Abstract
Despite surfactant and mechanical ventilation being the standard of care for preterm infants with respiratory failure, non-invasive respiratory support is increasingly being employed in neonatal units. The latter can be accomplished in a variety of ways but none of them have been proven so far to be superior to intubation and mechanical ventilation. Nonetheless, they appear to be safe and effective in experienced hands. This article relates to the use of non-invasive forms of respiratory support and evidence is reviewed from the clinical trials which have evaluated the use of these techniques.
Keywords: Newborn, non-invasive respiratory support, respiratory distress syndrome, respiratory failure
INTRODUCTION
Although life-saving, invasive mechanical ventilation in preterm neonates is a major risk factor for the development of Broncho-pulmonary Dysplasia (BPD)[1] and Ventilator-Induced Lung Injury (VILI). These concerns have prompted neonatologist to use non-invasive modes of ventilation, and this has been increasingly gaining acceptance in most neonatal units. Non-invasive Ventilation (NIV) in neonates has mainly been used to maintain effective breathing after a period of extubation and to avoid extubation failure. There has also been a recent trend to use NIV as primary mode of ventilation for early management of Respiratory Distress Syndrome (RDS) as an alternative to intubation and ventilation, but evidence of its superiority over traditional CPAP or intubation and ventilation is still lacking. Although the results of smaller studies have shown positive results in favor of NIV in preterm infants, this is not supported by the results of larger studies or systematic reviews. Moreover, the long-term safety and outcomes of this promising mode of ventilation need to be established before its widespread use as the primary mode of ventilation in this population can be recommended. This article aims to review the current available evidence for the use of non-invasive ventilation for treatment of RDS in preterm babies.
Historical background of NIV in neonates
The use of NIV in neonates is not a completely new concept and has been in use for almost over half a century. The first report on possible use of NIV in neonates[2] was published about 20 years before the Gregory's paper on continuous positive airway pressure (CPAP).[3] Negative pressure-assisted ventilation was used as a form of non-invasive ventilation but did not prove to be too beneficial.[4] NIV was found to achieve better gaseous exchange than simple oxygen therapy but was shown to be associated with significant head molding and cerebral hemorrhage due to the use of face mask straps.[5] Similarly, the reports of gastric perforations[6] with use of non-invasive ventilation made neonatologists reluctant to use NIV. With the advent of newer interfaces and devices, these complications are now less common,[7,8] and the clinicians are once again more interested in exploring the new ways of providing NIV as highlighted by recent surveys.[9,10] Various modes and ways of delivering NIV (synchronous or asynchronous) are being tested, and one can hope that this will further improve our understanding of use of NIV in preterm babies.
PHYSIOLOGICAL EFFECTS AND POTENTIAL BENEFITS OF NIV
The exact mechanism of action by which NIV works in preterm babies is not clear, but several physiological effects have been postulated. The immaturity of the respiratory system of preterm neonates along with unstable chest wall makes complaint airways easy to collapse and cause respiratory failure. One of the effects of NIV is simply to provide support and anchorage to these airways. This can be helpful in cases of obstructive apneas by stimulating the upper airways and make extubation successful after a period of invasive ventilation.[11,12] Non-invasive ventilation also helps to maintain functional residual capacity such as in the surfactant deficient alveoli of premature babies by augmenting their spontaneous respiratory effort and minute ventilation.[13,14]
The main proposed benefit of non-invasive ventilation is to avoid VILI and prevent development of BPD, but this has not been proven in large controlled trials. The other possible benefit is to prevent harmful effects of endotracheal intubation[15] including hemodynamic instability, increased airway resistance, acute and chronic airway trauma (potentially resulting in sub-glottic stenosis), increased infection risk due to colonization of trachea, and reduced clearance of secretions making frequent and traumatic suctioning less necessary, but there is not sufficient safety data to prove these hypotheses.
