Abstract
Background. Factors responsible for myeloid-derived suppressor cell (MDSC) expansion and T-cell dysfunction during human immunodeficiency virus type 1 (HIV) infection are unknown. This study investigated the role of MDSCs during HIV infection.
Methods. Peripheral blood mononuclear cells (PBMCs) were cultured with gp120 and infectious or inactivated HIV, with or without anti–interleukin 6 (IL-6) antibody. CD33+, CD4+, and CD8+ cells were isolated from PBMCs and cocultured in the presence or absence of inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and arginase 1 inhibitors. CD11b+CD33+CD14+HLA-DR−/lo MDSCs, phosphorylated STAT3 (pSTAT3), and CD4+CD25+FoxP3+ cells were evaluated by flow cytometry. IL-6, interferon γ (IFN-γ), interleukin 10 (IL-10), and gp120 levels were quantified by an enzyme-linked immunosorbent assay.
Results. MDSCs expanded when PBMCs were exposed to infectious or inactivated HIV. Exposure to gp120 led to MDSC expansion, with increases in IL-6 levels and pSTAT3 expression. Anti–IL-6 abrogated MDSC expansion and pSTAT3 expression. gp120-expanded CD33+ MDSCs inhibited IFN-γ release from autologous T cells, which was restored upon ROS and iNOS inhibition. gp120-expanded CD33+ MDSCs increased IL-10 and CD4+CD25+FoxP3+ regulatory T-cell levels in CD4+ T-cell cocultures. Finally, high frequencies of MDSCs were present in HIV–infected persons, compared with healthy controls.
Conclusions. These findings demonstrate that HIV gp120 induces IL-6 and MDSC expansion, which contributes to immune suppression by modulating cytokine and cellular responses.
Keywords: HIV-1, gp120, IL-6, Myeloid Derived Suppressor Cells
Human immunodeficiency virus type 1 (HIV) infection causes profound immune suppression leading to progressive destruction of the immune system in untreated patients. Recently, a subset of myeloid cells known as myeloid-derived suppressor cells (MDSCs) was found to negatively regulate immune functions [1, 2]. MDSCs express common myeloid markers (CD11b+CD33+HLA-DR−/lo) [1–3] and, depending on the presence of CD15 or CD14, are divided into granulocytic or monocytic subsets, respectively [3–5]. These cells suppress innate and adaptive immunity either by arginase 1 (Arg1), reactive oxygen species (ROS), and reactive nitrogen species generation and/or by mediation of CD4+CD25+FoxP3+ regulatory T-cell (Treg) expansion [6–10]. Expansion and function of MDSCs is regulated by the transcription factor STAT3, which induces expression of antiapoptotic genes and prevents differentiation of myeloid progenitor cells into mature myeloid cells [1]. Considerable research, predominantly performed in animal models, has demonstrated inhibition of antitumor and antimicrobial activity by MDSCs. Additionally, recent evidence suggests that this inhibitory activity is present in patients with certain malignancies [5, 6, 9, 10]. Limited information is available on the role of MDSCs in human infections, including HIV infection. In vitro and in vivo differentiation of MDSCs is regulated by various cytokines, including interleukin 6 (IL-6), interleukin 10 (IL-10), prostaglandins, stem-cell factor, granulocyte macrophage colony-stimulating factor, transforming growth factor β, vascular endothelial growth factor, and tumor necrosis factor α (TNF-α) [8, 9]. Levels of a number of these cytokines, including IL-6, are elevated in serum and cerebrospinal fluid of HIV–infected persons. Levels of IL-6 decline following successful suppression of replicating HIV with administration of combination antiretroviral therapy, suggesting the potential detrimental effects of IL-6 in HIV pathogenesis [11–14].
In the present study, we hypothesized that IL-6 produced during HIV infection drives the expansion of MDSCs, which contributes to HIV–associated immune suppression. Our findings indicate that when peripheral blood mononuclear cells (PBMCs) are exposed to HIV gp120, inhibitory MDSCs expand in an IL-6–dependent manner, suppressing T-cell functions and inducing regulatory T-cell expansion. Additionally, an increased number of MDSCs was observed in HIV–infected persons with detectable viral loads.
MATERIALS and METHODS
Patient Population
Blood was obtained after receipt of informed consent from HIV–seronegative donors and untreated HIV–infected persons. All studies were approved by the institutional review board of the University of California, San Diego. Cell culture studies were conducted on blood specimens from healthy donors. MDSC numbers were evaluated in the blood of HIV–infected persons.
Cell Isolation and Culture
PBMCs were isolated from freshly obtained blood by Ficoll density centrifugation (GE Healthcare, Uppsala, Sweden) and cultured in Roswell Park Memorial Institute 1640 medium (Gibco) and 10% human serum (MP Biomedicals, Solon, OH) in the presence or absence of 1 µg/mL gp120 or gp41 (both from Abcam, Cambridge, MA) or HIVBaL (obtained through the National Institutes of Health AIDS Research and Reference Reagent Program).
Antibodies and Other Reagents
Antibodies used for flow cytometry were fluorescein isothiocyanate (FITC)–anti-CD11b, phycoerythrin (PE)–anti-HLA-DR, PE/Cy7–anti-CD14, APC–anti-CD33, PerCP/Cy5.5–anti-CD33 (all from Biolegend, San Diego, CA); FITC–anti-CD4, APC–anti-CD25, PE–anti-FoxP3, and APC–anti-IL-10 (all from eBioscience, San Diego, CA); and Alexa Fluor 647–anti-phospho-STAT3 (BD Biosciences). For neutralization studies, monoclonal antibody to IL-6 or isotype control was used (10 µg/mL; Biolegend, San Diego, CA). Chemical inhibitors used were catalase (Sigma-Aldrich, St. Louis, MO), NG-monomethyl-L-arginineacetate (Sigma-Aldrich), and N(ϖ)-hydroxy-nor-L-arginine (Cayman Chemicals, Ann Arbor, MI).
Isolation of CD4, CD8, and MDSCs
CD33+, CD4+, and CD8+ cells were isolated from PBMCs, using magnetic beads conjugated to anti-CD33, anti-CD4, and anti-CD8 (Miltenyi Biotec, Auburn, CA), respectively. Selected cells were >95% positive.
