Abstract
Background:
Reticulated platelets (RPs) are newly synthesized platelets. Recently, an automatic method was established to detect RPs as a percentage of the immature platelet fraction (IPF%). Although, neonates often develop thrombocytopenia at some time during their hospitalization, the details of IPF% in neonates remain unclear. We, therefore, studied the relations between IPF% and other factors to gain a more detailed understanding of IPF% in neonates.
Methods:
The following clinical data were obtained from the medical records of 105 neonates who met our inclusion criteria: Gestational age, birth weight, IPF% and platelet count of neonatal peripheral blood at birth, and perinatal data. The subjects were divided into three groups: Group A, birth weight standard deviation score (SDS) ≥ −2 standard deviation (SD) and ≤ +2 SD; Group S, < −2 SD; and Group L, > +2 SD.
Results:
IPF% correlated negatively with platelet count at birth in the whole study population. IPF% was 2.8 ± 1.3% in term neonates, and IPF correlated negatively with gestational age and birth weight. Platelet count correlated positively with birth weight SDS in the whole study population and in Group S. IPF% correlated negatively with birth weight SDS in the whole study population and in Group S. In neonates with a platelet count below 25 × 104/μl, IPF% correlated negatively with platelet count. Among other neonates, however, IPF% remained almost constant.
Conclusion:
Monitoring of IPF% is useful for estimating the function of thrombocytopoiesis in neonates and preterm infants.
Keywords: Birth weight standard deviation score, immature platelet fraction, small for gestational age infant, thrombocytopenia, XE-2100
INTRODUCTION
Reticulated platelets (RPs) are newly synthesized platelets. Platelet production can be inferred by evaluation of megakaryocytes in bone marrow aspirates or sections.[1] A simple peripheral blood assay for estimating platelet production, analogous to red blood cell reticulocyte counts, would solve this problem. Recently, an automatic method was established to detect RPs as the percentage of immature platelet fraction (IPF%).
Previous studies have shown that IPF% could be used as a marker of platelet count for clinical medicine.[2,3,4] One study indicated that IPF is a marker for platelet recovery after stem cell transplantation in children and that IPF should be considered a useful marker of imminent platelet recovery so that unnecessary platelet transfusions can be avoided.[2] Another report showed the same to be true in adults.[3] De Blasi et al.[4] reported that IPF% is a cellular marker predicting sepsis according to changes in IPF% before manifestation of sepsis; they also described the IPF% time course after intensive care unit admission.
Neonates often develop thrombocytopenia during their hospitalization. In neonates admitted to a neonatal intensive care unit (NICU), thrombocytopenia develops in 22-35% of all admissions, with the rate increasing as gestational age decreases.[5,6] Thrombocytopenia, which presents after the 1st 3 days of life, occurs due to sepsis or necrotizing enterocolitis in >80% of cases.[7,8] Platelet transfusions are frequently administered, often unnecessarily, yet the details of IPF% in neonates or preterm infants remain unclear. We studied the relations between IPF% and platelet count, gestational age, birth weight, and birth weight standard deviation score (birth weight SDS) at birth.
METHODS
Between January and October 2011, 105 neonates not meeting the below exclusion criteria were admitted to the NICU of the Japanese Red Cross Hospital, Tokyo, Japan. Their gestational age was 35.0 ± 4.7 weeks and birth weight was 2126 ± 883 g. Exclusion criteria included neonates with chromosomal abnormalities, perinatal infection, early-onset sepsis, disseminated intravascular coagulation, or neonatal thrombocytopenia (e.g., chronic fetal hypoxia, perinatal asphyxia, alloimmune, autoimmune, thrombosis, bone marrow replacement, Kasabach-Merritt syndrome, and inheritance[6,9]), and neonates admitted at night or on holidays (our hospital did not use the hematology analyzer at these times). IPF% was analyzed soon after phlebotomy using a fully automated hematology analyzer (XE-2100; Sysmex, Japan) with specially designed software. Data relating to the study population were reviewed retrospectively. The following clinical data were obtained from the medical records of the neonates in the study population: Gestational age, birth weight, IPF% and platelet count level of neonatal peripheral venous samples before 24 h of life (on admission to NICU), and perinatal data.
Because of the retrospective nature of this study, approval of the research ethics committee in our hospital was not required. Under Japanese legislation, written parental consent was not required for this retrospective study.
Neonates were divided into three groups according to birth weight SDS: Group appropriate (Group A, birth weight SDS ≥ −2 standard deviation (SD) and ≤ +2 SD); Group small (Group S, < −2 SD); and Group large (Group L, > +2 SD).
