Abstract
Invasive aspergillosis is a deadly infection for which new antifungal therapies are needed. Heat shock protein 90 (Hsp90) is an essential chaperone in Aspergillus fumigatus representing an attractive antifungal target. Using a thiamine-repressible promoter (pthiA), we showed that genetic repression of Hsp90 significantly reduced virulence in a murine model of invasive aspergillosis. Moreover, substituting the A. fumigatus hsp90 promoter with 2 artificial promoters (potef, pthiA) and the Candida albicans hsp90 promoter resulted in hypersensitivity to caspofungin and abolition of the paradoxical effect (resistance at high caspofungin concentrations). By inducing truncations in the hsp90 promoter, we identified a 100–base pair proximal sequence that triggers a significant increase of hsp90 expression (≥1.5-fold) and is essential for the paradoxical effect. Preventing this increase of hsp90 expression was sufficient to abolish the paradoxical effect and therefore optimize the antifungal activity of caspofungin.
Keywords: aspergillus, heat shock protein 90, caspofungin, paradoxical effect, virulence, invasive aspergillosis, echinocandin, antifungal resistance
Invasive aspergillosis, caused by the ubiquitous mold Aspergillus fumigatus, is a frequent and life-threatening infection in immunosuppressed patients [1, 2]. Because of its high mortality rate and the limited number of therapeutic options, new antifungal strategies are needed. Heat shock protein 90 (Hsp90) has generated interest as a target for new antifungal therapies [3–5]. Hsp90 is an essential molecular chaperone in eukaryotes, controlling a large network of client proteins [6, 7]. The Hsp90 inhibitor geldanamycin and its derivatives 17-dimethylaminoethylamino-17-demethoxygeldanamycin and 17-allylamino-17-demethoxygeldanamycin demonstrated in vitro antifungal activity and a potentiating effect on both azole and echinocandin antifungals against Candida albicans [4, 5, 8]. Genetic repression of Hsp90 was achieved in a murine model of invasive candidiasis via a tetracycline-repressible promoter (tetO) and resulted in complete clearance of the infection [9]. The effect of both azoles and echinocandins was enhanced in vivo by loss of the native C. albicans hsp90 promoter, suggesting that Hsp90 is involved in a stress response to these drugs [5, 8]. Hsp90 inhibitors only had poor antifungal activity against A. fumigatus, but they enhanced the activity of the echinocandin drug caspofungin in vitro and in an invertebrate model of invasive aspergillosis [5, 10, 11]. We have recently shown that genetic repression of A. fumigatus Hsp90 by a nitrogen-dependent promoter (pniiA) resulted in an in vitro growth and conidiation defect [10], Decreased 1,3-β-D-glucan content of the cell wall and increased susceptibility to caspofungin, with abolition of the paradoxical effect of this drug were also observed after Hsp90 repression [10].
The echinocandin antifungals (caspofungin, anidulafungin, and micafungin) target the fungal cell wall by inhibiting the synthesis of one of its major components, 1,3-β-D-glucan. The paradoxical effect refers to the attenuation of echinocandin antifungal activity at elevated concentrations [12]. This well-described in vitro phenomenon is drug specific and is not consistently present among fungal species (eg, in A. fumigatus, only caspofungin displays a paradoxical effect) [12, 13]. An increase in the chitin content of the cell wall after caspofungin exposure has been involved in this compensatory response [13, 14]. Calcineurin, a client protein of Hsp90, was shown to be an important trigger of the paradoxical effect, whereas the protein kinase C and the high-osmolarity glycerol pathways have also been implicated in yeasts [13–17]. Although the clinical significance of the paradoxical effect is unclear, decreased efficacy of caspofungin at elevated doses was demonstrated in a murine model of invasive aspergillosis [18].
In this study, we investigated the potential of targeting Hsp90 as a new antifungal strategy against A. fumigatus. Through genetic modifications of the hsp90 promoter, we assessed the effect of Hsp90 repression on A. fumigatus virulence and the impact of compromising Hsp90-mediated stress responses under specific stress conditions, such as caspofungin treatment.
