Abstract
We investigated thymidine kinase (tk) mutants isolated during multiple episodes of recurrent bilateral acyclovir resistant herpes simplex keratitis in an immunocompetent patient. From one eye, we found a single guanine insertion, previously shown to greatly reduce TK expression, and from the other, a previously unidentified substitution, which genetic experiments confirmed confers drug resistance. The substitution, although distant from substrate binding sites, reduced thymidine phosphorylation 10–20-fold, and acyclovir phosphorylation >100-fold. This phenotype should permit reactivation from latency to cause recurrent disease. The results may have implications for the prevalence and prevention of acyclovir resistance in patients with herpes simplex keratitis.
Keywords: herpes simplex virus, acyclovir, antiviral drug resistance, thymidine kinase, herpes simplex keratitits
Herpes simplex virus (HSV) causes a variety of diseases including the recurrent ulceration, inflammation, and scarring of the cornea known as herpes simplex keratitis, which is a major cause of blindness worldwide [1]. Providing the virus is susceptible, treatment with topical antiviral agents such as acyclovir (ACV) hastens resolution and improves outcome. Prophylaxis with oral ACV reduces the risk of recurrent disease [2]. An important limitation of ACV therapy, however, is ACV resistance (ACVr), which is generally considered to be much more important in immunocompromised patients than in immunocompetent hosts where ACVr isolates are rare and only weakly associated with treatment failures (reviewed in [3]).
Overall, 95% of clinical isolates exhibiting ACVr contain mutations in the viral thymidine kinase (tk) gene, which encodes the enzyme (TK) that phosphorylates the drug thereby activating it [3]. ACVr tk mutants are usually cross-resistant to other drugs that depend on TK for activation, so when a patient fails to respond to ACV, drugs such as topical foscarnet (FOS), which inhibits the viral DNA polymerase directly, are administered. ACVr tk mutants can totally lack TK activity (TK negative), have substantially reduced TK activity (TK low), or have TK that phosphorylates thymidine relatively efficiently, but not ACV (TK altered) [3]. Half of ACVr tk mutations are additions and deletions of nucleotides, frequently in runs of guanines and cytosines, and half are substitutions [3]. Substitution mutations in tk usually alter conserved regions including an ATP-binding site and a nucleoside binding site [3].
Differences among TK mutants affect viral pathogenesis. Although TK is dispensable for replication in cell culture or peripheral tissues, it is crucial for replication in neurons and essential for reactivation of well-studied HSV-1 strains from latency in mouse models (reviewed in [4]). To retain pathogenicity while evading drug action, different ACVr mutants use different strategies [4]. For example, the most clinically common ACVr mutant, termed G7 + 1G (an insertion of a G into a run of 7 guanines between nucleotides 430 and 437), expresses approximately 0.1% of the level of TK expressed by wild-type (WT) virus by ribosomal frameshifting [5]. These amounts are biologically relevant, because they permit some reactivation from latency in mouse ganglia [6]. TK altered viruses exhibit pathogenicities that are similar to or only slightly lower than that of WT in mouse models, and at least one such mutant was associated with ACVr genital herpes in an immunocompetent host (reviewed in [7]).
Here we report on an immunocompetent patient who suffered from recurrent bilateral herpes simplex keratitis despite years of antiviral treatment, and the ACVr mutants responsible for his disease.
MATERIALS AND METHODS
Materials and Methods are described in Supplementary Material online.
