | … | … | … | |
| HI- Multitypic | ||||
| Subclinical | 58 | 1.60 (0.75) | 1 (1–3) | .41 |
| DF | 13 | 1.77 (0.93) | 2 (1–4) | |
| DHF | 2 | 1.00 (0.00) | 1 (1–1) |
Abbreviations: DF, dengue fever; DHF, dengue hemorrhagic fever; HI, hemagglutination inhibition; SD, standard deviation.
*P values were obtained using nonparametric Wilcoxon tests with SAS' NPAR1WAY procedure.
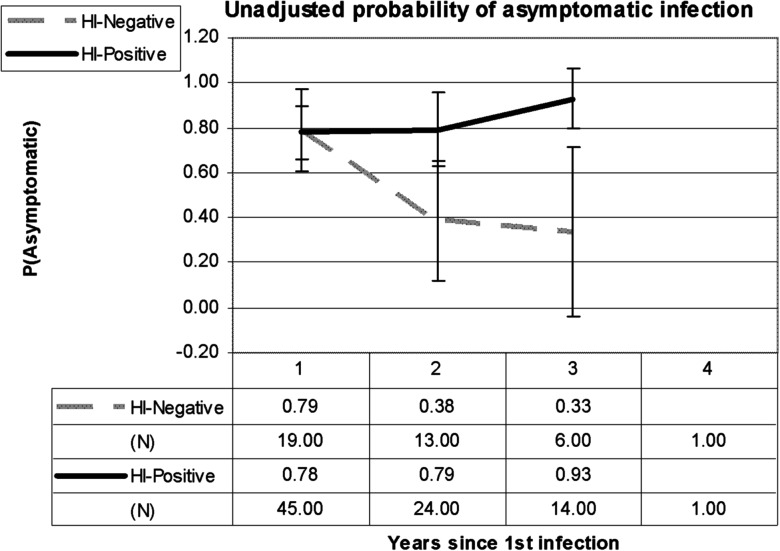
Given the low number of second-detected infections that were DHF, DF, and DHF cases were combined as “symptomatic” dengue infections for remaining analyses. The probability of subclinical infection decreased each year for children that were HI-negative at enrollment, from 79% at 1 year post-first infection, to 38% at 2 years, to 33% at 3 years (P = .042 by χ2, Figure 3). There was no significant change in the probability of subclinical infection over time in those children with some HI immunity at enrollment.
Figure 3.
Probability of asymptomatic infection by year since the first-detected infection in the cohort studies, by whether a child had detectable hemagglutination inhibition (HI) antibodies at enrollment (HI-positive) or was negative by HI at enrollment (HI-negative). Error bars indicate the 95% confidence intervals for the proportions.
Predictors of the Time to Infection and the Severity of Second-detected Infection
Age at first-detected infection was not associated with the clinical severity of second-detected infection in bivariate analysis but younger children experienced a longer time interval between infections (P < .01, Table 2). This is likely an artifact of the study design as older children would have had less time to experience a second-detected infection before graduating from the study. Enrollment HI profile was associated with clinical severity; children that were HI-negative at enrollment were more likely to be symptomatic with their second-detected infection (43.6% symptomatic vs 10% for HI-monotypics and 21.6% for HI-multitypics; P = .020). Enrollment HI profile was not associated with time to second infection. Second infections were more likely to be symptomatic in KPS2 than KPS1 (41.4% vs 23.4%, P = .058). Finally, the severity of the first infection was not associated with the severity of the second infection or time to infection.
Table 2.
