Abstract
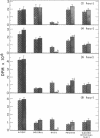
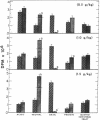
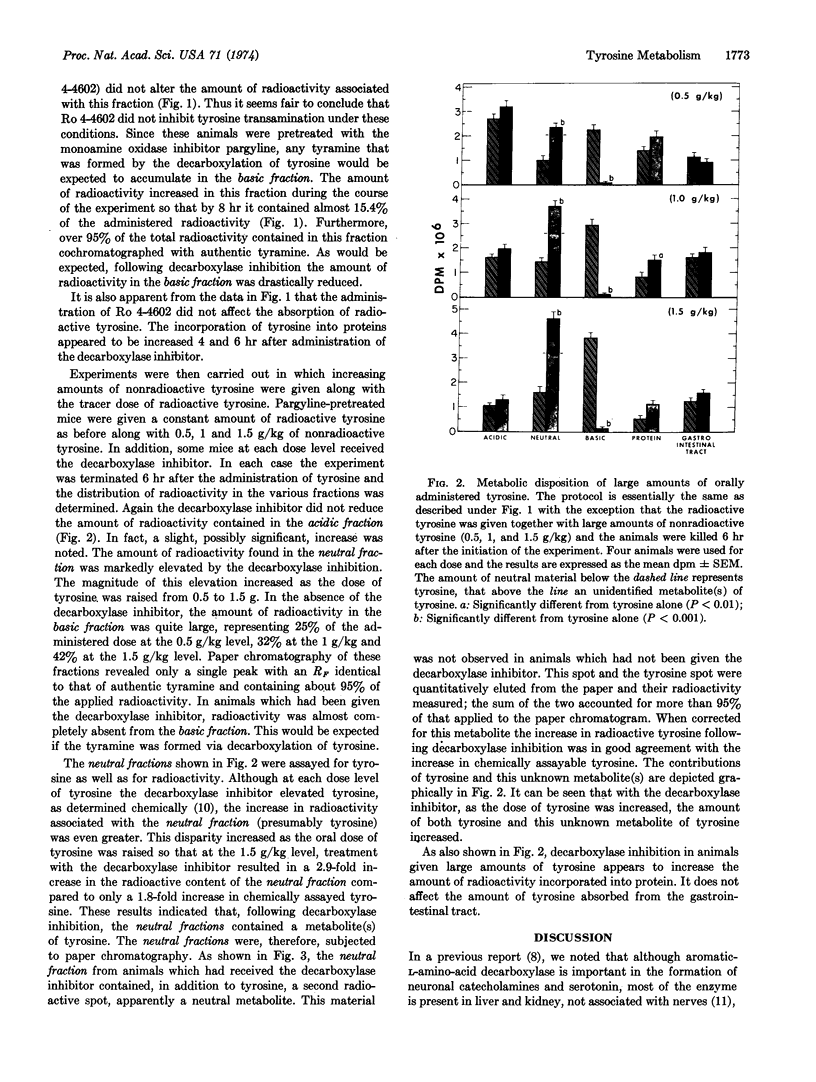
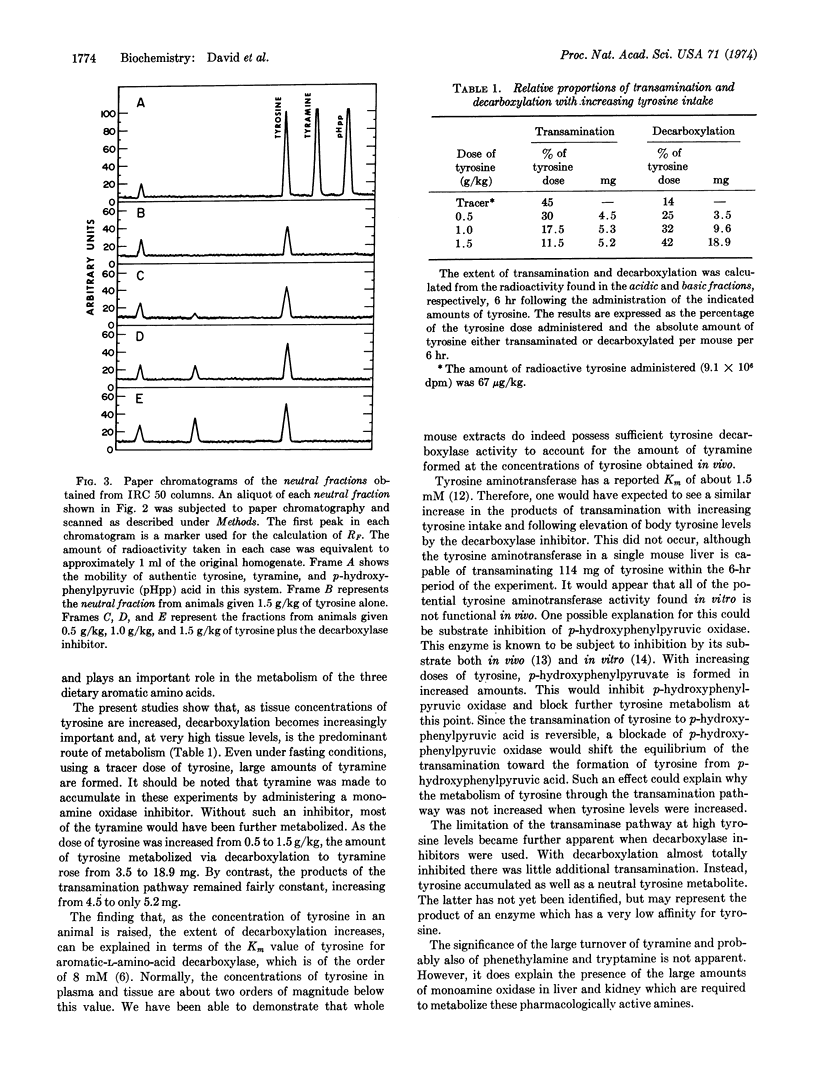
Metabolism of tyrosine was examined in mice, some of which had been treated with an inhibitor of aromatic-L-amino-acid decarboxylase. The results of the study indicate that as the plasma and tissue levels of tyrosine are elevated, decarboxylation to tyramine becomes the predominant route of metabolism. At the highest dose of tyrosine used (1.5 g/kg), it was found that 42% of the administered dose was decarboxylated within 6 hr and only 11.5% was metabolized by the tyrosine aminotransferase pathway.
Keywords: tyrosine aminotransferase pathway, decarboxylase inhibitor
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christenson J. G., Dairman W., Udenfriend S. Preparation and properties of a homogeneous aromatic L-amino acid decarboxylase from hog kidney. Arch Biochem Biophys. 1970 Nov;141(1):356–367. doi: 10.1016/0003-9861(70)90144-x. [DOI] [PubMed] [Google Scholar]
- David J. C., Dairman W., Udenfriend S. On the importance of decarboxylation in the metabolism of phenylalanine, tyrosine, and tryptophan. Arch Biochem Biophys. 1974 Feb;160(2):561–568. doi: 10.1016/0003-9861(74)90432-9. [DOI] [PubMed] [Google Scholar]