METHODS
Strains and Growth Conditions
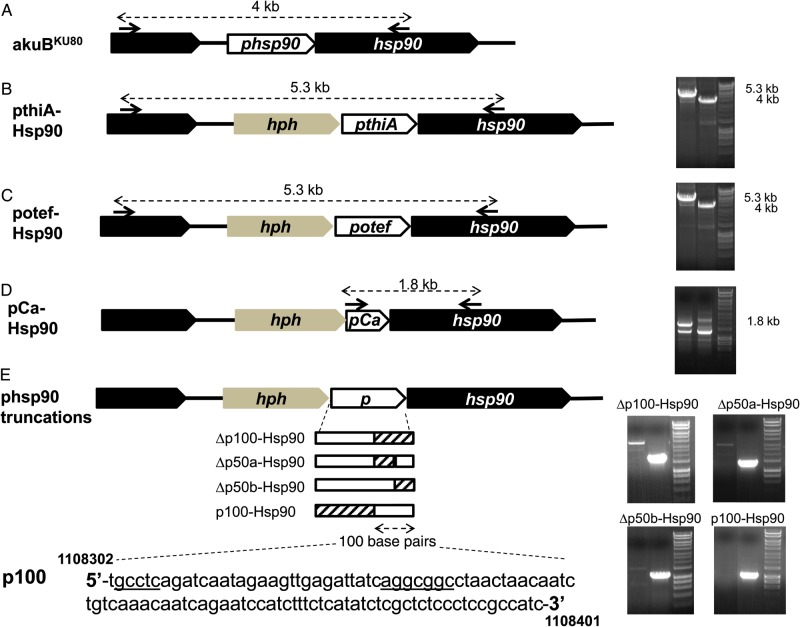
Various strains were generated by genetic substitutions and truncations of the hsp90 promoter of A. fumigatus. To accomplish this, a vector was generated from the pBluescript II SK(−) plasmid containing the hygromycin resistance cassette [19] and flanking regions of the 1.1-kb noncoding sequence, corresponding to the putative site of the hsp90 promoter (Figure 1A and Supplementary Figure 1). Other promoters were then cloned upstream of the start codon of hsp90 to replace the native hsp90 promoter of A. fumigatus. The promoter of the thiA gene (Afu6g08360; www.aspergillusgenome.org) was used for repressible expression of hsp90 in the pthiA-Hsp90 strain (Figure 1B). This gene is involved in thiamine biosynthesis and is transcriptionally repressed by thiamine [20]. The otef promoter from the pUCGH plasmid [21] was used for constitutively active expression of hsp90 in the potef-Hsp90 strain (Figure 1C). The hsp90 promoter of C. albicans (ie, the 0.3-kb noncoding sequence located between the hsp90 gene of C. albicans and the next upstream encoding sequence) was used to generate the pCa-Hsp90 strain (Figure 1D). Truncations of the native A. fumigatus hsp90 promoter were obtained by cloning the 1.1-kb noncoding sequence located upstream of hsp90, excluding (1) the last 100 base pairs (bp; Δp100-Hsp90); (2) the first 50 bp of this 100-bp sequence, conserving the last 50 bp (Δp50a-Hsp90); (3) the last 50 bp (Δp50b-Hsp90); and (4) the entire promoter region, conserving only the last 100-bp (p100-Hsp90; Figure 1E).
Figure 1.
Schematic representation of the genomic locus of hsp90 and its promoter in the different strains used in this study. A, akuBKU80 strain. In the wild-type strain, hsp90 is separated from the next upstream gene (black box) by a 1.6-kb noncoding (intergenic) sequence. The putative promoter of hsp90 (phsp90) was defined as the 1.1-kb sequence located upstream of hsp90. A 0.5-kb sequence was conserved for the terminator elements of the upstream gene. B, pthiA-Hsp90 strain; hsp90 is placed under the control of the 1-kb thiA promoter (pthiA) after removal of the putative hsp90 promoter. hph, hygromycin B resistance cassette (used as selection marker). Polymerase chain reaction (PCR) analysis shows the difference in size between the pthiA-Hsp90 strain on the left (5.3 kb) and the Aspergillus fumigatus wild-type strain on the right (4 kb). C, potef-Hsp90 strain. hsp90 is under the control of the 0.9-kb constitutively active otef promoter (potef). PCR shows the difference in size between the potef-Hsp90 strain on the left (5.3 kb) and the A. fumigatus wild-type strain on the right (4 kb). D, pCa-Hsp90 strain. The hsp90 promoter is substituted by the 0.3-kb promoter of Candida albicans hsp90 (pCa). PCR shows the presence of a 1.8-kb fragment corresponding to the amplification of a segment including the C. albicans promoter and a proximal sequence of hsp90 (left) that is absent in the wild-type strain (right). E, phsp90 truncations. The approximately 1.1-kb noncoding sequence located upstream of hsp90 and corresponding to the putative hsp90 promoter (phsp90) is cloned between the hygromycin resistance cassette (hph) and hsp90 with truncations of the last 100 base pairs (bp) preceding the start codon of hsp90 (Δp100-Hsp90), the first and the last 50 bp of this 100-bp sequence (Δp50a-Hsp90 and Δp50b-Hsp90, respectively), and the first 1000 bp, conserving the last 100-bp sequence (p100-Hsp90). Parts of the hsp90 promoter that have been removed are represented as dashed bars, and conserved parts as plain white bars. PCR, performed with 1 primer within the deleted sequence for each strain, confirmed the absence of these sequences in the transformants (left), compared with the amplification present in the wild-type strain (right). The 100-bp sequence immediately preceding the start codon of hsp90 (p100) is shown at the bottom (chromosomal coordinates 1 108 302–1 108 401 on chromosome 5), with putative calcineurin-dependent response elements underlined.