Statistical analysis was performed and P values were derived from Fisher's test, the Mann-Whitney U-test, and Pearson's correlation coefficient. A P ≤ 0.05 was considered statistically significant. Analysis was performed with International Business Machines (IBM) Statistical Package Social Sciences Statistics 20 (IBM, New York).
RESULTS
A total of 602 neonates were admitted to the NICU of the Japanese Red Cross Hospital, Tokyo, Japan, between January and October 2011. After application of the exclusion criteria, 105 (17%) neonates remained and were included in the study. IPF% was measured at birth (in the first 24 h of life), and neonates were categorized into Group A (n = 81), Group S (n = 21), or Group L (n = 3) based on this measurement.
Patient characteristics in Groups A and S are shown in Table 1. The neonates in these two groups did not differ significantly in gestational age or sex. Incidence of polycyesis was significantly higher in Group S than Group A. There were only three neonates in Group L. They were included in the analysis but only as part of the whole study population.
Table 1.
Patient characteristics in Groups A and S*
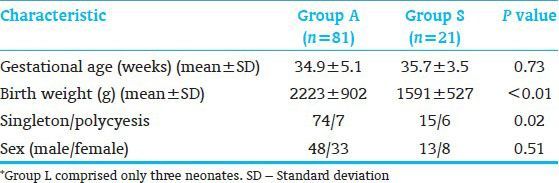
Analysis of IPF% and platelet count at birth in the neonates is shown in Figure 1. IPF% correlated negatively with platelet count in the whole study population (r = −0.33, P < 0.01) [Figure 1a] (---) and with platelet count in Group S (r = −0.71, P < 0.01) [Figure 1b]. There was no correlation between IPF% and platelet count in Group A.
Figure 1.
Correlation of immature platelet fraction percentage (IPF%) with platelet count at birth in the neonates. (a) IPF% correlated negatively with platelet count in the whole study population (r = −0.33, P < 0.01). IPF% at birth in the neonates. IPF% correlated negatively with platelet count in neonates with platelet counts under 250 × 109/L (r = −0.27, P = 0.03), whereas IPF% did not correlate significantly with neonates with platelet count over 250 × 109/L (r = −0.26, P = 0.08). A platelet count of 250 × 109/L was approximately the average count of Group A. (b) IPF% correlated negatively with platelet count in Group S (r = −0.71, P < 0.01)
We used 45 neonates from Group A, who were of gestational age 37-41 weeks, in the comparison of IPF% between Group A and Group S at birth. IPF% was 2.8 ± 1.3% in term neonates. IPF% was 2.5 ± 0.9% in Group A and 3.7 ± 2.0% in Group S. This difference was not significant.
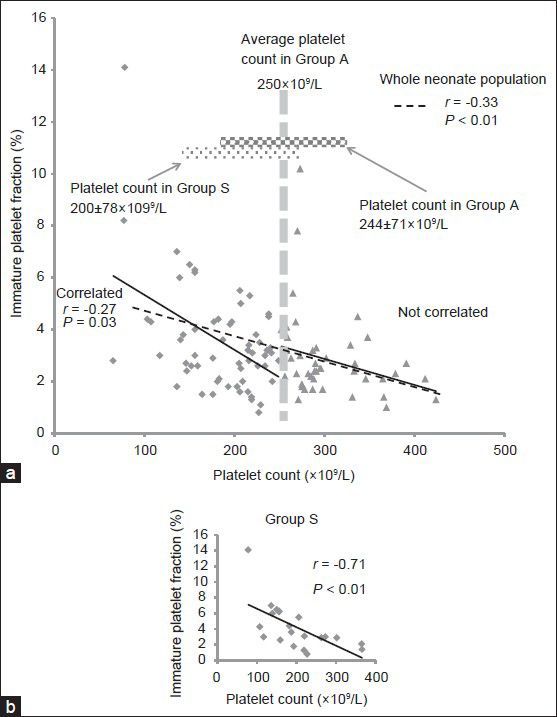
Figure 2 documents the relation of IPF% and platelet count at birth with gestational age and birth weight in the neonates. Although there was no significant negative correlation between platelet count and gestational age (r = 0.06, P = 0.48) [Figure 2a], there was a significant negative correlation between IPF% and gestational age (r = −0.24, P = 0.01) [Figure 2b]. There was a significant positive correlation between platelet count and birth weight (r = 0.19, P = 0.04) [Figure 2c] and a significant negative correlation between IPF% and birth weight (r = −0.31, P < 0.01) [Figure 2d].
Figure 2.