Immunolabeling and Flow Cytometry
Cells were surface stained for CD11b+, CD14+, CD33+, HLA-DR−/lo, and CD4+, using cell staining buffer (Biolegend, San Diego, CA). For intracellular phosphorylated STAT3 (pSTAT3), cells were fixed with fixation buffer and permeabilized with Phosphoflow Perm Buffer III (both from BD Biosciences), followed by staining with AF647-mouse anti-STAT3 or AF647-mouse immunoglobulin G2a. CD4+ cells cocultured with CD33+ cells were stained for intracellular FoxP3 and IL-10, using the Cytofix/Cytoperm Plus kit and anti-FoxP3 and anti–IL-10 (all from eBioscience, San Diego, CA). Flow cytometry was done on a FACS Calibur, and data were analyzed using CellQuest Pro (BD Biosciences). Controls for each experiment included unstained cells, cells that were single stained for either surface markers or intracellular proteins, and isotype-matched antibodies. For MDSC analysis, a total of 105 cells were used, and relative numbers were calculated per 105 cells. Absolute MDSC numbers in HIV–infected persons were calculated as follows: [(total white blood cell count) × (percentage of MDSCs)]/100.
Coculture of CD4+, CD8+, and CD33+ Cells
A total of 2.5 × 105 CD33+ cells were cocultured with 5 × 105 autologous CD4+ or CD8+ cells for 3 days in the presence of anti-CD3 (5.0 µg/mL) and anti-CD28 (2 µg/mL; both from eBioscience, San Diego, CA). Plates were centrifuged, and supernatants were harvested and analyzed for cytokines by enzyme-linked immunosorbent assay (ELISA). In some experiments, CD33+ cells were cultured in transwell inserts (pore diameter, 0.4 µm) and CD4+ or CD8+ T cells in wells of 24-well plates (Costar, Milpitas, CA) for 3 days.
Quantification of Cytokines and gp120
Supernatants collected and stored at −80°C were used to determine levels of IL-6, IFN-γ, and IL-10 by ELISA (Biolegend, San Diego, CA). Plasma IL-6 levels were measured using the IL-6HS kit (R&D Systems, Minneapolis, MN). HIV gp120 was quantified in plasma, using the HIV gp120 Clade B ELISA kit (Immune Technology, New York, NY).
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA from CD33+ cells was quantified by qPCR. Primers and hydrolysis probe sequences used for p47phox, inducible nitric oxide synthase (iNOS), Arg1, and hypoxanthine-guanine phosphoribosyltransferase (Supplementary Methods) were synthesized from TIB MOLBIOL.
Statistical Analysis
Data are expressed as mean values ± standard error of the mean (SEM). Paired Student t tests were used to determine the statistical significance for in vitro experiments. The MDSC frequency between healthy donors and patients was compared by the nonparametric Mann–Whitney U test. Statistical analysis was performed using Prism 5 (La Jolla, CA). A P value of <.05 was considered statistically significant.
RESULTS
MDSCs Expand Following Exposure to HIV
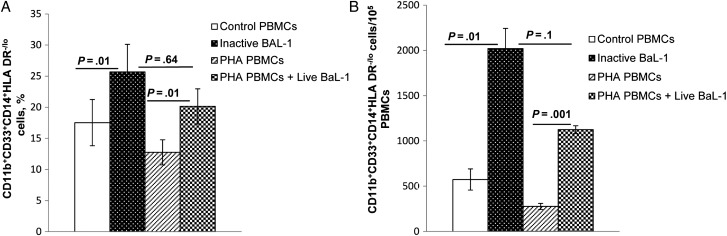
To investigate whether MDSCs expand when exposed to HIV, we treated PBMCs from healthy donors with heat-inactivated or infectious HIV and examined the frequency of CD11b+CD33+CD14+HLA-DR−/lo cells. There was a significantly increased expansion of monocytic MDSCs following exposure to infectious HIV (mean [±SEM], 12.75% ± 2.02% among unexposed PBMCs vs 20.14% ± 2.82% among exposed PBMCs; P = .01) and to heat-inactivated virus (mean [±SEM], 17.53% ± 3.72% among unexposed PBMCs vs 25.66% ± 4.45% among exposed PBMCs; P = .01; Figure 1A) at a multiplicity of infection of 0.01. This was associated with increased numbers of CD11b+CD33+CD14+HLA-DR−/lo cells (Figure 1B). Total MDSC expansion upon exposure to heat-inactivated HIV was comparable to total MDSC expansion in infected cells (P = .64 and P = .1), suggesting that MDSC expansion does not require viral replication.
Figure 1.
CD11b+CD33+CD14+HLA-DR−/lo myeloid-derived suppressor cell expansion by human immunodeficiency virus type 1 does not require viral replication. Peripheral blood mononuclear cells (PBMCs) from healthy donors were treated with heat-inactivated HIVBaL (multiplicity of infection, 0.01) or stimulated with phytohemagglutinin (PHA; 10 µg/mL; Sigma–Aldrich, St. Louis, MO) for 48 hours and infected with HIVBaL (multiplicity of infection, 0.01) in the presence of recombinant interleukin 2 (10 units/mL; Roche Diagnostics, Mannheim, Germany). After 5 days, the percentages of CD11b+CD33+CD14+HLA-DR−/lo cells (A) and the relative numbers of CD11b+CD33+CD14+HLA-DR−/lo cells (B) were determined and compared to those for controls. Mean values and standard errors of the mean are shown for 4 healthy donors.