Modes of non-invasive ventilation
Non-invasive forms of ventilation in neonates can be provided either as a single level support such as CPAP and High Flow Nasal Cannula (HFNC) or bilevel support such as Nasal Intermittent Positive Pressure Ventilation (NIPPV). In NIPPV, CPAP provides a constant distending pressure (both during inspiration and expiration), while superadded ventilatory inflation (high level as in NIPPV or low level as in BiPAP and SiPAP) augments the tidal ventilation. The ventilator rate and inspiratory time (Ti) can be fixed as in traditional ventilation. The manufacturers use different names to describe these modes of NIV making the nomenclature confusing although the mechanism remains the same. Various modes of NIPPV include Synchronized Nasal Intermittent Positive Pressure Ventilation (SNIPPV),[16] Nasal Synchronized Intermittent Mandatory Ventilation (N-SIMV),[17] Nasopharyngeal Synchronized Intermittent Mandatory Ventilation (NP-SIMV),[18] Nasal Bi-level Positive Airway Pressure (N-BiPAP),[19] Nasal Intermittent Mandatory Ventilation (NIMV),[20] and Non-invasive Pressure Support Ventilation (NI-PSV).[21]
Nasal cannula oxygen with a flow of more than 2 liters per min (HFNC) has been shown to provide CPAP at these high flow rates[22] and has been becoming a favorable mean of providing single level non-invasive ventilation although the recent Cochrane Review by Wilkinson et al.,[23] has highlighted the insufficient evidence available at present to suggest long-term safety and efficacy of this modality. A recent review by Roehr et al.[24] highlighted the need to wait for the results of ongoing trials before HFNC use can be recommended widely. The main drawback of using HFNC is that the pressure generated is not measurable and cannot be regulated. One of the perceived benefits of HFNC is its ease of use and less nasal trauma, making it more popular amongst neonatal nurses.
Nasal high-frequency oscillation has been tested in animal models[25] as well as in preterm babies,[26] but its routine use as a non-invasive mode of ventilation warrants further studies.
CPAP on the other hand has been a time tested widely used modality of NIV and can be delivered by several different mechanisms and devices. The main difference between these delivery systems to provide CPAP is dependent on variations in the flow and/or pressure delivered. The conventional ventilator and bubble CPAP are considered as “constant flow” systems, but the pressure achieved varies. Infant flow driver (IFD), on the other hand, is considered to be a variable flow system generating “constant pressure.” Gupta et al.[27] compared the two modalities in a randomized controlled trial in 140 preterm infants (24 to 29 weeks gestational age), who were being weaned from mechanical ventilation (MV) and found no significant difference in extubation failure rate. However, in a sub-group analysis of infants ventilated for less than 14 days (N = 127), the extubation failure rate was significantly lower in those infants randomized to bubble CPAP (14.1% vs. 28.6%, P = 0.046). No published trials have compared the effectiveness of bubble CPAP with that of IFD CPAP when used as the initial mode of respiratory support in preterm infants with RDS. Large multicenter RCTs comparing the effectiveness of these devices will be required to detect differences between them.
Various interfaces have been used to deliver NIV such as by face masks, nasopharyngeal and nasal interfaces. The main drawback of these interfaces is the difficulty to maintain constant seal and to achieve the adequate pressure. The endotracheal tubes and nasopharyngeal interfaces have minimal leaks but increase the work of breathing significantly. Nasal interfaces are now commonly used, and short bi-nasal prongs are shown to be most effective and generate least amount of airway resistance and are minimally invasive.
CPAP VS. MECHANICAL VENTILATION
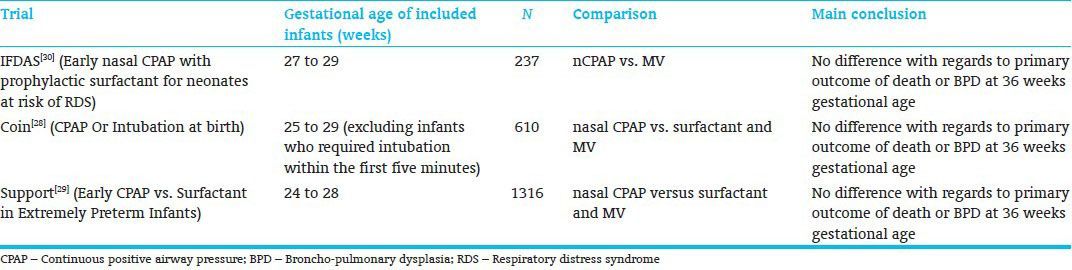
With better antenatal and perinatal care, it has become possible to manage even the smallest of the babies on CPAP from birth avoiding intubation and mechanical ventilation. Large randomized controlled trials published recently[28,29,30] [Table 1] have shown safety of this approach, but CPAP has not been proven to show any significant long-term benefit in prevention of BPD and/or death over mechanical ventilation. Moreover, CPAP is not fully safe and can still result in serious complications in not managed properly. The clinicians, therefore, have to be careful in selecting and monitoring the babies managed on CPAP as the incidence of pneumothorax was significantly higher in the babies randomized to CPAP group in COIN Trail.[28] This might have been related to difference in standard of care provided in different units taking part in this trial.