RESULTS
Virus Genotypes
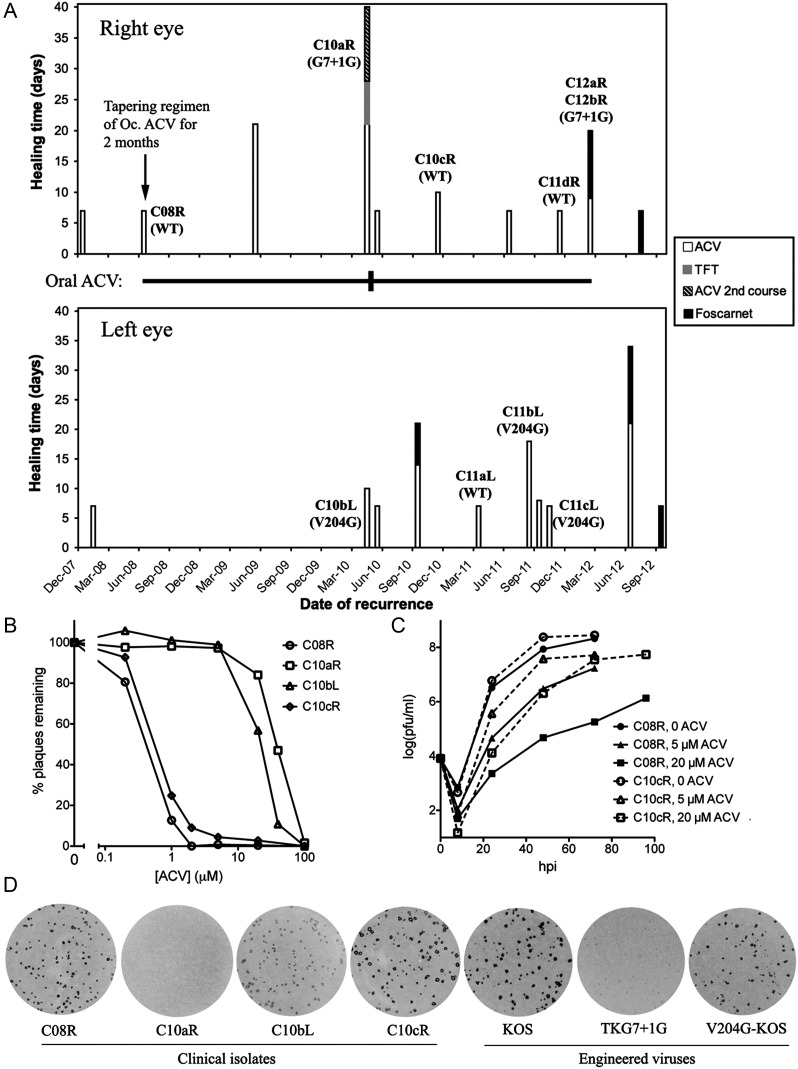
A history of an immunocompetent patient with recurrent bilateral herpes simplex keratitis is summarized in Figure 1A and is detailed in Supplementary Material online. Eye images from selected eposides are shown in Supplementary Figure 1. HSV-1 was isolated from the right eye in June 2008 prior to successful topical ACV therapy (C08R), April 2010 (C10aR), November 2010 (C10cR), November 2011 (C11dR), and February 2012 (C12aR and C12bR), and from the left eye in April 2010 (same episode as C10aR; C10bL), March 2011 (C11aL), September 2011 (C11bL), and October 2011 (C11cL). The tk genes from the isolates were amplified by PCR and sequenced. The C08R tk gene (GenBank accession no. KC881055) differed from all 393 other HSV-1 tk sequences in the National Center for Biotechnology Information (NCBI) database. It differed from the reference HSV-1 KOS strain tk gene by 15 nucleotides. C08R was susceptible to ACV, with a dose that reduced plaque formation by 50% (ED50) similar to that of KOS (Table 1, Figure 1B). Thus, the differences with KOS are TK polymorphisms, and C08R serves as the WT strain for this set of isolates.
Figure 1.
Characterization of isolates from the patient. A, Time lines of disease and drug treatment for the right eye (top) and the left eye (bottom). Each vertical bar represents an episode, with the length of the bar indicating the healing time following administration of different topical drugs (as indicated in the key to the right of the panels). The topical antivirals were discontinued within a week following healing of the ulcer except for the June 2008 episode when the topical (Oc.) ACV was tapered over a 2-month period as indicated by the arrow. The horizontal line between the 2 panels indicates the regimen of systemic oral ACV with the thin line representing 400 mg BD and the short thicker vertical line representing 800 mg 5 times a day for 10 days. Isolate names are labeled above or next to the bars for the episodes whose isolates were obtained. Tk genotypes of the isolates are shown below isolate names in parentheses, with WT meaning the sequence of the C08R isolate. C08R differs from KOS in tk by 6 silent nucleotide changes (A528G, C672T, T694C, G723A, T915C, C1053T) and 9 amino acid changes (C6G, R41H, Q89R, A192V, G251V, V267L, P268T, D286E, N376H). C12aR and C12bR were obtained 7 days separate from each other in the same episode. B, Plaque reduction assay showing ACV susceptibilities of the clinical isolates. Plaque counts are normalized to the non-drug control. The symbols representing the viruses are indicated to the right of the panel. C, Replication kinetics of C08R and C10cR in Vero cells in the presence or absence of ACV (MOI = 0.02). Solid lines represent C08R, and dashed lines represent C10cR. The symbols representing different ACV concentrations are indicated to the right of the panel. D, Plaque autoradiography showing in situ thymidine phosphorylation activities of plaques. The virus names are below each autoradiograph. Abbreviations: ACV, acyclovir; MOI, multiplicity of infection; WT, wild type.