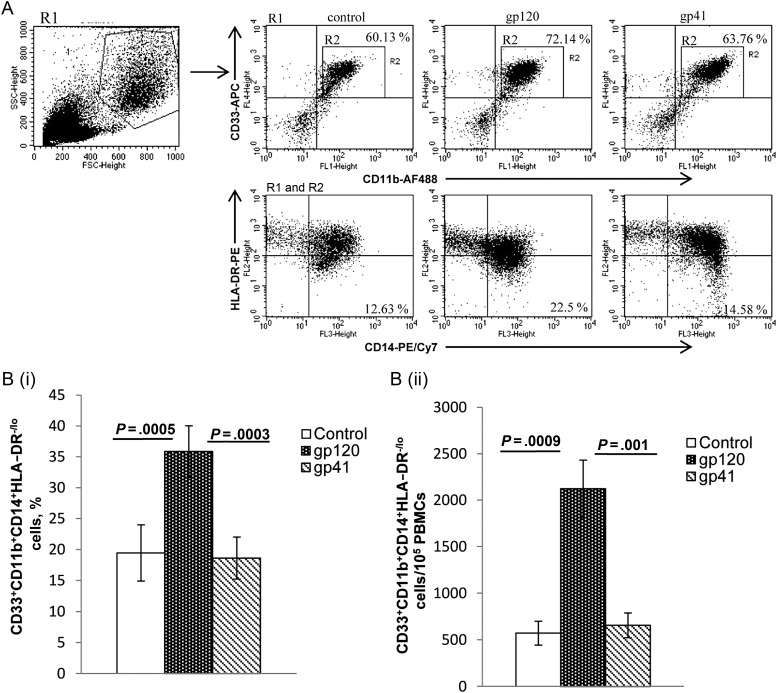
Exposure of PBMCs to HIV gp120 but Not HIV gp41 Expands MDSCs
To examine the role of HIV surface proteins in the expansion of MDSCs, PBMCs were cultured in the presence of gp120 or gp41. When PBMCs were treated with gp120, there was expansion of CD33+CD11b+CD14+HLADR−/lo cells, compared with untreated controls and gp41-treated PBMCs (mean [±SEM], 35.9% ± 4.17% among gp120-treated PBMCs vs 19.4% ± 4.54% among untreated PBMCs [P = .0005] and 18.6% ± 3.4% among gp41-treated PBMCs [P = .0003]; Figure 2A and 2Bi). This was associated with increased numbers of CD11b+CD33+CD14+HLA-DR−/lo cells (Figure 2Bii). MDSC expansion with gp120 exposure increased significantly by day 3 and peaked at day 5.
Figure 2.
Effect of envelope protein gp120 on expansion of CD11b+CD33+CD14+HLA-DR−/lo myeloid-derived suppressor cells. Peripheral blood mononuclear cells from healthy donors were treated with gp120 (1 µg/mL) or gp41 (1 µg/mL) and compared to untreated controls. After 5 days, cells were analyzed by flow cytometry using logical gating. A, Representative dot plot is shown. B and C, Percentages (B) and relative numbers (C) of CD11b+CD33+CD14+HLA-DR−/lo cells were determined. Mean values and standard errors of the mean are shown for 9 healthy donors. Abbreviation: PE, phycoerythrin.
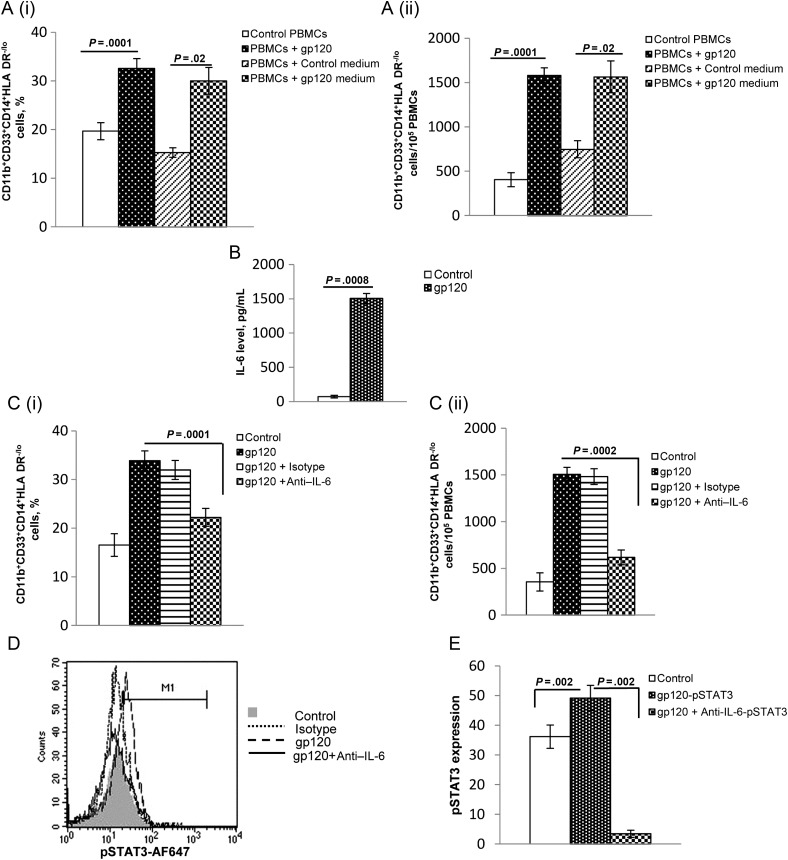
Soluble Factors Mediate MDSC Expansion
We next examined whether soluble factors produced by gp120 are responsible for the generation of MDSCs during HIV infection. PBMCs were cultured in control medium or gp120-conditioned culture supernatant for 5 days, and the expansion of CD11b+CD33+CD14+HLA-DR−/lo cells was assessed by flow cytometry. As previously observed, CD11b+CD33+CD14+HLA-DR− /lo cells expanded when PBMCs were treated with gp120, compared with controls (mean [±SEM], 19.7 ± 1.75 vs 32.6 ± 2.0; P = .0001). Importantly, a significant expansion of MDSCs was observed when PBMCs were cultured in gp120-conditioned culture medium, compared with control medium (mean [±SEM], 15.3 ± 2.0 vs 30.0 ± 2.75; P = .02; Figure 3Ai and 3Aii). These findings suggest that soluble factors produced by gp120 mediate MDSC expansion.
Figure 3.