- JACOBY G. A., LADU B. N. STUDIES ON THE SPECIFICITY OF TYROSINE-ALPHA-KETOGLUTARATE TRANSAMINASE. J Biol Chem. 1964 Feb;239:419–424. [PubMed] [Google Scholar]
- KLINGMAN G. I. CATECHOLAMINE LEVELS AND DOPA-DECARBOXYLASE ACTIVITY IN PERIPHERAL ORGANS AND ADRENERGIC TISSUES IN THE RAT AFTER IMMUNOSYMPATHECTOMY. J Pharmacol Exp Ther. 1965 Apr;148:14–21. [PubMed] [Google Scholar]
- KNOX W. E., LeMAY-KNOX M. The oxidation in liver of l-tyrosine to acetoacetate through p-hydroxyphenylpyruvate and homogentisic acid. Biochem J. 1951 Oct;49(5):686–693. doi: 10.1042/bj0490686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA DU B. N., Jr, GREENBERG D. M. The tyrosine oxidation system of liver. I. Extracts of rat liver acetone powder. J Biol Chem. 1951 May;190(1):245–255. [PubMed] [Google Scholar]
- LA DU B. N., ZANNONI V. G. The role of ascorbic acid in tyrosine metabolism. Ann N Y Acad Sci. 1961 Apr 21;92:175–191. doi: 10.1111/j.1749-6632.1961.tb46117.x. [DOI] [PubMed] [Google Scholar]
- LA DU B. N., ZANNONI V. G. The tyrosine oxidation system of liver. II. Oxidation of p-hydroxyphenylpyruvic acid to homogentisic acid. J Biol Chem. 1955 Dec;217(2):777–787. [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- SCHEPARTZ B., BOYLE A. Transamination as a step in tyrosine metabolism. J Biol Chem. 1951 Nov;193(1):293–298. [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]