Table 1.
Drug Susceptibility and TK Activity Assays of the Clinical Isolates and the Laboratory Engineered Virusesa
| Viruses | C08R | C10aR | C10bL | C10cR | KOS | TKG7 + 1G | V204G-KOS |
|---|---|---|---|---|---|---|---|
| ED50 ACV (µM)b | 0.39 ± 0.06 | 41 ± 5 | 25 ± 6 | 0.6 ± 0.1 | 0.40 ± 0.07 | 70 ± 20 | 50 ± 10 |
| ED50 foscarnet (µM) | 200 ± 40 | 180 ± 20 | 220 ± 40 | 140 ± 40 | 350 ± 50 | n.d. | n.d. |
| ED50 TFT (µM) | 2.0 ± 0.2 | 4.2 ± 0.8 | 4.0 ± 0.7 | 1.9 ± 0.1 | 3.5 ± 0.7 | n.d. | n.d. |
| Relative thymidine phosphorylation activityc | 140 ± 10 | <0.3 | 10.3 ± 1.4 | n.d. | 100 | <0.3 | 4.7 ± 0.6 |
| Relative ACV phosphorylation activity | 73 ± 3 | <1 | <1 | n.d. | 100 | <1 | <1 |
Abbreviations: ACV, acyclovir; n.d., no data; TFT, trifluorothymidine.
a Each value was obtained by 2 to 3 independent experiments with duplicates for each experiment. Mean values ± standard deviations are shown.
b ED50 values were obtained by fitting the normalized plaque reduction curves with the following equation in the program Prism: y = Max/(1 + (x/ED)50−Hillslope).
c Reaction rates were calculated from the initial slopes plotting production of phosphorylated thymidine or ACV as a function of enzyme reaction time, and normalized to the values for KOS.
Compared to C08R, the tk sequences of isolates C10aR, C12aR, and C12bR from the right eye contained the aforementioned common single mutation, the nucleotide insertion, G7 + 1G. In contrast, the tk sequences of C10bL, C11bL, and C11cL from the left eye, contained a single point mutation, T611G, resulting in a valine to glycine substitution at amino acid residue 204 (V204G), which, to our knowledge, has never been reported (GenBank accession no. KC881056). The remaining 3 isolates, C10cR, C11aL, and C11dR, had the same tk sequence as C08R (but see below).
Drug Susceptibilities
Five isolates, C08R, C10aR, C10cR, C10bL, and C11cL, gave rise to amplifiable virus. In plaque reduction assays, C10cR was inhibited as potently by ACV as C08R and WT reference strain KOS, with an ED50 < 1 µM (but see below), whereas isolate C10aR with the G7 + 1G mutation and isolate C10bL with the V204G mutation had ED50 values 50–100-fold higher (Figure 1B, Table 1). All 5 clinical isolates tested were susceptible to FOS, consistent with the apparent efficacy of FOS in treating the patient's disease, and exhibited approximately 2-fold differences in ED50 for TFT (Table 1).
Interestingly, C10cR showed a higher proportion of plaques at concentrations of 1–20 µM ACV than the 2 WT viruses (Figure 1B). We therefore assayed the replication of C08R and C10cR in different concentrations of ACV following infection at a low multiplicity of infection (MOI) (Figure 1C). In the absence of the drug, the 2 viruses grew similarly, but in the presence of 5 µM or 20 µM ACV, C10cR replicated to titers that were 1–2 orders of magnitude higher than those of C08R at 24–72 hours postinfection, consistent with C10cR containing a larger subpopulation of ACVr mutants than C08R.
Engineering V204G
We engineered the V204G substitution into the KOS strain. The resulting mutant virus, V204G-KOS, had an ACV ED50 50- to 100-fold higher than that of KOS (Table 1) and similar to that of TKG7 + 1G, a previously constructed KOS mutant containing the G7 + 1G mutation (TKG7 + 1G) [6]. Thus, the V204G mutation confers ACVr.