Predictors of the Severity of Second Detected Infections and Predictors of the Mean Time From First to Second Detected Infection
| n | Sub-clinical | Symptomatica | P | Time (years) | Pb | ||
|---|---|---|---|---|---|---|---|
| Age at 1st-detected infection | |||||||
| 7–8 | 42 | 32 (76.2%) | 10 (23.8%) | .715 | 7–8 | 1.93 | <.001 |
| 9–10 | 58 | 40 (69.0%) | 18 (31.0%) | 9–10 | 1.71 | ||
| 11–15 | 23 | 17 (73.9%) | 6 (26.1%) | 11–15 | 1.13 | ||
| Enrollment DENV antibody status (by HI) | |||||||
| HI-Negative | 39 | 22 (56.4%) | 17 (43.6%) | .020 | HI-Negative | 1.72 | .56 |
| HI-Monotypic | 10 | 9 (90.0%) | 1 (10.0%) | HI-Monotypic | 1.91 | ||
| HI-Multitypic | 74 | 58 (78.4%) | 16 (21.6%) | HI-Multitypic | 1.62 | ||
| Study phase | |||||||
| Kps1 (1998–2002) | 94 | 72 (76.6%) | 22 (23.4%) | .058 | Kps1 | 1.70 | .69 |
| Kps2 (2004–2007) | 29 | 17 (58.6%) | 12 (41.4%) | Kps2 | 1.59 | ||
| Severity of 1st-detected infection | |||||||
| Asx | 33 | 29 (87.9%) | 4 (12.1%) | .267 | Asx | 1.73 | .46 |
| DF | 25 | 18 (72.0%) | 7 (28.0%) | DF | 1.72 | ||
| DHF | 3 | 2 (66.7%) | 1 (33.3%) | DHF | 1.33 |
Abbreviations: DENV,dengue virus; DF,dengue fever; DHF, dengue hemorrhagic fever; HI,hemagglutination inhibition; SD, standard deviation.
a “Symptomatic” DENV infection was defined as a documented history of febrile illness with virologic or serologic evidence of acute DENV infection, combining DHF and non-DHF cases.
b P values were obtained using exact χ2 methods for categorical variables and NPAR1WAY for continuous variables.
Immunological Predictors of Subclinical Infection
The summed HI response following first-detected infection as well as the summed HI titer prior to second-detected infection was not significantly associated with subclinical second infection in crude or stratified analysis, nor was the total seropositivity (number of DENV serotypes with HI > 20) following first-detected infection (Table 3). Total seropositivity prior to second-detected infection was positively associated with the probability of a subclinical infection. Antibody decay rate was not significantly associated with subclinical infection in crude or stratified analysis. A separate crude analysis was performed comparing immunological response patterns by enrollment HI profile: children that were HI negative had significantly higher summed antibody titers following first-detected infection, lower summed titers and lower seropositivity prior to second-detected infection, and a faster antibody decay rate compared to individuals that were HI positive on enrollment.
Table 3.
Immunological Predictors of Clinical Severity for the Second Detected Infection
| Unstratified Analysis for Enrollment HI Profile |
Unstratified Analysis for Symptomatic or Subclinical Infection |
HI-negative at Enrollment |
Some Immunity at Enrollment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI-neg | HI-pos | Pa | Asx | Sx | P | Asx | Sx | P | Asx | Sx | P | |
| (N) | (39) | (84) | (89) | (33) | (22) | (16) | (67) | (17) | ||||
| Post-1st-detected infection | ||||||||||||
| Rise in summed HAI titers (Log10) | 2.86 | 2.59 | .051 | 2.70 | 2.59 | .381 | 2.96 | 2.73 | .288 | 2.62 | 2.46 | .311 |
| Total seropositivity (no. serotypes) | 3.72 | 3.87 | .138 | 3.88 | 3.67 | .163 | 3.77 | 3.64 | .689 | 3.91 | 3.71 | .283 |
| Pre-2nd-detected infection | ||||||||||||
| Summed HAI titers (Log10) | 2.10 | 2.40 | <.01 | 2.31 | 2.24 | .254 | 2.10 | 2.09 | .642 | 2.37 | 2.50 | .730 |
| Total seropositivity (# serotypes) | 3.10 | 3.67 | <.01 | 3.61 | 3.14 | .014 | 3.27 | 2.88 | .223 | 3.73 | 3.41 | .397 |
| Decay rate | ||||||||||||
| % decrease in summed titers from post-1st to pre-2nd detected infection by year | 54.7% | 26.1% | <.01 | 38.7% | 25.7% | .125 | 72.9% | 31.1% | .097 | 27.5% | 20.0% | .747 |
Abbreviations: DENV, dengue virus; HAI,hemagglutination inhibition assay; HI,hemagglutination inhibition; Asx, Asymptomatic (subclinical) second-detected DENV infection; Sx, symptomatic second-detected DENV infection.
a P values were obtained using non parametric Wilcoxon tests with SAS' NPAR1WAY procedure.