Soluble factors from gp120-treated peripheral blood mononuclear cells (PBMCs) mediate CD11b+CD33+CD14+HLA-DR−/lo myeloid-derived suppressor cell (MDSC) expansion. A, PBMCs were cultured in the absence or presence of gp120 (1 µg/mL), and untreated (control medium), and gp120-treated (gp120 medium) supernatants were used to culture PBMCs from different healthy donors. As additional controls, PBMCs from the same donors were left untreated or were treated with gp120 (1 µg/mL). After 5 days, the percentages (i) and relative numbers (ii) of CD11b+CD33+CD14+HLA-DR−/lo cells were determined by flow cytometry. Mean values and standard errors of the mean (SEM) from 3 donors are shown. B, PBMCs from healthy donors were left untreated or were treated with gp120. After 5 days, interleukin 6 (IL-6) concentrations were determined in culture supernatants by enzyme-linked immunosorbent assay. C, IL-6–neutralizing antibody was added to cultures to determine the effect of blocking IL-6 on MDSC expansion. PBMCs from healthy donors were cultured in medium alone or with gp120 (1 µg/mL) and either anti–IL-6 (10 µg/mL) or isotype control antibody (10 µg/mL) was added to some gp120 treated wells. After 5 days, the percentages (i) and relative numbers (ii) of CD11b+CD33+CD14+HLA-DR−/lo cells were determined. Mean values and SEM are shown for 4 healthy donors. D and E, PBMCs were treated with gp120 in presence of IL-6–neutralizing antibody for 5 days. Cells were surface stained with anti-CD11b and anti-CD33, followed by intracellular staining with anti-pSTAT3 or isotype control antibody. Phosphorylated STAT3 (pSTAT3) was analyzed in CD11b+CD33+ cells by flow cytometry. A representative histogram is shown. Abbreviation: MFI, mean fluorescence intensity, and mean values and SEM are shown for 4 healthy donors.
IL-6 Induction by gp120 Is Responsible for Expansion of MDSCs
To evaluate whether gp120-induced IL-6 activity leads to CD11b+CD33+CD14+HLA-DR−/lo cell expansion, we quantified IL-6 in supernatants of gp120-treated PBMCs. These supernatants had significantly increased levels of IL-6, compared with control PBMCs (mean [±SEM], 73 ± 20 vs 1503 ± 74 pg/mL; P = .0008; Figure 3B). Additionally, neutralization of IL-6 in gp120 treated PBMCs inhibited expansion of CD33+CD11b+CD14+HLA-DR−/lo cells, compared to gp120 treated PBMCs without IL-6 neutralization (mean [±SEM], 34.0% ± 2% vs 22.0% ± 1.8%; P = .0001; Figure 3Ci and Cii).
To determine whether MDSC expansion during HIV infection is regulated by IL-6–mediated STAT3 [15], we assessed pSTAT3 expression in gp120-expanded myeloid suppressor cells. A significant increase in pSTAT3 mean fluorescence intensity was observed in PBMCs treated with gp120, compared to untreated cells (mean [±SEM], 36.1 ± 3.9 vs 49.2 ± 4.25; P = .002); furthermore, neutralization of IL-6 completely abrogated pSTAT3 expression, compared with cells unexposed to anti–IL-6 (mean [±SEM], 49.2 ± 4.25 vs 3.5 ± 1.2; P = .002; Figure 3D and 3E).
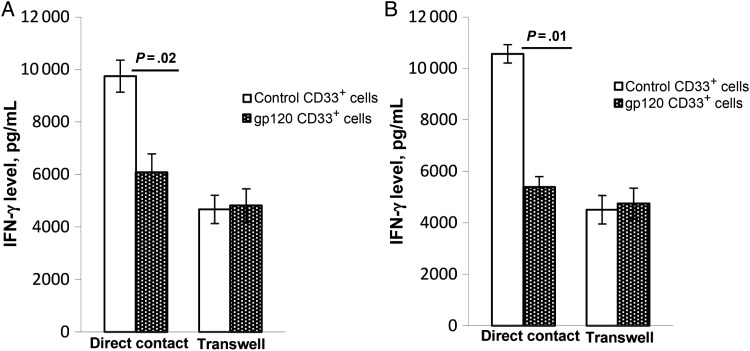
gp120-Expanded CD33+ Cells Inhibit CD4+ and CD8+ T-Cell Function and Require Cell-to-Cell Contact
Since STAT3 regulates the generation and function of MDSCs and STAT3+ CD33+ cells suppress T-cell functions [1, 5, 16], we assessed whether HIV gp120–expanded CD33+ MDSCs can impair the capacity of T cells to produce IFN-γ. PBMCs were cultured in the presence or absence of gp120 for 5 days. CD33+ cells were then isolated, and HLA-DR expression and pSTAT3 expression were analyzed by flow cytometry. A significant decrease in surface expression of HLA-DR and increased expression of intracellular pSTAT3 was observed in gp120-expanded CD33+ cells, compared with control CD33+ cells (Supplementary Figure 1).
To determine whether gp120-expanded CD33+ cells could abrogate IFN-γ induction in T lymphocytes, control or gp120-expanded CD33+ cells were cultured with autologous CD4+ and CD8+ T cells for 3 days, and IFN-γ induction was determined in culture supernatants. IFN-γ production by CD4+ T cells was inhibited when gp120-expanded CD33+ cells were cocultured with CD4+ T cells, compared with production by CD4+ cells cultured with control CD33+ cells (mean [±SEM], 9745 ± 611 vs 6078 ± 705 pg/mL; P = .02; Figure 4A). However, when gp120 CD33+ cells and CD4+ T cells were cultured in different chambers of transwells, IFN-γ production by CD4+ T cells was not inhibited, compared with production by control CD33+ cells (mean [±SEM], 4668 ± 540 vs 4818 ± 636 pg/mL, P = .46; Figure 4A). Similarly, IFN-γ production by CD8+ T cells was inhibited when gp120-expanded CD33+ cells, rather than control CD33+ cells, were in direct contact with CD8+ T cells (mean [±SEM], 10 563 ± 359 vs 5385 ± 404 pg/mL, P = .01; Figure 4B); culturing them in transwells did not inhibit IFN-γ production from CD8+ T cells, compared with control CD33+ cells (mean [±SEM], 4504 ± 554 vs 4742 ± 601 pg/mL, P = .17; Figure 4B). These findings suggest that gp120-expanded CD33+ cells require cell-to-cell contact for their inhibitory activity on T cells. Furthermore, in our CD4+/CD33+ and CD8+/CD33+ coculture system, we observed that total IFN-γ production from CD4+ and CD8+ T cells decreased when CD33+ cells were cultured in the upper chamber of a transwell, compared with cells cultured in direct contact with CD33+ cells. This suggests that CD33+ cells provide accessory cell function like conventional antigen-presenting cells and regulate T-cell responses.