TK Activities
We performed 3H thymidine plaque autoradiography, in which TK activities in infected cells are reflected by the intensities of the autoradiographic signals over the plaques (Figure 1D). The plaques from isolates C08R and C10cR, which are predominantly ACV sensitive, gave slightly weaker signals than those from KOS, which is likely due to their forming smaller plaques than KOS. Consistent with this, C10aR, which contains the G7 + 1G mutation showed no signal even though TKG7 + 1G's signal was low but detectable (Figure 1D). Interestingly, for both the clinical strain and the KOS strain, the V204G mutants showed signals only slightly weaker than those of their corresponding WT viruses.
Viruses that exhibit as little as 5%–10% TK expression can give strong plaque autoradiographic signals [8]. We therefore performed TK enzyme assays. In these assays, the rates of phosphorylation of thymidine by extracts of cells infected with C10bL, which contains the V204G mutation, and V204G-KOS were 7% and 5% those of cells infected with C08R and KOS, respectively (Table 1). The rates of thymidine phosphorylation by extracts of cells infected with C10aR and TKG7 + 1G were no greater than those in extracts of mock-infected cells, <0.3% of KOS, consistent with the TKG7 + 1G mutant expressing approximately 0.1% as much TK protein as KOS [5]. We then measured ACV phosphorylation activities using the enzyme assay. Extracts of cells infected with KOS and C08R phosphorylated ACV similarly (Table 1). However, ACV phosphorylation from extracts of cells infected with the G7 + 1G or V204G mutants of either strain was not detected above that of extracts of mock-infected cells (approximately 1% of WT-infected cells). We could detect ACV phosphorylation in extracts from mock-infected cells spiked with 1% vol/vol with extracts from cells infected with either WT virus (not shown). These results indicate that the ACV phosphorylation activities of V204G and C10bL are, conservatively, <1% those of their respective WT strains (Table 1). Thus, the V204G mutation not only reduced TK's enzyme activity but also altered its substrate specificity to drastically reduce drug activation.
DISCUSSION
We have characterized ACVr and ACV susceptible HSV-1 isolates obtained over the course of 4 years from an immunocompetent patient with bilateral herpetic eye disease. Recurrences associated with ACVr virus usually healed more slowly with ACV therapy than did those with ACV susceptible virus. Based on our results, we infer that the same ACV susceptible virus infected both eyes, established latency in each of the trigeminal ganglia innervating the eyes, and that the 2 ACVr mutants evolved separately. This differs from what was observed in a recent analysis of autopsy specimens from 5 individuals where the same major ACVr tk variant was found in both ganglia from each individual [9]. ACVr emerged after suppressive oral ACV prophylaxis for nearly 2 years. Although this is standard practice based on a clinical trial, the dose used often does not suppress recurrences of HSV eye disease [2]. We speculate that this contributed to the emergence of resistance.
Some isolates contained mainly WT virus, even in the face of prophylactic ACV. This is consistent with the results of a survey of sequential isolates from cases of herpetic keratitis [10]. Such recurrences of WT virus could be due to insufficient concentrations of ACV in infected tissue or to the presence of subpopulations of ACVr virus that can complement WT virus for drug resistance and pathogenesis [7]. Indeed, isolate C10cR appeared to include such an ACVr subpopulation. Analyses of autopsy specimens have shown the coexistence of tk mutant and WT viral genomes in individual human trigeminal ganglia [9].
The residual thymidine phosphorylation of the V204G mutant (5%–10%) should permit considerable viral pathogenicity, as a mutant with similar thymidine phosphorylation, exhibits a WT phenotype for acute and latent infections in a mouse model [11]. Indeed, even viruses expressing vastly less TK can reactivate from latency in mouse ganglia, albeit inefficiently [5, 6, 8].
In the crystal structure of HSV-1 TK, the V204 residue is situated within a β-strand distant from the nucleoside binding site, the ATP binding site or the dimer interface [12] (Supplementary Figure 2A). Val204 makes van der Waals contacts with Val52, Leu327, and Met347, respectively (Supplementary Figure 2B). A Gly204 residue would lose these contacts and might render the β-strand more labile. Therefore, the mutation may affect the overall integrity and/or the dynamics of the enzyme, explaining V204G's reduced thymidine phosphorylation activity. Notably, the crystal structures of TK in complex with ACV and TK in complex with thymidine show that fewer contacts are made between the enzyme and ACV than with thymidine [12]. We therefore hypothesize that perturbations brought about by the mutation might affect ACV phosphorylation much more than thymidine phosphorylation because thymidine's multiple interactions with the enzyme could still permit productive binding.