Multivariate Model of the Odds of Symptomatic Infection Over Time
The final multivariate model evaluated the association between time between infections and symptomatic DENV infection, controlling for enrollment DENV immunity (by HI) with an interaction term for DENV immunity and time between infections, and conditioning on the subdistrict, age, and study period (see appendix detailing model construction and output). Time to second infection was independently associated with symptomatic infection, as was enrollment DENV immunity. The summed rise in titers following first-detected infection was inversely associated with the odds of symptomatic second-detected infection (Figure 4); this effect was most pronounced for children who were HI negative on enrollment, who were more likely to be symptomatic with a low HI response to their first-detected infection. For each time point, children that were HI positive on enrollment were less likely to experience symptomatic infection than HI-negative children.
Figure 4.
Odds ratios (ORs) for experiencing a symptomatic versus an subclinical second infection, given that a second infection occurred. Results are stratified by hemagglutination inhibition (HI) antibody profile at enrollment (HI positive [Pos] or HI negative [Neg]) and whether the total HI antibody response to the first infection was higher than the mean (High rise) or lower than the mean (Low rise). ORs compare the odds of symptomatic infection for years 2 and 3 in each stratum to the odds of symptomatic infection in year 1 for the reference stratum (children who were HI-positive and demonstrated a high antibody response to infection).
DISCUSSION
The strong association between DHF and secondary DENV infection has been repeatedly demonstrated. However, the majority of secondary infections are mild or subclinical, and the factors determining severity within secondary infections remain poorly understood. In this study, we used longitudinal data on the occurrence of subclinical, mild, and severe DENV infections in school-children in Thailand to evaluate whether the time interval between infections is associated with the clinical severity of second-detected DENV infections within the cohort.
The time interval between infections was an important predictor of the severity of second-detected DENV infections in children that were HI antibody-negative at enrollment, with subclinical infections occurring at shorter time intervals (mean, 1.41 years) and DF and DHF occurring at longer intervals (mean, 1.92 and 2.60 years, respectively). The presence of this trend in HI-negative children is noteworthy because for most of these children, their second-detected infection may be more likely to reflect a true second DENV infection.
Additionally, this study found evidence of both short-term (as above) and long-term effects of cross-reactive immunity. That is, while the effects of temporary cross-protection were evident for HI-negative children, no temporal trends in disease risk were observed in HI-positive children, who were also less likely to be symptomatic at each time point than HI-negative children. This is consistent with suggestions that the cross-protection afforded by the accumulation of immune responses to multiple DENV infections may attenuate the clinical severity of a third or fourth infection [11]. These short-term and long-term effects may lend insight into the complicated dynamics of DENV epidemics, as population-level shifts in cross-protection may underlie observed and unpredictable fluctuations in epidemic incidence and severity [24–26]
Controlling for time to infection, higher antibody response to the first infection was independently associated with subclinical second infection. This is consistent with a model of risk where antibodies decay over time; a higher peak in the response to infection may allow antibodies to persist longer at high titers and thereby increase the duration of cross-protection. Alternately, the magnitude of the antibody response to infection may serve as a marker of the child's underlying immune state with unmeasured factors influencing the risk of illness (eg, prior immunization against Japanese encephalitis virus). It is interesting that time to second infection was significant after controlling for antibody response, suggesting that time to infection itself may be associated with some shifts in disease risk, perhaps through shifts in antibody specificity.
The presumed protective capability of high-titer cross-reactive antibodies is an important finding in this study. As DENV vaccine development efforts intensify, there is concern that some antibody responses to vaccination may reflect cross-reactivity and that these antibodies may have unintended disease-enhancing effects [27]. The evidence that cross-reactive antibodies provide some protection against DENV illness is encouraging. However, this analysis also found that cross-protection appeared to decline over time; it may be that boosting by intermittent virus exposure (or vaccination) is critical to maintaining the high-titer responses necessary for a protective immune profile. Our findings caution that some vaccine programs might increase the proportion of unvaccinated individuals experiencing symptomatic infections by lengthening the interval between sequential infections. Future studies should investigate the importance of natural boosting in contributing to individual cross-protective immunity.