Primers used in this study are listed in Supplementary Table 1. Escherichia coli DH5α competent cells (New England Biolabs) were used for cloning as previously described [10]. All genetic constructs were verified by sequencing (Eton Biosciences) for correct integration and absence of mutations. Transformations were performed in the A. fumigatus akuBKU80 strain, possessing an increased rate of homologous recombination compared with the wild-type strain AF293 [22], as described elsewhere [19, 23]. Homologous recombination of the genetic constructs in the akuBKU80 strain was verified by polymerase chain reaction (PCR) (Figure 1).
For phenotypic testing, 104 conidia of each strain were inoculated on glucose minimal medium (GMM) agar plates [23]. Growth was assessed after 5 days of incubation under basal conditions (37°C in the absence of any drug) and various stress conditions, including heat shock (55°C) and exposure to antifungals (caspofungin, voriconazole, FK506, amphotericin B, and nikkomycin Z). The paradoxical effect of caspofungin was defined as a substantial increase of fungal growth between caspofungin concentrations of 1 and 4 µg/mL, according to the radial diameter of the colony.
Gene Expression by Real-Time Reverse-Transcription PCR
Expression of hsp90 was assessed in selected strains in the absence of any drug and in the presence of caspofungin at different concentrations. Each strain was grown at a concentration of 106 conidia per milliliter in GMM broth at 37°C for 20 hours in the absence of any drug, and then for 4 hours in the presence of caspofungin at concentrations of 0, 1, and 4 µg/mL. RNA extraction, complementary DNA synthesis and real-time reverse-transcription PCR (RT-PCR) assay were performed as described elsewhere [13]. The 2−ΔΔCt analytic method [24], normalized to β-tubulin, was used to calculate expression changes. Results were expressed as the mean (± SD) of triplicate assays. Statistical significance was defined at P < .05 (unpaired t test).
Murine Inhalational Model of Invasive Aspergillosis
Virulence of the pthiA-Hsp90 and akuBKU80 strains was compared in 20 male mice (CD1; Charles River Laboratories) in each group. Mice were immunosuppressed with cyclophosphamide (Cytoxan; Bristol-Myers Squibb; 150 mg/kg body weight given intraperitoneally 2 days before and 3 days after infection) and triamcilonone acetonide (Kenalog-40; Bristol-Myers Squibb; 40 mg/kg given subcutaneously 1 day before and 6 days after infection). Infection was induced by exposure to a 40-mL aerosolized suspension of 109 conidia per milliliter for each strain for 30 minutes in a Hinners inhalational chamber, as described elsewhere [19, 25].
Mice were housed under sterile conditions and evaluated daily for mortality. Survival was plotted on a Kaplan-Meier curve, using the log-rank test for pairwise comparison. Histopathological examination of the lungs was performed in 1 mouse from each group, euthanized 7 days after infection. Lungs were embedded in 10% neutral buffered formalin and subsequently sectioned and stained with Gomori methenamine silver and hematoxylin-eosin to assess histological signs of infection. The animal model and experiments were conducted in accordance with the Animal Care and Use Program of the Duke University Medical Center.
RESULTS
Hsp90 Repression and A. fumigatus Virulence
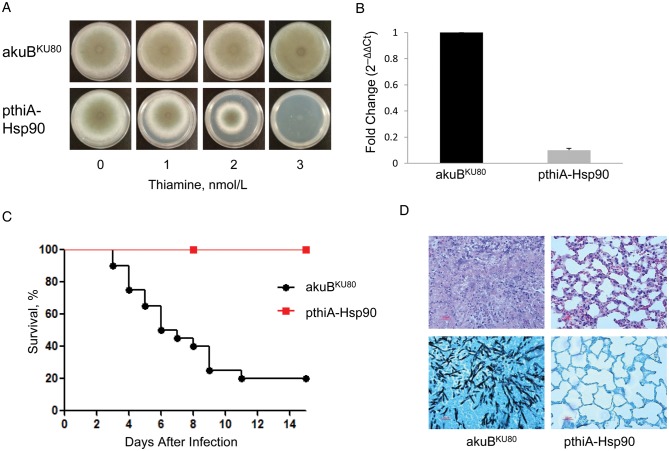
We previously achieved genetic repression of Hsp90 in A. fumigatus via a nitrogen-dependent promoter (pniiA) [10]. Because ammonium is required to repress Hsp90, this strain was not suitable for virulence studies in a murine model of invasive aspergillosis. To assess the effect of genetically repressing Hsp90 in vivo, we generated a strain in which the native hsp90 promoter was substituted by the thiA promoter (pthiA-Hsp90; Figure 1B), which is repressed in the presence of thiamine [20]. The Aspergillus oryzae thiA promoter was successfully used in A. oryzae and Aspergillus nidulans, achieving near-complete genetic repression in vitro at thiamine concentrations between 10 nmol/L and 10 µmol/L [20, 26]. In the present study, we tested for the first time the ability of the A. fumigatus thiA promoter to achieve repression of an essential gene in A. fumigatus. Because thiamine is present in the blood of mice at variable concentrations (100–500 nmol/L) [27, 28], we hypothesized that this genetic model of repression would be suitable for in vivo application.