ACVr HSV has rarely been isolated from immunocompetent patients and has even more rarely been associated with ACVr disease [3]. It is plausible that the V204G mutant retains enough pathogenicity to cause recurrent disease in an immunocompetent setting but is considerably less plausible for the G7 + 1G mutant, which expresses very low levels of TK [5] and reactivates from latency only weakly in animal models of HSV disease [6]. Perhaps these viruses contain alleles in other genes that compensate for the loss of TK (reviewed in [4]).
A final possibility is that ACVr virus is much more likely to cause recurrent corneal disease than other diseases such as herpes labialis or herpes genitalis, on which most previous studies of the prevalence of ACVr in immunocompetent individuals have focused [3]. This possibility is supported by a variety of case reports [3, 13, 14] and a 2010 study that indicated a high prevalence (6.4%) of ACVr herpes simplex keratitis in seemingly otherwise healthy patients [15]. The authors’ hypothesis that recurrent ACVr disease is facilitated by the immune-privileged status of the cornea deserves strong consideration.
FOS and other currently approved drugs that are active against most ACVr mutants have major limitations in terms of toxicities and pharmacokinetic liabilities that prevent their prophylactic use, and are not active against all ACVr mutants [3]. Especially if ACVr corneal disease is much more common than previously appreciated, new antiviral anti-HSV drugs are needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank our colleagues and friends David Leib (for arranging the collaboration between Boston and Liverpool) and Clyde Crumpacker (for helpful comments on the article). We also acknowledge with regret that we were unable to cite many relevant papers due to the constraints of the Brief Report format.
Financial support. This work was supported by grants from the National Institute of Allergy and Infectious Diseases; and National Institute of Neurological Diseases and Stroke (grants R01 AI26126, P01 NS35138) at the National Institutes of Health to D. M. C.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–80. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–6. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 3.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–72. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths A. Slipping and sliding: frameshift mutations in herpes simplex virus thymidine kinase and drug-resistance. Drug Resist Updat. 2011;14:251–9. doi: 10.1016/j.drup.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D, Coen DM. Quantification and analysis of thymidine kinase expression from acyclovir-resistant G-string insertion and deletion mutants in herpes simplex virus-infected cells. J Virol. 2012;86:4518–26. doi: 10.1128/JVI.06995-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths A, Chen SH, Horsburgh BC, Coen DM. Translational compensation of a frameshift mutation affecting herpes simplex virus thymidine kinase is sufficient to permit reactivation from latency. J Virol. 2003;77:4703–9. doi: 10.1128/JVI.77.8.4703-4709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen DM. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 1994;2:481–5. doi: 10.1016/0966-842x(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths A, Coen DM. High-frequency phenotypic reversion and pathogenicity of an acyclovir-resistant herpes simplex virus mutant. J Virol. 2003;77:2282–6. doi: 10.1128/JVI.77.3.2282-2286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Velzen M, van Loenen FB, Meesters RJ, et al. Latent acyclovir-resistant herpes simplex virus type 1 in trigeminal ganglia of immunocompetent individuals. J Infect Dis. 2012;205:1539–43. doi: 10.1093/infdis/jis237. [DOI] [PubMed] [Google Scholar]
- 10.Duan R, de Vries RD, van Dun JM, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–14. doi: 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- 11.Coen DM, Irmiere AF, Jacobson JG, Kerns KM. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989;168:221–31. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- 12.Bennett MS, Wien F, Champness JN, et al. Structure to 1.9 A resolution of a complex with herpes simplex virus type-1 thymidine kinase of a novel, non-substrate inhibitor: X-ray crystallographic comparison with binding of aciclovir. FEBS Lett. 1999;443:121–5. doi: 10.1016/s0014-5793(98)01619-6. [DOI] [PubMed] [Google Scholar]
- 13.Choong K, Walker NJ, Apel AJ, Whitby M. Aciclovir-resistant herpes keratitis. Clin Experiment Ophthalmol. 2010;38:309–13. doi: 10.1111/j.1442-9071.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 14.Burrel S, Boutolleau D, Azar G, et al. Phenotypic and genotypic characterization of acyclovir-resistant corneal HSV-1 isolates from immunocompetent patients with recurrent herpetic keratitis. J Clin Virol. 2013;58:321–4. doi: 10.1016/j.jcv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–63. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.