There were several limitations to this analysis. The first is the relatively limited timescale of the studies: 1998–2002 and 2004–2007. There is a significant yearly variability in the incidence of DENV infection, with large epidemics occurring approximately every 3–5 years [24]. There is also yearly variability in the clinical severity of DENV epidemics [26] and the relative dominance of DENV serotypes in circulation [28]. We attempted to control for this variation by incorporating study period into the models, but it is possible that there was residual confounding. A second important constraint is the limited number of second-detected infections available for analysis, made more limited by the large number of cases occurring outside of the active surveillance period (approximately one-half). It is well known that DENV transmission occurs year-round in tropical endemic regions; however, the fact that such a high degree of transmission was occurring throughout the year is perhaps reflective of climate change and warmer winter months in tropical countries [29]. Third, for reasons of cost and time it was not possible to perform assays for neutralizing antibodies on all routine specimens. Although HI assays are not specific for neutralizing antibody, they have been used extensively in field studies to efficiently detect primary and secondary DENV infections. Fourth, as acute specimens could not be collected for subclinical infections, the infecting serotype and viremia are not known for the majority of these cases. This would be of interest as studies have demonstrated increasing virus titer with duration of time between the first and second doses of live attenuated DENV vaccines [30]. Finally, these findings may not be translatable to other regions where transmission rates are not as high as in Thailand and where this temporary cross-protection may not be a significant aspect of the epidemiology of DENV.
The prospective characterization of individuals as protected from DENV illness or at risk of enhanced disease with DENV infection remains an unsolved problem and one that looms large as DENV vaccines approach licensure and implementation. In this analysis we provide evidence that for secondary DENV infections, individuals are more likely to be subclinically infected with a shorter interval between infections and with a more robust antibody response to the previous infection. This study suggests that there may exist a temporary period of cross-protection following DENV infection, using data on natural human infections on the individual level.
Supplementary Material
Notes
Acknowledgments. We thank Alan Rothman, John McGowan, Ruth Berkelman, and Dana Flanders for their careful review and helpful comments on the analysis and the article. We thank the staff at the Department of Virology, Armed Forces Research Institute of Medical Science (Bangkok, Thailand) for their careful diagnostic testing and data collection and entry. We acknowledge the support of the Office of the Provincial Public Health, Kamphaeng Phet province, and the clinical research nurses at AFRIMS and the support staff at the Kamphaeng Phet Field Station for all their efforts. This project and publication was made possible by Dissertation grant 1R36CK00104 from the Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH) grant P01 AI034533, the Military Infectious Diseases Research Program (MIDRP) and the United States Army Medical Research and Materiel Command, Ft Detrick, MD, USA. The opinions expressed in this manuscript do not necessarily represent the official views of the US NIH, the US Department of Defense, or the US Department of the Army.
Financial support. The clinical trial was supported by the United States Army Medical Research and Materiel Command, Ft Detrick, MD, USA. The analysis was supported by Dissertation grant 1R36CK00104 from the CDC, NIH Grant P01 AI034533, and the Military Infectious Diseases Research Program (MIDRP). The opinions expressed in this manuscript do not necessarily represent the official views of the US Department of Defense, or the US Department of the Army.
Financial Disclosure. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix.