Figure 4.
gp120-expanded CD33+ cells inhibit T-cell–associated interferon γ (IFN-γ) production and require cell-to-cell contact. Peripheral blood mononuclear cells (PBMCs) from healthy donors were treated with gp120 or medium for 5 days, and CD33+ cells were subsequently isolated by positive selection, using magnetic beads. CD33+ cells from gp120-treated or control PBMCs were either cocultured with freshly isolated autologous T cells or cultured in transwell and T cells in wells of a 24-well plate in the presence of anti-CD3/CD28 for 3 days. IFN-γ concentrations were then determined by enzyme-linked immunosorbent assay. gp120-expanded CD33+ cells were cultured with CD4+ cells (A), and gp120-expanded CD33+ cells were cultured with CD8+ T cells (B). Mean values and standard errors of the mean are shown for 7 healthy donors.
gp120-Expanded CD33+ Cell–Mediated T-Cell Suppression Is Dependent on iNOS and ROS
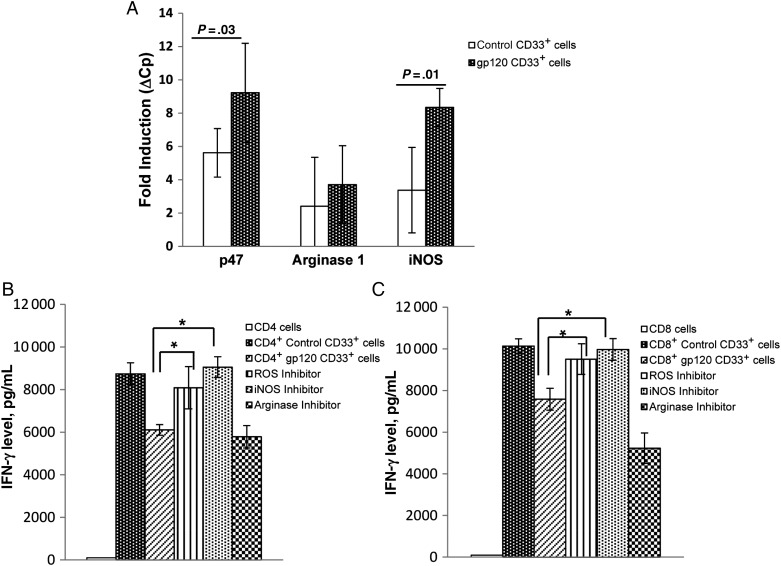
MDSCs use the biochemical and metabolic soluble mediators ROS, iNOS, and Arg1 to confer their suppressor activity [5–7, 17]. To delineate which of these mechanisms is used by gp120-expanded MDSCs to inhibit T-cell activity, we quantified messenger RNA for Arg1, iNOS, and p47phox in purified CD33+ cells by qPCR. gp120-expanded CD33+ cells exhibited 2.5-fold increased expression of iNOS and 2-fold increased expression of p47phox, compared with control CD33+ cells (Figure 5A). No difference was observed in Arg1 expression. Of the 3 molecules analyzed, ROS expression was highest in CD33+ cells, followed by iNOS and Arg1 expression. Interestingly, expression of Arg1 was minimal and was undetectable in some donor CD33+ cells.
Figure 5.
gp120-expanded CD33+ cell mediated suppression is induced by reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS). Peripheral blood mononuclear cells (PBMCs) from healthy donors were cultured in the presence or absence of gp120 (1 µg/mL) for 5 days, and CD33+ cells were isolated. A, Expression of p47phox, arginase 1, and iNOS was assessed by quantitative polymerase chain reaction, relative to hypoxanthine–guanine phosphoribosyltransferase. Gene induction was compared to CD33+ cells isolated from untreated PBMCs. Isolated CD33+ cells were cocultured with autologous CD4+ (B) and CD8+ (C) T cells as described previously in presence of ROS inhibitor (catalase; 100 U/mL), arginase inhibitor (nor-NOHA; 0.5 mM), or iNOS inhibitor (L-NMMA; 0.5 mM) for 3 days. Supernatants were collected, and interferon γ (IFN-γ) concentrations were measured by enzyme-linked immunosorbent assay. Mean values and standard errors of the mean are for 5 separate donors. *P < .05.
To explore the relative contribution of these molecules on the function of gp120-expanded MDSCs, ROS inhibitor catalase, iNOS inhibitor nor-NOHA, and Arg1 inhibitor NG-monomethyl-L-arginineacetate were added to CD33+ and CD4+ or CD8+ T-cell cocultures. As previously observed, IFN-γ production was inhibited when CD4+ cells were cultured with gp120-expanded CD33+ cells, compared with control CD33+ cells (mean [±SEM], 8739 ± 519 vs 6108 ± 253 pg/mL; P = .002). Consistent with our gene expression findings, IFN-γ production was restored in CD4+ cells following neutralization of ROS and iNOS but not Arg1. In similar experiments, IFN-γ production was also inhibited when CD8+ cells were cultured with gp120-expanded CD33+ cells, compared with control CD33+ cells (mean [±SEM], 10 134 ± 345.12 vs 7584 ± 528 pg/mL; P = .01) and was restored following neutralization of ROS and iNOS but not Arg1 (Figure 5B and 5C).