Under standard in vitro growth conditions (GMM agar without thiamine), the pthiA-Hsp90 strain did not exhibit any growth defect compared with the control akuBKU80 strain (Figure 2A). The addition of thiamine in the growth medium (3 nmol/L) resulted in a complete lack of growth, indicating effective Hsp90 repression (Figure 2A). Owing to this severe growth defect, we were not able to analyze the expression of hsp90 in the presence of thiamine. However, RT-PCR analysis in the absence of thiamine revealed that hsp90 expression was 9.8-fold (±1.4) lower in the pthiA-Hsp90 strain compared with the akuBKU80 strain (P < .001; Figure 2B).
Figure 2.
Genetic repression of Hsp90 in the pthiA-Hsp90 strain. A, hsp90 under the control of the thiA promoter (pthiA-Hsp90 strain) is repressed by the addition of thiamine to the growth medium, which results in the complete lack of growth of the pthiA-Hsp90 strain at a thiamine concentration of 3 nmol/L. In comparison, the growth of the wild-type strain (akuBKU80), in which hsp90 is under the control of its native promoter, is unaltered by the presence of thiamine. Photographs were taken after 5 days of growth at 37°C. B, Real-time reverse-transcription polymerase chain reaction shows an approximately 10-fold decrease of basal hsp90 expression in the pthiA-Hsp90 strain compared with the akuBKU80 strain. Each strain was grown for 24 hours in glucose minimal medium in the absence of thiamine. C, In a murine inhalational model of invasive aspergillosis, the survival rate in mice infected by the pthiA-Hsp90 strain (red line) was significantly higher than that in mice infected by the akuBKU80 strain (black line) (100% vs 20%, respectively; P < .0001). D, Histopathological examination of the lungs of infected mice at day 7 after infection shows extensive hyphal proliferation and inflammation for the akuBKU80 strain but not the pthiA-Hsp90 strain with hematoxylin-eosin (top row) and Gomori methenamine silver (bottom row) staining.
The virulence of the pthiA-Hsp90 strain was investigated in our inhalational model of invasive aspergillosis. Because very low thiamine concentrations (below the basal thiamine blood level in mice) were sufficient to achieve Hsp90 repression, we did not deliver supplemental thiamine to the mice. At 14 days after infection, survival among mice infected with the pthiA-Hsp90 strain was significantly greater than among those infected with the control strain (100% vs 20%, respectively, P < .0001; Figure 2C). Extensive hyphal proliferation and inflammation was observed in lung sections of the mice infected with the akuBKU80 strain, whereas only minor inflammatory signs and no obvious hyphae were observed in mice infected with the pthiA-Hsp90 strain (Figure 2D). We thus demonstrated the important role of Hsp90 in A. fumigatus virulence, as has been shown in C. albicans [5, 9].
Loss of the Native Promoter of hsp90 and Resulting Hypersensitivity to Caspofungin and Abolition of the Paradoxical Effect
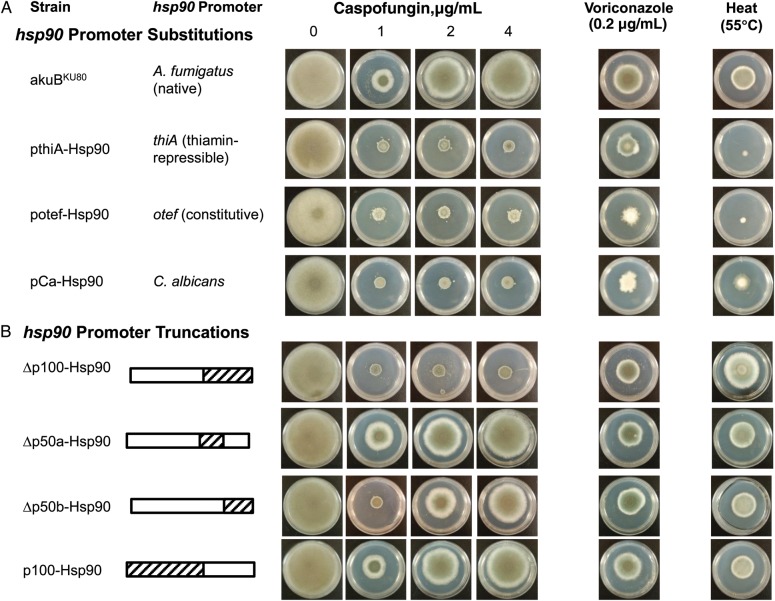
In our previous work, we highlighted the role of Hsp90 in compensatory mechanisms in response to cell wall stress [10]. In the current study, we assessed the effect of more targeted inhibition of Hsp90 by compromising Hsp90-mediated stress responses. Various strains with substitutions of the A. fumigatus hsp90 promoter by other promoters were generated (Figure 1). These strains did not exhibit any growth defect under basal conditions despite various degrees of conidiation defects, as shown by the whitish appearance of the colonies compared with the wild-type strain (Figure 3A). As expected, heat stress induced a significant growth defect in all strains (Figure 3A), confirming the role of Hsp90 in heat stress tolerance. We observed an increased susceptibility to caspofungin, with abolition of this drug's paradoxical effect, along with a moderate increase in susceptibility to voriconazole (Figure 3A); the effects of amphotericin B, nikkomycin Z, and FK506, however, remained unaltered (data not shown).