Table 1.
Trials comparing CPAP with Mechanical ventilation in preterm infants
NIPPV VS. CPAP
Whilst more and more neonatologists are using CPAP for primary treatment of RDS, this still fails in a significant proportion of babies necessitating re-intubation and invasive mechanical ventilation. This has prompted the use of NIPPV in many neonatal units with a hope that will reduce the chances of failure as compared to CPAP by improving respiratory mechanics due to increased minute ventilation and reduced work of breathing.
Several studies have shown short-term benefits of NIPPV over CPAP, but the data on incidence of BPD or long-term outcomes is not consistent. In some studies, NIPPV as compared to nasal CPAP has been shown to reduce extubation failure and apnea rates in preterm babies.[31,32] The evidence is in favor of NIPPV in reducing the need for invasive ventilation in the first few days of life.[33,34,35,36] A recent meta-analysis by Meneses et al.[37] found that NIPPV significantly decreases the need for invasive ventilation within the first 72 hours of life compared with nasal CPAP, but no difference between groups was found in the incidence of broncho-pulmonary dysplasia (risk ratio, 0.56; 95% CI, 0.09-3.49).
In the largest trial to date (1009 babies <30 weeks and/or <1000 gm) by Kirpalani et al.,[38] no significant difference was found in death or BPD rate in NIPPV or nasal CPAP group (published only in abstract form). Another recently finished randomized controlled trial comparing primary use of nasal CPAP vs. SiPAP (CoSi Trial,[39] abstract publication) for respiratory distress in premature babies (28-32 weeks gestation) again showed no significant difference in the primary outcome (failure of non-invasive respiratory support, necessitating intubation and ventilation, in the first 72 hours of treatment). The incidence of pneumothorax or BPD was not significantly different either in this trial.
How to wean patients from NIV
Preterm infants should be ready to wean from NIV once they reach the target PaO2 or saturations with minimal oxygen requirement (e.g. below 0.3 for acute respiratory failure) and they have not experienced any apneas in previous 24 hours. Although there is not always a consensus as to the best practice of weaning, clinicians should familiarize themselves with the methods and equipments they are using on their units.
Weaning from CPAP may be done by decreasing the pressure by 1 cm of H2O and closely monitoring for any clinical deterioration. Once the pressure has reached 4 cm of H2O and infant is stable, they can safely be taken off. The nursing observations at the time of cares provide valuable information in deciding how likely a premature infant will manage off CPAP in addition to other parameters. The weaning from NIPPV is similar to that of MV (pressure, back up rate, and oxygen).
Future of non-invasive ventilation in neonates
NIV seems to be an attractive option of respiratory support in preterm infants and can prove to be effective in reducing the need for invasive mechanical ventilation and the complications associated with invasive mode including BPD. Although useful developments have been made over last 20 years in the understanding of NIV use in neonates, further research is still needed in the following areas for the best use of this approach:
Should NIV be used with synchronization and, if yes, how best that can be achieved? New forms of respiratory support, which are designed to improve synchronization of breaths between the patient and the ventilator are being developed, and it will be important to consider and evaluate their use in preterm infants. Neurally Adjusted Ventilatory Assist (NAVA)[40] is a novel form of non-invasive ventilation that is designed to improve synchronization and works by sensing the electrical activity of the diaphragm (electrode placed in esophagus).
Best ventilatory settings and weaning strategies of non-invasive ventilation to avoid failure of NIV and need for intubation and mechanical ventilation.
Can HFNC/CPAP/NIPPV be used as primary mode of ventilation and will they improve the long-term outcome?