gp120-Expanded CD33+ MDSCs Induce IL-10 From CD4+ T Cells
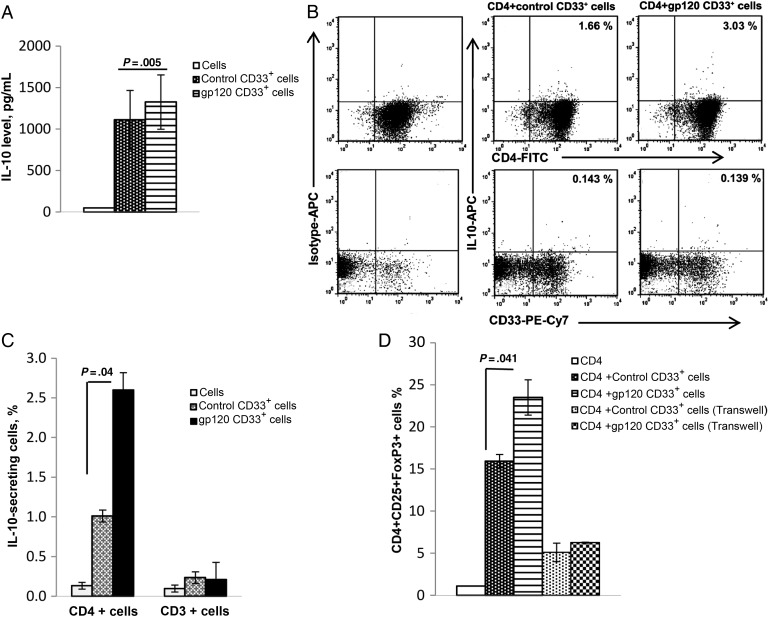
IL-10 plays a central role in T-cell impairment during HIV infection and is associated with high viral loads [18–20]. As such, we sought to investigate whether gp120-expanded CD33+ cells influence IL-10 production. For these experiments, autologous CD4+ T cells were cultured for 3 days with CD33+ cells isolated from PBMCs that were or were not treated with gp120, and IL-10 levels were quantified in culture supernatants. IL-10 levels in supernatants of cells cultured with gp120-expanded CD33+ cells increased significantly, compared with those cultured with control CD33+ cells (mean [±SEM], 1112 ± 340 vs 1326 ± 315 pg/mL; P = .005; Figure 6A).
Figure 6.
gp120-expanded CD33+ cells increase interleukin 10 (IL-10) expression in CD4 cocultures and induce T-regulatory cell expansion. A, Peripheral blood mononuclear cells (PBMCs) from healthy donors were treated with gp120 or medium for 5 days, and CD33+ cells were subsequently isolated by positive selection, using magnetic beads. CD33+ cells from untreated and gp120-treated PBMCs were cocultured with autologous CD4+ T cells in the presence of anti-CD3/CD28 for 3 days. IL-10 concentrations were then determined by enzyme-linked immunosorbent assay. Mean values and standard errors of the mean (SEM) shown are for 7 separate donors. B, Autologous CD4+ T cells were cultured with control or gp120-expanded CD33+ cells in the presence of anti-CD3/CD28 for 3 days, and IL-10 secretion was analyzed by intracellular fluorescence-activated cell sorter analysis, as shown in the representative plot. C, IL-10 secretion was analyzed by gating on CD4+ T cells and CD33+ cells, and the percentage of IL-10–secreting cells was quantified. Mean values and SEM from 3 independent experiments are shown. D, Control and gp120-expanded CD33+ cells were cultured with CD4+ T cells either together or in transwells in the presence of anti-CD3/CD28 for 3 days. Staining for surface CD4 and CD25 and intracellular FoxP3 was performed using respective antibodies and percentages of CD4+CD25+FoxP3+ cells was analyzed by flow cytometry. Mean values and SEM are for 3 separate donors.
Since IL-10 is also produced by macrophages and dendritic cells in HIV infection [20, 21], we further examined whether gp120-expanded CD33+ cells also produce IL-10. We performed intracellular staining to identify the cell type producing IL-10 in CD4+ and CD33+ cell cocultures. In these studies, neither CD33+ nor CD4+ cells were found to produce IL-10 on their own. However, coculture of CD4+ lymphocytes with gp120-expanded CD33+ cells led to a significant increase in IL-10 production, compared with control cells (mean [±SEM], 1.0% ± 0.18% vs 2.6% ± 0.37% cells; P = .02). No significant amount of IL-10 was produced by CD33+ cells, even when cultured with CD4+ T cells (Figure 6B and 6C)
gp120-Expanded CD33+ MDSCs Induce Treg Expansion
Because gp120-expanded CD33+ cells induce IL-10 production by CD4+ cells, we hypothesized that the induction of IL-10 is due to Treg expansion. For these experiments, PBMCs were cultured with or without gp120 for 5 days. CD33+ cells were then isolated and cultured with anti-CD3/CD28–stimulated autologous CD4+ T cells for 3 days. An increased frequency of CD4+CD25+FoxP3+ T regulatory cells was observed when CD4+ cells were cocultured with gp120-expanded CD33+ cells, compared with control cells (mean [±SEM], 15.9% ± 1.3% vs 23.5% ± 2.1%; P = .041). Furthermore, Treg expansion was abrogated when CD33+ cells were cultured in transwells and CD4+ T cells in wells of a 24-well plate (Figure 6D).
MDSC Levels Are Increased in Patients With Active HIV Replication
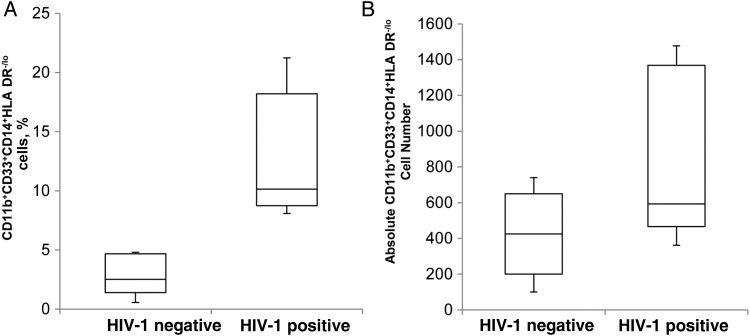
Our data demonstrate that HIV infection of PBMCs from healthy donors results in expansion of MDSCs. To determine whether these findings occur in vivo, we examined the frequency of CD11b+CD33+CD14+HLA-DR−/lo cells in the blood of 5 patients with untreated HIV infection. Our results demonstrate that the frequency of MDSCs was significantly higher in HIV–infected persons with detectable replicating virus in their plasma, compared with HIV–uninfected controls (mean [±SEM], 12.81% ± 2.31% vs 3.1% ± 1.0%; P = .008; Figure 7A). This was associated with high absolute numbers of CD11b+CD33+CD14+HLA-DR−/lo cells in the HIV–infected group (Figure 7B). Furthermore, these patients had high plasma IL-6 levels, and gp120 was detected in plasma of 4 of the 5 patients (Supplementary Table 1). These results suggest that in vivo IL-6 is associated with MDSC expansion during HIV infection.