Figure 3.
Effect of various stress conditions after genetic substitutions or truncations of the hsp90 promoter of Aspergillus fumigatus. A, Resistance to caspofungin at high concentrations (>1 μg/mL), known as the paradoxical effect, is observed in the akuBKU80 strain. Substitutions of the native hsp90 promoter of A. fumigatus by the thiA promoter (pthiA-Hsp90), the constitutively active otef promoter (potef-Hsp90) and the hsp90 promoter of Candida albicans (pCa-Hsp90) result in hypersensitivity to caspofungin and loss of the paradoxical growth at high concentrations. Impaired adaptation to the stress induced by heat shock (55°C) and voriconazole is also observed. B, Truncation of the 100–base pair (bp) sequence (p100) preceding the start codon of hsp90 (Δp100-Hsp90 strain) induces hypersensitivity to caspofungin and abolition of the paradoxical effect. Removal of only the first 50 bp of p100 (Δp50a-Hsp90) does not alter the susceptibility to caspofungin or the paradoxical effect. Although removal of the last 50 bp (Δp50b-Hsp90) is sufficient to increase the susceptibility to caspofungin, the paradoxical growth at higher concentrations is still present. The paradoxical effect was also present when the hsp90 promoter was limited to p100 (p100-Hsp90 strain), which highlights the essential role of this part of the hsp90 promoter in caspofungin resistance. The effect of heat stress and voriconazole stress on these strains is shown in the last column. Compensatory responses to these stress conditions were conserved in all strains, as shown by the absence of growth defect compared with the akuBKU80 strain, suggesting that p100 does not have an essential role in hsp90-mediated stress responses other than the caspofungin stress. Parts of the hsp90 promoter that have been removed are represented as dashed bars, and the conserved parts as plain white bars. Photographs were taken after 5 days of growth at 37°C. The antifungal activity of voriconazole was tested against the akuBKU80 strain for concentrations ranging from 0.1 to 1 μg/mL, and the concentration of 0.2 μg/mL was chosen for photographs.
RT-PCR analyses under basal growth conditions revealed a decrease in hsp90 expression in the potef-Hsp90 and pCa-Hsp90 strains as observed in the pthiA-Hsp90 strain (fold decreased compared with the akuBKU80 strain, 7.5 ± 0.3 and 6.9 ± 0.7, respectively; P < .001). Thus, substitution of the native hsp90 promoter of A. fumigatus by other promoters, including a constitutively active promoter or an hsp90 promoter from another pathogenic fungus, resulted in a decrease in hsp90 expression in all cases with similar phenotypes, with hypersensitivity to caspofungin and loss of the paradoxical effect the most relevant traits.
Proximal 100-bp Region of the hsp90 Promoter in Regulation of the Paradoxical Response to Caspofungin
To investigate whether Hsp90 regulates the paradoxical response to caspofungin at the transcriptional level, we generated 4 strains with various truncations in the hsp90 promoter (Figure 1E). Deletion of the 100-bp sequence preceding the start codon of hsp90 (hereafter referred as p100; Figure 1E) was sufficient to induce hypersensitivity to caspofungin and the complete abolition of the paradoxical effect (Figure 3B), but exposure to other stress conditions, such as heat stress and voriconazole, did not result in a substantial growth defect compared with the control strain (Figure 3B).
We further dissected this promoter region by truncating the first and the last 50 bp of p100 (Δp50a-Hsp90 and Δp50b-Hsp90, respectively; Figure 1E). Only the Δp50b-Hsp90 strain exhibited increased susceptibility to caspofungin (Figure 3B). However, the paradoxical response was present in both strains (Figure 3B), indicating that the key regulatory elements of caspofungin resistance are contained in both the proximal and distal portions of p100, although the proximal region may be predominant in this response. To confirm these results, we then deleted the entire hsp90 promoter except the proximal 100-bp region (p100-Hsp90) and found that the paradoxical effect was conserved, as was the response to other stress conditions (Figure 3B). We thus have identified a critical region on the hsp90 promoter that seems essential for the resistance to caspofungin and the paradoxical effect but not for other Hsp90-mediated stress responses.