Long-term respiratory and neuro-developmental outcomes of NIV as compared to MV needs to be evaluated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–72. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donald I, Lord J. Augmented respiration; studies in atelectasis neonatorum. Lancet. 1953;1:9–17. doi: 10.1016/s0140-6736(53)92511-2. [DOI] [PubMed] [Google Scholar]
- 3.Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med. 1971;284:1333–40. doi: 10.1056/NEJM197106172842401. [DOI] [PubMed] [Google Scholar]
- 4.Stern L, Ramos AD, Outerbridge EW, Beaudry PH. Negative pressure artificial respiration: Use in treatment of respiratory failure of the newborn. Can Med Assoc J. 1970;102:595–601. [PMC free article] [PubMed] [Google Scholar]
- 5.Pape KE, Armstrong DL, Fitzhardinge PM. Central nervous system pathology associated with mask ventilation in the very low birth weight infant: A new etiology for intracerebellar hemorrhages. Pediatrics. 1976;58:473–83. [PubMed] [Google Scholar]
- 6.Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics. 1985;76:406–10. [PubMed] [Google Scholar]
- 7.Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants after extubation. J Perinatol. 1999;19(6 Pt 1):413–8. doi: 10.1038/sj.jp.7200205. [DOI] [PubMed] [Google Scholar]
- 8.Meneses J, Bhandari V, Alves JG. Nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome. Arch Pediatr Adolesc Med. 2012;166:372–6. doi: 10.1001/archpediatrics.2011.1142. [DOI] [PubMed] [Google Scholar]
- 9.Owen LS, Morley CJ, Davis PG. Neonatal nasal intermittent positive pressure ventilation: A survey of practice in England. Arch Dis Child Fetal Neonatal Ed. 2008;93:F148–50. doi: 10.1136/adc.2007.118109. [DOI] [PubMed] [Google Scholar]
- 10.Manley BJ, Owen L, Doyle LW, Davis PG. High-flow nasal cannulae and nasal continuous positive airway pressure use in non-tertiary special care nurseries in Australia and New Zealand. J Paediatr Child Health. 2012;48:16–21. doi: 10.1111/j.1440-1754.2011.02186.x. [DOI] [PubMed] [Google Scholar]
- 11.Finer N. Nasal Cannula use in the preterm infant: Oxygen or pressure? Pediatrics. 2005;166:1216–7. doi: 10.1542/peds.2005-1741. [DOI] [PubMed] [Google Scholar]
- 12.Miller MJ, DiFiore JM, Strohl KP, Martin RJ. Effects of nasal CPAP on supraglottic and total pulmonary resistance in preterm infants. J Appl Physiol. 1990;68:141–6. doi: 10.1152/jappl.1990.68.1.141. [DOI] [PubMed] [Google Scholar]
- 13.Lin CH, Wang ST, Lin YJ, Yeh TF. Efficacy of nasal intermittent positive pressure ventilation in treating apnea of prematurity. Pediatr Pulmonol. 1998;26:349–53. doi: 10.1002/(sici)1099-0496(199811)26:5<349::aid-ppul8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Greenough A, Morley CJ, Davis JA. Provoked augmented inspirations in ventilated premature babies. Early Hum Dev. 1984;9:111–7. doi: 10.1016/0378-3782(84)90091-4. [DOI] [PubMed] [Google Scholar]
- 15.Aly H. Ventilation without tracheal intubation. Pediatrics. 2009;124:786–9. doi: 10.1542/peds.2009-0256. [DOI] [PubMed] [Google Scholar]
- 16.Santin R, Brodsky N, Bhandari V. A prospective observational pilot study of synchronized nasal intermittent positive pressure ventilation (SNIPPV) as a primary mode of ventilation in infants>or=28 weeks with respiratory distress syndrome (RDS) J Perinatol. 2004;24:487–93. doi: 10.1038/sj.jp.7211131. [DOI] [PubMed] [Google Scholar]
- 17.Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatr Pulmonol. 1998;25:175–81. doi: 10.1002/(sici)1099-0496(199803)25:3<175::aid-ppul7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants after extubation. J Perinatol. 1999;19:413–8. doi: 10.1038/sj.jp.7200205. [DOI] [PubMed] [Google Scholar]
- 19.Migliori C, Motta M, Angeli A, Chirico G. Nasal bilevel vs. continuous positive airway pressure in preterm infants. Pediatr Pulmonol. 2005;40:426–30. doi: 10.1002/ppul.20276. [DOI] [PubMed] [Google Scholar]
- 20.Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: A randomized, controlled, prospective study. J Pediatr. 2007;150:521–6. doi: 10.1016/j.jpeds.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Ali N, Claure N, Alegria X, D’Ugard C, Organero R, Bancalari E. Effects of noninvasive pressure support ventilation (NI-PSV) on ventilation and respiratory effort in very low birth weight infants. Pediatr Pulmonol. 2007;42:704–10. doi: 10.1002/ppul.20641. [DOI] [PubMed] [Google Scholar]