Figure 7.
CD11b+CD33+CD14+HLA-DR−/lo cell counts are increased in human immunodeficiency virus type 1–infected patients. Whole blood specimens from 5 HIV–infected persons and 5 healthy controls were stained with antibody to CD11b, CD33, CD14, and HLA-DR. Flow cytometry was used to determine percentages (A) and absolute counts (B) of CD11b+CD33+CD14+HLA-DR−/lo cells. The upper and lower limits of the boxes denote the interquartile ranges. The horizontal lines within the boxes denote median values. The upper and lower lines outside the boxes denote the highest and lowest values, respectively.
DISCUSSION
Although the hallmark of HIV immunosuppression is characterized by the destruction of CD4+ lymphocytes, the virus uses multiple approaches to suppress the host immune response. In this research, we demonstrate that infectious or noninfectious HIV or gp120-induced IL-6 result in expansion of CD11b+CD33+CD14+HLA-DR−/lo MDSCs, leading to additional HIV-related suppression of immune system. We have also shown that gp120-expanded CD33+ cells exhibit a HLA-DR−/lo and pSTAT3hi phenotype and upregulate iNOS and ROS, further suppressing immune responses. Additionally, we have found that the gp120-associated MDSCs facilitate CD4+ Treg expansion. This suggests that the increased frequency of MDSCs is yet another mechanism through which HIV downmodulates the immune system. Consistent with this hypothesis, we have found higher MDSC and plasma IL-6 levels in untreated HIV–infected persons, compared with uninfected healthy individuals.
Altered accessory cell functions, particularly reduced HLA-DR expression and dysregulated cytokine production in HIV infection, are attributed to the presence of gp120 in vitro or to infectious virus during active infection [22, 23]. To our knowledge, these findings are the first to demonstrate that gp120-induced IL-6 expands a myeloid cell subset with reduced HLA-DR that makes T cells dysfunctional. Furthermore, comparable in vitro expansion of CD33+ MDSCs with live and heat-inactive HIV affirms an important “bystander” effect during HIV infection, in which the interaction of gp120 with immune cells profoundly influences cellular functions in vivo, contributing to the severe immune suppression observed in patients with AIDS. Recently, Qin et al found HIV Tat protein to be responsible for in vitro MDSC expansion. However, the Tat concentration used in their studies (10 µg/mL) far exceeds the levels found in the plasma of HIV–infected persons and, thus, may not exist in vivo [24]. In our experiments, we have demonstrated that MDSC expansion is induced by gp120 at concentrations that have been detected in plasma from HIV–infected persons. Of note, we observed MDSC expansion upon treatment of PBMCs with gp120, whereas gp120 treatment of isolated CD33+ cells failed to induce MDSC expansion. Interestingly, we found that adding very low numbers of CD3+ T cells to CD33+ cells promoted MDSC expansion (A. Garg, unpublished data). We speculate from these preliminary findings that factors produced by T cells regulate MDSC expansion and function in HIV infection.
Elevated IL-6 and TNF-α levels are present in serum and cerebrospinal fluid from HIV–infected patients, and their decline is an indicator of an improved immunologic response associated with viral suppression during combination antiretroviral therapy [13, 25, 26, 12]. IL-6–regulated transcription factor NF-IL6, also known as C/EBPβ, binds to the HIV long terminal repeat and facilitates its transcription in monocytes/macrophages and CD4+ T cells by inhibiting APOBEC3G. Furthermore, NF- IL6 induces replication of Δvif-HIV in nonpermissive CD4+ T cells [27, 28]. IL-6, through the gp130/JAK/STAT pathway, phosphorylates STAT3, which translocates to the nucleus and regulates differentiation, mobilization, and survival of MDSCs in mice and human disease conditions [5, 15, 16, 29, 30]. Here, we demonstrate that IL-6 production by HIV gp120 drives the expansion of pSTAT3 expressing MDSCs. Importantly, neutralizing IL-6 abrogates both MDSC expansion and pSTAT3 expression, suggesting that MDSC expansion in HIV is regulated by autocrine production of IL-6. This is consistent with the observation that HIV–infected patients have high IL-6 levels associated with high frequencies of MDSCs. To our knowledge, this is the first demonstration of the previously unexplained immunoregulatory role of IL-6 in HIV infection.
STAT3 directly regulates the p47phox subunit of ROS, producing the NADPH oxidase (NOX2) complex [7, 31], and our finding that p47phox and iNOS upregulated in gp120-expanded STAT3 expressing CD33+ MDSCs is similar to that observed in hepatitis C virus infection and simian immunodeficiency virus infection [18, 32]. Efficient T-cell signaling and subsequent immune effector function requires proper interaction between APC and T-cell receptor (TCR)-CD3 complex on T cells. ROS alone or in combination with nitric oxide contributes to the generation of peroxynitrite, which causes nitration of the tyrosine residue at the TCR-CD3 complex in CD8+ cells, thereby weakening CD8-TCR interaction [33, 34]. Whether ROS and iNOS produced by gp120-expanded MDSCs during HIV infection also modify tyrosine or any other amino acid at the TCR-CD3 junction is unknown. Here, we propose that iNOS and ROS produced by HIV–expanded MDSCs are the mediators of T-cell suppression. The extremely short half-life of iNOS and ROS helps to explain our findings that gp120-expanded CD33+ cells inhibit IFN-γ production from autologous CD4+ and CD8+ T cells when cultured directly but not in transwell experiments, suggesting that cell-to-cell contact may be required for the greatest effect.