Relation of immature platelet fraction percentage (IPF%) and platelet count at birth with gestational age and birth weight in the neonates. (a) There was no significant negative correlation of platelet count with gestational age (r = 0.06, P = 0.48). (b) IPF% correlated negatively with gestational age (r = −0.24, P = 0.01). (c) Platelet count correlated positively with birth weight (r = 0.19, P = 0.04). (d) IPF% correlated negatively with birth weight (r = −0.31, P = 0.00)
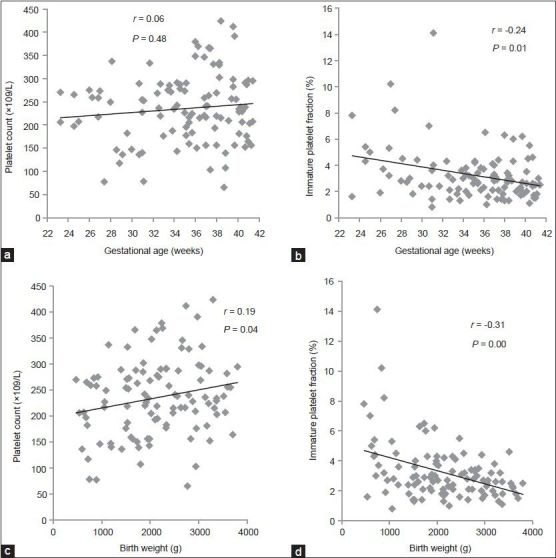
Analysis of platelet count and IPF% in the neonates at birth is illustrated in Figure 3. Platelet count correlated positively with birth weight SDS in both the whole study population (r = 0.29, P < 0.01) [Figure 3a] and in Group S (r = 0.54, P = 0.01) [Figure 3b] but not in Group A (r = 0.19, P = 0.08). IPF% correlated negatively with birth weight SDS in both the whole study population (r = −0.22, P = 0.02) [Figure 3c] and in Group S (r = −0.52, P = 0.01) [Figure 3d] but did not correlate in Group A (r = −0.17, P = 0.12).
Figure 3.
Platelet count and immature platelet fraction percentage (IPF%) at birth in the neonates. (a) Platelet count correlated positively with birth weight standard deviation score (SDS) in the whole neonate population (r = 0.29, P < 0.01). (b) Platelet count correlated positively with birth weight SDS in Group S neonates (circled in a) (r = 0.54, P = 0.01). (c) IPF% correlated negatively with birth weight SDS in the whole neonate population (r = −0.22, P = 0.02). (d) IPF% correlated negatively with birth weight SDS in Group S neonates circled in c) (r = −0.52, P = 0.01)
Alteration of IPF% associated with platelet count is also shown in Figure 1a (—), which shows both the alteration of IPF% associated with platelet count and the mean of the platelet counts in Groups A and S. Mean platelet counts were 200 ± 78 × 109/L in Group S and 244 ± 71 × 109/L in Group A. IPF% was divided into two sections according to the average of the platelet count in Group A of 250 × 109/L. In the neonates with a platelet count below 250 × 109/L, IPF% correlated negatively with platelet count (r = −0.46, P = 0.03), whereas there was no significant correlation between IPF% and platelet count in the other neonates. In short, IPF% remained almost constant.
DISCUSSION
A previous report suggested the examination of RPs as a marker of thrombocytopenia.[10] We showed that IPF% was associated with platelet count in neonates at birth, and we produced reference values of IPF% in neonates. In the future, IPF% could potentially be used as a cellular marker for indirectly predicting thrombopoiesis.
We examined the influence of immaturity on the factors of gestational age and birth weight. These results showed a tendency for an increase of IPF% in instances of greater immaturity. In a previous study, RP percentages, which were quantified with flow cytometry, were measured at birth in neonates without thrombocytopenia in three gestational age groups (<30, 30-36, and > 36 weeks). Neonates younger than 30 weeks of gestation were found to have had significantly higher RP percentages than older neonates.[11] Similarly, Kihara et al.[12] investigated the IPF% and platelet count at birth of non-thrombocytopenic neonates and found that IPF% correlated negatively with gestational age. Our results also indicated a similar relation between IPF% and platelet count.
Special attention should be paid to one point, which is the increase in hematopoietic cells or immature nucleated cells in the umbilical cord blood of preterm infants and which causes a variety of perinatal stresses. Saxonhouse et al.[13] reported the inhibitory effect of hypoxia on the proliferation of megakaryocyte progenitors through hypoxia-induced changes in the environment of fetal hematopoiesis. The reason for this increase of IPF% in peripheral blood collected within 24 h after birth, which reflects bone marrow function, is not clear.[14] Larger studies are needed to elucidate the relation between bone marrow function and thrombocytopoiesis in neonates.