- 22.Locke RG, Wolfson MR, Shaffer TH, Rubenstein SD, Greenspan JS. Inadvertent administration of positive end distending pressure during nasal cannula flow. Pediatrics. 1993;91:135–8. [PubMed] [Google Scholar]
- 23.Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2011;5:CD006405. doi: 10.1002/14651858.CD006405.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Roehr CC, Manley BJ, Dold SK, Davis PG. High-Flow Nasal Cannulae for respiratory support of preterm infants: A review of the evidence. Arch Dis Child. 2012;97(Suppl 2):A512–3. doi: 10.1159/000341754. [DOI] [PubMed] [Google Scholar]
- 25.Rehan VK, Fong J, Lee R, Sakurai R, Wang ZM, Dahl MJ, et al. Mechanism of reduced lung injury by high-frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr Res. 2011;70:462–6. doi: 10.1203/PDR.0b013e31822f58a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colaizy TT, Younis UM, Bell EF, Klein JM. Nasal high-frequency ventilation for premature infants. Acta Paediatr. 2008;97:1518–22. doi: 10.1111/j.1651-2227.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Sinha SK, Tin W, Donn SM. A randomized controlled trial of post-extubation bubble continuous positive airway pressure versus Infant Flow Driver continuous positive airway pressure in preterm infants with respiratory distress syndrome. J Pediatr. 2009;154:645–50. doi: 10.1016/j.jpeds.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 29.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson MA. Early nasal continuous positive airways pressure (nCPAP) with prophylactic surfactant for neonates at risk of RDS. The IFDAS multi-centre randomised trial. Pediatr Res. 2002;51:379A. [Google Scholar]
- 31.Davis PG, Lemyre B, de Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev. 2001;3:CD003212. doi: 10.1002/14651858.CD003212. [DOI] [PubMed] [Google Scholar]
- 32.Lemyre B, Davis PG, de Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database Syst Rev. 2002;1:CD002272. doi: 10.1002/14651858.CD002272. [DOI] [PubMed] [Google Scholar]
- 33.Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: A randomized, controlled, prospective study. J Pediatr. 2007;150:521–6. doi: 10.1016/j.jpeds.2007.01.032. 526.e1. [DOI] [PubMed] [Google Scholar]
- 34.Sai Sunil Kishore M, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatr. 2009;98:1412–5. doi: 10.1111/j.1651-2227.2009.01348.x. [DOI] [PubMed] [Google Scholar]
- 35.Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: A randomized controlled trial. Pediatrics. 2011;127:300–7. doi: 10.1542/peds.2010-0922. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan R, Sekar KC, Rasmussen M, Bhatia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants<30 weeks’ gestation: A randomized, controlled trial. J Perinatol. 2012;32:336–43. doi: 10.1038/jp.2012.1. [DOI] [PubMed] [Google Scholar]
- 37.Meneses J, Bhandari V, Alves JG. Nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome. Arch Pediatr Adolesc Med. 2012;166:372–6. doi: 10.1001/archpediatrics.2011.1142. [DOI] [PubMed] [Google Scholar]
- 38.Kirpalani H, Millar D, Lemyre B, Yoder B, Chiu A, Roberts A. Nasal Intermittent Positive Pressure (NIPPV) Does Not Confer Benefit above Nasal CPAP (nCPAP) in Extremely Low Birth Weight (ELBW) Infants<1000 g BW – an international randomised trial. Arch Dis Child. 2012;97(Suppl 1):A133–4. [Google Scholar]
- 39.Wood FE, Gupta S, Win T, Sinha S. Randomised Controlled Trial of Synchronised Intermittent Positive Airway Pressure (SiPAP™) Versus Continuous Positive Airway Pressure (CPAP) as a Primary Mode of Respiratory Support in Preterm Infants with Respiratory Distress Syndrome. E-PAS. 2013:3500. 8. [Google Scholar]
- 40.Biban P, Serra A, Polese G, Soffiati M, Santuz P. Neurally adjusted ventilatory assist: A new approach to mechanically ventilated infants. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):38–40. doi: 10.3109/14767058.2010.510018. [DOI] [PubMed] [Google Scholar]