HIV infection of cells of the myeloid lineage (ie, macrophages and dendritic cells) is known to induce IL-10 production [19, 21, 22, 35]. In contrast, we observed that CD33+ MDSCs do not produce IL-10 but instead induce IL-10–producing Tregs. This suggests that although MDSCs in HIV infection are functionally distinct from other myeloid cells, they contribute to HIV–induced immune suppression by facilitating Treg expansion. Treg activity in HIV infection is modulated by costimulatory molecules [36, 37], and we observed that gp120-expanded CD33+ MDSCs express B7-H1, B7-DC, B7-H3, and CD80/86, which are known to be involved in Treg function (unpublished data). These observations further help to explain the requirement of cell-to-cell contact for Treg expansion by CD33+ MDSCs. Our CD33+ and T-cell coculture experiments suggest that HIV MDSC–mediated immune suppression involves a complex cellular network, which, on one hand, can inhibit IFN-γ and, on the other, can facilitate Treg expansion. However, a limitation of our experimental system is that the use of purified cells may not fully represent what occurs in blood, where other cell types may also modulate MDSC activity. As such, it is important to investigate the role of MDSCs in HIV–infected persons by using whole-blood PBMCs.
In summary, our findings provide a mechanism of MDSC expansion and function in HIV infection. Additionally, we have identified that gp120-induced IL-6 expands CD33+ MDSCs that dampen the immune response through multiple pathways, including ROS, iNOS, and Treg expansion, and requires MDSC and T-cell contact. Our findings suggest a 2-signal model for MDSCs during HIV infection, in which MDSC generation and expansion are mediated by soluble factors but suppressor activity requires cell-to-cell contact with the target cell. An improved understanding of MDSC biology can contribute to novel approaches to restore normal immune function for HIV–infected persons.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Neurological Disorders and Stroke (grant R01 NS084912) and the International Maternal Perinatal Adolescent AIDS Clinical Trials Network (through the National Institute of Allergy and Infectious Diseases [contract U01 AI068632] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [contract N01-DK-9-001/HHSN267200800001C]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gabrilovich DI, Nagraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:163–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation of granulocyte-macrophage colony -stimulation factor-based-antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 4.Youn JI, Nagraj S, Collazo M, Gabrilovich DI. Subsets of myeloid derived suppressor cells in tumor bearing mice. J Immunol. 2008;181:5791–02. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase 1-producing myeloid derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induces myeloid- derived suppressor cells. J Immunol. 2009;182:5693–01. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechner MG, Megiel C, Russell SM, et al. Functional characterization of human CD33+ and CD11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Translational Med. 2011;9:2–20. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Alessandro CL, French MA, Baxter J, et al. Resumption of HIV-1 replication is associated with monocyte/macrophage derived cytokine and chemokine changes: results from a large international clinical trial. AIDS. 2011;25:1207–17. doi: 10.1097/QAD.0b013e3283471f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervia JS, Chantry CJ, Hughes MD, et al. Associations of proinflammatory cytokine levels with lipid profiles, growth and body composition in HIV-1-infected children initiating or changing antiretroviral therapy. Pediatr Infect Dis J. 2010;29:1118–22. doi: 10.1097/INF.0b013e3181ed9f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reychert J, Strick D, Bazner S, Robinson J, Rosenberg E. Detection of HIV-1 gp120 in plasma during early HIV-1 infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res Hum Retroviruses. 2010;26:1139–45. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santosuosso M, Righi E, Lindstorm V, Leblanc PR, Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J infect Dis. 2009;200:1050–53. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 15.Hovarth CM. The JAK-STAT pathway stimulated by interleukin 6. Sci STKE. 2004;260:tr9. doi: 10.1126/stke.2602004tr9. [DOI] [PubMed] [Google Scholar]
- 16.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2010;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacke R, Lee HC, Goh C, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–53. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schols D, Clercq ED. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin -10 production. J Virol. 1996;70:4953–60. doi: 10.1128/jvi.70.8.4953-4960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacey AR, Norris PJ, Qin J, et al. Induction of a striking systemic cytokine cascade prior to peak virema in acute human immunodeficiency virus Type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–59. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buisson S, Benlahrech A, Gazzard B, Gotch F, Kelleher P, Patterson S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis. 2009;199:1862–71. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 22.Zembala M, Bach S, Szczepanek A, Mancino G, Colizzi V. Phenotypic changes of monocytes induced by HIV-1-1 gp120 molecule and its fragments. Immunobiology. 1997;197:110–21. doi: 10.1016/S0171-2985(97)80061-7. [DOI] [PubMed] [Google Scholar]
- 23.Taoufik Y, Lantz O, Wallon C, Charles A, Dussaix E, Delfraissy JF. Human immunodeficiency virus gp120 inhibits interleukin-12 secretion by human monocytes: an indirect interleukin-10 mediated effect. Blood. 1997;89:2842–48. [PubMed] [Google Scholar]
- 24.Qin A, Cai W, Pan T, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1 seropositive individuals. J Virol. 2013;87:1477–90. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung MC, Pulliam L, Lau AS. The HIV-1 envelope protein gp120 is toxic to human brain cell-cultures through the induction of interleukn-6 and tumor growth factor-alpha. AIDS. 1995;9:137–43. [PubMed] [Google Scholar]
- 26.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV-1 is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–84. [PubMed] [Google Scholar]
- 27.Kinoshita SM, Taguchi S. NF-IL-6 (C/EBPb) induces HIV-1 replication by inhibiting cytidine deaminase APOBEC3G. Proc Natl Acad Sci U S A. 2008;105:15002–27. doi: 10.1073/pnas.0807269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson AJ, Zou X, Calame KL. C/EBP proteins activate transcription from the human immunodefeciency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–44. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander LE, Sackett SD, Dierssen U, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sareila O, Kelkka T, Pizolla A, Hultqvist M, Holmdahl R. NOX2 complex-derived ROS as immune regulators. Antiox Redox Signal. 2011;15:2197–08. doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- 32.Gama L, Shirk EN, Russell JN, et al. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV-1 infection. J Leukoc Biol. 2012;91:803–16. doi: 10.1189/jlb.1111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of C8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 35.Kwon DS, Angin M, Hongo T, et al. CD4+ CD25+ regulatory T cells impair HIV-1specific CD4 T cell responses by upregulating interleukin-10 production in monocytes. J Virol. 2012;86:6586–94. doi: 10.1128/JVI.06251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito T, Yang M, Wang YH, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manches O, Munn D, Fallahi A, et al. HIV-1-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase–dependent mechanism. J Clin Invest. 2008;118:3431–39. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.