The relation between IPF% and platelet count was remarkable in Group S. Platelet count increased in association with increasing birth weight SDS, and IPF% significantly decreased in association with increasing birth weight SDS. Cremer et al.[15] reported that small for gestational age (SGA) neonates with moderate and severe thrombocytopenia might have pronounced suppression of megakaryopoiesis compared with neonates with infection. Thrombopoiesis in SGA infants is known as a passing phenomenon at an early stage after birth that is poorly recognized. Wasiluk et al.[16] suggested that thrombocytopenia in SGA infants may be due to placental vascular pathology, fetal consumptive coagulopathy and platelet destruction, and a local imbalance of thromboxane A2 causing placental vasoconstriction and platelet aggregation. Fuse et al.[17,18] suggested that one cause in SGA infants was accelerated blood coagulation and fibrinolysis. Although platelet function did not differ significantly, we hypothesize that the IPF% in the SGA infants of Group S increased to compensate for the consumption of platelets in utero or in the placenta.
Thrombocytopenia is one of the most common hematological problems in neonates,[6] to whom platelet transfusions are frequently administered. Platelet transfusions are often administered when a neonate's platelet count is lower than the indication for platelet transfusion. Although it is too difficult to predict whether neonates with thrombocytopenia who have a severe reduction in platelet count will improve, the monitoring of IPF has been proposed as a method for predicting platelet recovery and thereby reducing the need for transfusion. Hennel et al.[19] noted that the time to platelet recovery depends on the source of stem cells and found no significant differences between IPF% peak concentration and time between IPF peak concentration and platelet recovery. In the future, we will investigate the difference between the change in platelet count and IPF% over 24 h. Therefore, it is necessary to clarify the indicator of necessity for administration of platelet transfusions.
Figure 1a shows that IPF% increased with a decrease in platelet count, although it was almost constant in the neonates in whom the platelet count was more than 250 × 109/L. In other words, platelet production was maintained in spite of a high platelet count. Despite this limitation, IPF% is helpful for use as a predictive marker for platelet recovery, especially when making decisions regarding the administration of platelet transfusion.
Another report showed that although high IPF concentrations are generally followed by platelet recovery, wide variations exist between individuals, and there were even cases in which no recovery was recorded.[2] That study served as a warning on making judgments based only on increasing IPF%. We did not describe the IPF% time course in the present study, and thus further investigation will be required before IPF% can be used in clinical practice.
Establishing practical use of IPF% not only in adults and children but also in neonates and preterm infants will be useful to avoid unnecessary treatment and platelet transfusions and for the provision of early and appropriate treatment. A larger clinical study with endpoints is needed to assess the clinical value of IPF% measurement.
Preserved blood cannot be examined by the usual technique.[20,21] The present study was limited by the fact that neonates born after normal daytime working hours were excluded from the study, which resulted in only 105 neonates fulfilling the study criteria.
CONCLUSION
Our study examined the relation between IPF% and gestational age, birth weight, and platelet count in neonates at birth. IPF% correlated negatively with platelet count at birth in the whole neonate population. The monitoring of IPF% appears to be useful for estimating the function of thrombocytopoiesis in neonates and preterm infants. However, further studies from other centers will be required to confirm the relation between IPF% and the other factors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dale GL, Friese P, Hynes LA, Burstein SA. Demonstration that thiazole-orange-positive platelets in the dog are less than 24 hours old. Blood. 1995;85:1822–5. [PubMed] [Google Scholar]