Increased Expression of hsp90 as Requirement for the Paradoxical Response to Caspofungin
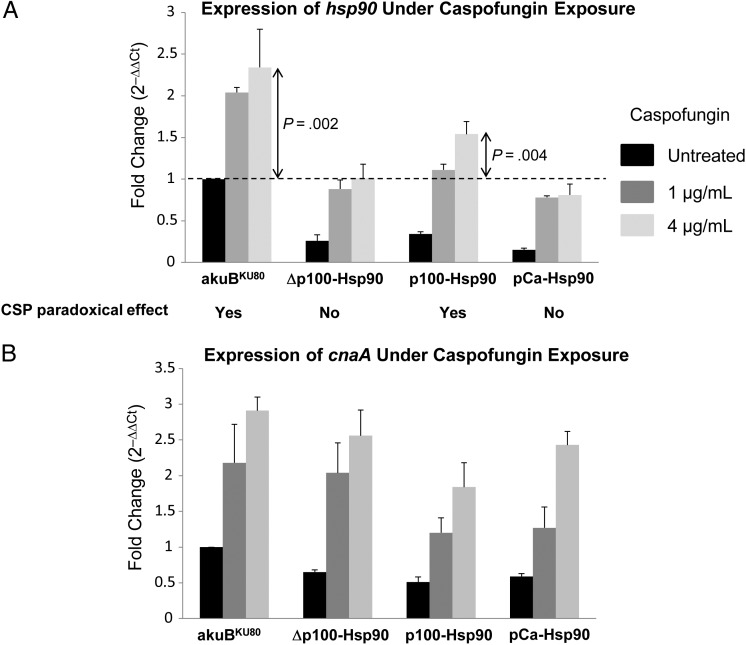
Expression analysis of hsp90 was performed to further characterize the role of p100 in controlling the paradoxical effect of caspofungin. We first measured hsp90 expression in the akuBKU80 strain and found a significant increase in hsp90 transcript levels after exposure to caspofungin at concentrations of 1 and 4 μg/mL, compared with the untreated condition (fold increase, 2.04 ± 0.06 and 2.34 ± 0.46-fold, respectively; P < .01; Figure 4A). We then assessed this caspofungin-induced increase of hsp90 expression in the Δp100-Hsp90 and p100-Hsp90 strains, as well as in the pCa-Hsp90 strain for comparison with the C. albicans hsp90 promoter that was not able to generate a paradoxical response in A. fumigatus.
Figure 4.
Correlation between hsp90 transcript levels and the CSP paradoxical effect. Real-time reverse-transcription polymerase chain reaction (PCR) analysis was performed on selected strains after conidia were grown for 20 hours in liquid glucose minimal medium (GMM) in the absence of any drug, with addition of caspofungin at a concentration of 0, 1, or 4 μg/mL for an additional 4-hour incubation. Results are presented as mean fold change (2−ΔΔCt; ± SD) compared with the untreated condition of the control strain (akuBKU80), with P values determined by unpaired t test. A, Expression of hsp90 significantly increases along with the escalation of caspofungin concentrations in the control akuBKU80 strain. Although all the other strains exhibit various degrees of decreased basal expression of hsp90, the ability to induce hsp90 expression in response to the stress induced by caspofungin is conserved. However, only the p100-Hsp90 strain, capable of a paradoxical response, shows a significant increase in hsp90 expression at a high concentration of caspofungin (4 μg/mL), compared with the basal expression of the control strain (akuBKU80 strain, untreated). At this concentration, the expression of hsp90 in the other strains that do not exhibit the paradoxical effect (Δp100-Hsp90 and pCa-Hsp90) does not exceed the basal expression level of the akuBKU80 strain. B, Increased caspofungin concentrations induce expression of the calcineurin A subunit gene (cnaA) in the akuBKU80 strain, as was observed for hsp90. Although the basal level of expression of cnaA is slightly decreased in the other strains harboring various modifications of the hsp90 promoter, the ability to respond to the stress induced by caspofungin with a significant increase in cnaA expression is conserved and cnaA transcript levels do not correlate with the presence or absence of the paradoxical effect.
Both the Δp100-Hsp90 and p100-Hsp90 strains exhibited a significant decrease in the basal level of hsp90 expression compared with the akuBKU80 strain (fold decrease, 3.94 ± 1.25 and 2.95 ± 0.26, respectively; P < .01) but were able to increase hsp90 transcript levels in the presence of caspofungin (Figure 4A). However, only the p100-Hsp90 strain showed a substantial increase of hsp90 expression at caspofungin concentrations ranging from 1 to 4 μg/mL, corresponding to the paradoxical effect.
Although the C. albicans promoter of hsp90 was able to respond to the caspofungin stress, hsp90 expression was considerably reduced in this strain and remained below the basal level of the akuBKU80 strain (Figure 4A). Compared with the basal expression level of hsp90 in the wild-type strain, only the akuBKU80 strain itself and the p100-Hsp90 strain capable of a paradoxical response demonstrated a significant increase in hsp90 expression at a caspofungin concentration of 4 μg/mL (fold increase, 2.34 ± 0.46 and 1.54 ± 0.15, respectively; P < .01; Figure 4A). These data indicate that an increase in hsp90 expression by approximately 1.5-fold is required and sufficient to regulate the paradoxical growth at higher concentrations of caspofungin.
Because calcineurin is a client protein of Hsp90 and was shown to have a role in the paradoxical response to caspofungin [3, 13], we also quantified the expression of the calcineurin A subunit gene (cnaA) under caspofungin exposure. Despite a slight decrease of the basal cnaA expression level in our mutant strains (1.5–2-fold decrease; P ≤ .01), all strains were able to produce a significant increase in cnaA expression in response to caspofungin that did not correlate with the presence or absence of the paradoxical effect (Figure 4B). Thus, the increased hsp90 expression was a major determinant to generate this compensatory response to caspofungin, independent of cnaA expression levels.