Multivariate Logistic Model Output
| Parameter | Coding | Coefficient | Standard Error | P Value** |
|---|---|---|---|---|
| Outcome: Symptomatic Infection | 1 = Symptomatic infection in active surveillance window (n = 34) | … | … | … |
| 0 = Subclinical infection in active surveillance window (n = 89) | ||||
| Enrollment antibody profile (“Some_imm”) | 1 = HI-negative | 1.593 | 0.467 | <.001 |
| 0 = HI-negative | ||||
| Two years post-1st infection† (“Time2”) | 1 = Infected in year 2 | 0.193 | 0.305 | .379 |
| 0 = Infected in year 1 or 3 | ||||
| Three years post-1st infection (“Time3”) | 1 = Infected in year 3 | 0.195 | 0.391 | .734 |
| 0 = Infected in year 1 or 2 | ||||
| Some_imm*Time2 | Interaction term for 2 years post-1st infection and immunity | 0.651 | 0.317 | .065 |
| Some_imm*Time3 | Interaction term for 3 years post-1st infection and immunity | 0.921 | 0.385 | .011 |
| Rise in summed HI titers following the 1st infection | 1 = Less than or equal to the mean | 0.643 | 0.317 | .040 |
| 0 = Greater than the mean |
Abbreviation: HI, hemagglutination inhibition.
* Conditioned on subdistrict of residence and study period of enrollment (KPS1 or KPS2).
** Exact P values were calculated.
† Year 4 was not assessed in this model because no subclinical infections occurred 4 years after the first.
References
- 1.US Department of Health and Human Services NIoH, National Institute of Allergy and Infectious Disease. NIAID Biodefense Research Agenda for CDC Category A Agents: 2006 Progress Report
- 2.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970;42:311–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Hammon WM, Rudnick A, Sather GE. Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science. 1960;131:1102–3. doi: 10.1126/science.131.3407.1102. [DOI] [PubMed] [Google Scholar]
- 4.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 5.Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 6.Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 7.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–84. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. Immunological parameters of togavirus disease syndromes. Togaviruses. 1980:107–73. [Google Scholar]
- 9.Guzman MG, Kouri G, Valdes L, et al. Epidemiologic studies on dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–9. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 10.Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–3. [PubMed] [Google Scholar]
- 12.Halstead SB. Dengue haemorrhagic fever—a public health problem and a field for research. Bull World Health Organ. 1980;58:1–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:S830–9. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 14.Kou Z, Quinn M, Chen H, et al. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol. 2008;80:134–6. doi: 10.1002/jmv.21051. [DOI] [PubMed] [Google Scholar]
- 15.Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 16.Halstead SB, Lan NT, Myint TT, et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–9. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Kouri G, Mas P, Guzman MG, Soler M, Goyenechea A, Morier L. Dengue hemorrhagic fever in Cuba, 1981: rapid diagnosis of the etiologic agent. Bull Pan Am Health Organ. 1983;17:126–32. [PubMed] [Google Scholar]
- 19.Kouri G, Guzman MG, Valdes L, et al. Reemergence of dengue in Cuba: a 1997 epidemic in Santiago de Cuba. Emerg Infect Dis. 1998;4:89–92. doi: 10.3201/eid0401.980111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman MG, Alvarez M, Rodriguez-Roche R, et al. Neutralizing antibodies after infection with dengue 1 virus. Emerg Infect Dis. 2007;13:282–6. doi: 10.3201/eid1302.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mammen MP, Pimgate C, Koenraadt CJ, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmannitya S. Dengue hemorrhagic fever: diagnosis and management. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. Cambridge: CAB International; 1997. pp. 133–45. [Google Scholar]
- 23.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–73. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 24.Nisalak A, Endy TP, Nimmannitya S, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 25.Tien N, Luxemburger C, Toan N, et al. A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans R Soc Trop Med Hyg. 2010;104:592–600. doi: 10.1016/j.trstmh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Endy T, Anderson K, Nisalak A, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5:e975. doi: 10.1371/journal.pntd.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci U S A. 2008;105:2238–43. doi: 10.1073/pnas.0709029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Mammen MP, Jr, Chinnawirotpisan P, et al. Clade replacements in dengue virus serotypes 1 and 3 are associated with changing serotype prevalence. J Virol. 2005;79:15123–30. doi: 10.1128/JVI.79.24.15123-15130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thammapalo S, Chongsuvivatwong V, Geater A, Dueravee M. Environmental factors and incidence of dengue fever and dengue haemorrhagic fever in an urban area, Southern Thailand. Epidemiol Infect. 2007:1–9. doi: 10.1017/S0950268807008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durbin AP, Schmidt A, Elwood D, et al. Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis. 2011;203:327–34. doi: 10.1093/infdis/jiq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.