- 2.Saigo K, Sakota Y, Masuda Y, Matsunaga K, Takenokuchi M, Nishimura K, et al. Automatic detection of immature platelets for decision making regarding platelet transfusion indications for pediatric patients. Transfus Apher Sci. 2008;38:127–32. doi: 10.1016/j.transci.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Nomura T, Kubota Y, Baba N, Izeki K, Arai T, Yamaoka G, et al. Immature platelet fraction measured using the automated hematology analyzer XE-2100: A possible predictive marker for platelet recovery after chemotherapy and hematopoietic stem cell transplantation. Jpn J Transfus Cell Ther. 2009;55:691–7. [Google Scholar]
- 4.De Blasi RA, Cardelli P, Costante A, Sandri M, Mercieri M, Arcioni R. Immature platelet fraction in predicting sepsis in critically ill patients. Intensive Care Med. 2013;39:636–43. doi: 10.1007/s00134-012-2725-7. [DOI] [PubMed] [Google Scholar]
- 5.Sola-Visner M, Saxonhouse MA, Brown RE. Neonatal thrombocytopenia: What we do and don’t know. Early Hum Dev. 2008;84:499–506. doi: 10.1016/j.earlhumdev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Roberts I, Stanworth S, Murray NA. Thrombocytopenia in the neonate. Blood Rev. 2008;22:173–86. doi: 10.1016/j.blre.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IA. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med. 2002;12:35–41. doi: 10.1046/j.1365-3148.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 8.Roberts IA, Murray NA. Neonatal thrombocytopenia: New insights into pathogenesis and implications for clinical management. Curr Opin Pediatr. 2001;13:16–21. doi: 10.1097/00008480-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Kumari S, Singhal A, Bahl A. Neonatal thrombocytopenia and platelets transfusion. Asian J Transfus Sci. 2012;6:161–4. doi: 10.4103/0973-6247.98924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinder HM, Munz UJ, Ault KA, Bonan JL, Smith BR. Reticulated platelets in the evaluation of thrombopoietic disorders. Arch Pathol Lab Med. 1993;117:606–10. [PubMed] [Google Scholar]
- 11.Peterec SM, Brennan SA, Rinder HM, Wnek JL, Beardsley DS. Reticulated platelet values in normal and thrombocytopenic neonates. J Pediatr. 1996;129:269–74. doi: 10.1016/s0022-3476(96)70253-6. [DOI] [PubMed] [Google Scholar]
- 12.Kihara H, Ohno N, Karakawa S, Mizoguchi Y, Fukuhara R, Hayashidani M, et al. Significance of immature platelet fraction and CD41-positive cells at birth in early onset neonatal thrombocytopenia. Int J Hematol. 2010;91:245–51. doi: 10.1007/s12185-009-0482-3. [DOI] [PubMed] [Google Scholar]
- 13.Saxonhouse MA, Rimsza LM, Stevens G, Jouei N, Christensen RD, Sola MC. Effects of hypoxia on megakaryocyte progenitors obtained from the umbilical cord blood of term and preterm neonates. Biol Neonate. 2006;89:104–8. doi: 10.1159/000088561. [DOI] [PubMed] [Google Scholar]
- 14.Lim FT, Scherjon SA, van Beckhoven JM, Brand A, Kanhai HH, Hermans JM, et al. Association of stress during delivery with increased numbers of nucleated cells and hematopoietic progenitor cells in umbilical cord blood. Am J Obstet Gynecol. 2000;183:1144–52. doi: 10.1067/mob.2000.108848. [DOI] [PubMed] [Google Scholar]
- 15.Cremer M, Weimann A, Schmalisch G, Hammer H, Bührer C, Dame C. Immature platelet values indicate impaired megakaryopoietic activity in neonatal early-onset thrombocytopenia. Thromb Haemost. 2010;103:1016–21. doi: 10.1160/TH09-03-0148. [DOI] [PubMed] [Google Scholar]
- 16.Wasiluk A, Mantur M, Kemona H, Szczepañski M, Jasiñska E, Milewski R. Thrombopoiesis in small for gestational age newborns. Platelets. 2009;20:520–4. doi: 10.3109/09537100903207505. [DOI] [PubMed] [Google Scholar]
- 17.Fuse Y, Hosono Y, Kakinuma S. A study of blood coagulation and fibrinolysis in a small-for- gestational-age infant birth group. Nihon Sanka Fujinka Gakkai Zasshi. 1986;38:643–6. [PubMed] [Google Scholar]
- 18.Fuse Y, Kakinuma S, Hosono Y. Blood coagulation and fibrinolysis in cord blood with reference to birth weight. Nihon Sanka Fujinka Gakkai Zasshi. 1985;37:1825–32. [PubMed] [Google Scholar]
- 19.Hennel E, Kentouche K, Beck J, Kiehntopf M, Boeer K. Immature platelet fraction as marker for platelet recovery after stem cell transplantation in children. Clin Biochem. 2012;45:749–52. doi: 10.1016/j.clinbiochem.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama M, Hayashi S, Kabutomori O, Yamanishi H, Suehisa E, Kurata Y, et al. Effects of anticoagulants and storage temperature on immature platelet fraction % (IPF%) values in stored samples measured by the automated hematology analyzer, XE-5000 – Utility of CTAD-anticoagulation and room temperature storage. Rinsho Byori. 2011;59:452–8. [PubMed] [Google Scholar]
- 21.Osei-Bimpong A, Saleh M, Sola-Visner M, Widness J, Veng-Pedersen P. Correction for effect of cold storage on immature platelet fraction. J Clin Lab Anal. 2010;24:431–3. doi: 10.1002/jcla.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]