To further investigate the link between Hsp90 and calcineurin in the paradoxical effect, we looked for the presence of calcineurin-dependent response elements (CDREs) in p100, which is suggestive of a transcriptional regulation by the calcineurin-responsive zinc finger transcription factor crzA [29–31], and we identified 2 putative CDRE regions in p100 (Figure 1E).
DISCUSSION
In the pursuit of novel antifungal therapies, intracellular proteins involved in signaling pathways and stress compensatory mechanisms, such as calcineurin and Hsp90, represent attractive targets [32]. The fact that hsp90 is an essential gene in eukaryotes is widely recognized. Among fungi, attempts and failures of hsp90 gene deletion have been reported in the yeast Saccharomyces cerevisiae [33] and in the molds Neurospora crassa and A. fumigatus [10, 34]. Our previous model of Hsp90 repression via the pniiA promoter (repressed by ammonium) resulted in a moderate growth defect under repression conditions [10]. The thiA promoter was used here for the first time for genetic repression in A. fumigatus and demonstrated better efficiency in achieving near-complete repression of an essential gene, as shown by the complete lack of growth of the pthiA-Hsp90 strain under thiamine exposure. Even in the absence of thiamine, this strain exhibited a 10-fold repression of hsp90 expression, but in vitro growth was not affected, which suggests that hsp90 is expressed at much higher level than required for basal growth. Hsp90 repression was achieved in vivo for the first time in A. fumigatus, in our murine model of invasive aspergillosis, significantly decreasing virulence and confirming that Hsp90 represents an attractive target for novel antifungal therapies.
Antifungal strategies targeting Hsp90 by competitive inhibitors have been hampered for 2 main reasons. First, although it is an essential gene, effective inhibition of Hsp90 is difficult to achieve because of its very high level of basal expression, as demonstrated in this study. Second, its highly conserved structure among eukaryotes prevents the easy development of drugs that are fungus specific and thus nontoxic for humans. Indeed, geldanamycin and its derivatives 17-allylamino-17-demethoxygeldanamycin and 17-dimethylaminoethylamino-17-demethoxygeldanamycin have poor antifungal activity as monotherapy against A. fumigatus, and their synergistic effect with caspofungin is obtained at concentrations above their toxic threshold [5, 10, 11].
For these reasons, we focused on a different approach, targeting the hsp90 promoter to alter Hsp90 function in specific compensatory pathways. Our findings show that compromising the ability of Hsp90 to sense and respond to various stress conditions may represent an alternative strategy to inhibiting the protein itself. Preventing the relatively modest increase of Hsp90 to 1.5-fold its basal expression was sufficient to potentiate the effect of caspofungin within its therapeutic range of concentrations and completely abolish the paradoxical response. This effect was attributed to a 100-bp proximal sequence of the promoter (p100) that was essential for the compensatory response to caspofungin but not for basal growth and adaptation to other stress conditions.
Further analyses to identify the transcription factors binding to p100 may reveal new antifungal targets rather than Hsp90 itself. The p100 sequence is unique to A. fumigatus and has no homology in C. albicans. The paradoxical effect was not conserved after substitution of the A. fumigatus hsp90 promoter by that of C. albicans, which was not able to boost hsp90 expression above the threshold required for this response. Whether Hsp90 repression abolishes the paradoxical effect of caspofungin in C. albicans is unknown. Compromising Hsp90 function resulted in different effects on antifungal resistance among fungi. For instance, the role of Hsp90 in azole resistance that was described in C. albicans [4, 5, 35] was not obvious in our work on A. fumigatus [10]. We also did not find an additive effect of Hsp90 inhibition on amphotericin B, as was reported for Aspergillus terreus [36].
Mechanisms of drug resistance in fungi are multiple and remain largely unknown. The paradoxical effect of caspofungin is an example of such complex mechanisms. Although Hsp90 seems essential in this response, other signaling proteins and pathways have been involved [13, 16, 17], and the role of the innate immune system in regulating caspofungin activity in a dose-dependent manner has also been highlighted recently [37]. The identification of 2 CDREs in p100 further supports the link between Hsp90 and calcineurin in this response via the crzA transcription factor. The paradoxical effect was also lacking in our crzA deletion strain [13], and CDRE sites were identified in the promoter sequences of the chitin synthases, whose activity is increased in a calcineurin-dependent manner on exposure to caspofungin [13]. In our current study, hsp90 expression level was the decisive trigger of the paradoxical effect, independent of cnaA transcript levels. However, results of RT-PCR analyses do not reflect the interactions between these 2 proteins at the posttranscriptional level.
Caspofungin is increasingly used as a second-line therapy of invasive aspergillosis and has the advantage of an excellent safety profile. Resistance has been rarely documented in A. fumigatus clinical isolates [38]. The limited efficacy of this drug against A. fumigatus seems to be mainly due to an inherent mode of resistance consisting of cell wall repair mechanisms, as illustrated by the paradoxical effect. Growing evidence indicates that caspofungin may require an adjuvant drug to counteract these compensatory responses and achieve an optimal therapeutic efficacy [32]. In our current study, we demonstrated the key role of Hsp90 in fine-tuning this response via a short regulatory element located upstream of the gene. Whereas fungus-specific inhibition of such an essential and conserved protein as Hsp90 may be difficult to achieve, preventing the increase of Hsp90 above a defined threshold is sufficient to optimize the activity of caspofungin. Further investigating the mechanistic processes of Hsp90 in the maintenance of the cell wall integrity may improve our understanding of the mechanisms of antifungal resistance and help identify new antifungal targets.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the Swiss National Science Foundation (grant PASMP3-142746 to F. L.) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant 1R21AI097541-01A1 to W. J. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 3.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol. 2008;6:187–98. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 4.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–9. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 5.Cowen LE, Singh SD, Kohler JR, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 2009;106:2818–23. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Davey M, Hsu YC, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–27. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009;5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro RS, Uppuluri P, Zaas AK, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–9. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot Cell. 2012;11:1324–32. doi: 10.1128/EC.00032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamoth F, Juvvadi PR, Gehrke C, Steinbach WJ. In vitro activity of calcineurin and heat-shock protein 90 (Hsp90) inhibitors against Aspergillus fumigatus azole- and echinocandin-resistant strains. Antimicrob Agents Chemother. 2013;57:1035–9. doi: 10.1128/AAC.01857-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiederhold NP. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr Opin Infect Dis. 2007;20:574–8. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 13.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother. 2010;54:1555–63. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortwendel JR, Juvvadi PR, Pinchai N, et al. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:476–82. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro CA, Selvaggini S, de Bruijn I, et al. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol. 2007;63:1399–413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother. 2005;49:5146–8. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis. 2004;190:1464–71. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 19.Pinchai N, Perfect BZ, Juvvadi PR, et al. Aspergillus fumigatus calcipressin CbpA is involved in hyphal growth and calcium homeostasis. Eukaryot Cell. 2009;8:511–9. doi: 10.1128/EC.00336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoji JY, Maruyama J, Arioka M, Kitamoto K. Development of Aspergillus oryzae thiA promoter as a tool for molecular biological studies. FEMS Microbiol Lett. 2005;244:41–6. doi: 10.1016/j.femsle.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Langfelder K, Philippe B, Jahn B, Latge JP, Brakhage AA. Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect Immun. 2001;69:6411–8. doi: 10.1128/IAI.69.10.6411-6418.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva Ferreira ME, Kress MR, Savoldi M, et al. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:207–11. doi: 10.1128/EC.5.1.207-211.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbach WJ, Cramer RA, Jr, Perfect BZ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Steinbach WJ, Benjamin DK, Jr, Trasi SA, et al. Value of an inhalational model of invasive aspergillosis. Med Mycol. 2004;42:417–25. doi: 10.1080/13693780410001712034. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi Y, Shoji JY, Arioka M, Kitamoto K. Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae. Eukaryot Cell. 2009;8:37–46. doi: 10.1128/EC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Itokawa Y. Determination of thiamine and its phosphate esters in human and rat blood by high-performance liquid chromatography with post-column derivatization. J Chromatogr. 1985;332:181–8. doi: 10.1016/s0021-9673(01)83295-7. [DOI] [PubMed] [Google Scholar]
- 28.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology. 2010;138:1802–9. doi: 10.1053/j.gastro.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendizabal I, Pascual-Ahuir A, Serrano R, de Larrinoa IF. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol Genet Genomics. 2001;265:801–11. doi: 10.1007/s004380100474. [DOI] [PubMed] [Google Scholar]
- 30.Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–44. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimoto H, Saltsman K, Gasch AP, et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–88. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- 32.Steinbach WJ, Juvvadi PR, Fortwendel JR, Rogg LE. Newer combination antifungal therapies for invasive aspergillosis. Med Mycol. 2011;49(Suppl 1):S77–81. doi: 10.3109/13693786.2010.499374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–30. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colot HV, Park G, Turner GE, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A. 2006;103:10352–7. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell. 2006;5:2184–8. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blum G, Kainzner B, Grif K, et al. In vitro and in vivo role of heat shock protein 90 in amphotericin B resistance of Aspergillus terreus. Clin Microbiol Infect. 2013;19:50–5. doi: 10.1111/j.1469-0691.2012.03848.x. [DOI] [PubMed] [Google Scholar]
- 37.Moretti S, Bozza S, D'Angelo C, et al. Role of innate immune receptors in paradoxical caspofungin activity in vivo in preclinical aspergillosis. Antimicrob Agents Chemother. 2012;56:4268–76. doi: 10.1128/AAC.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arendrup MC, Garcia-Effron G, Buzina W, et al. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob Agents Chemother. 2009;53:1185–93. doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.