Abstract
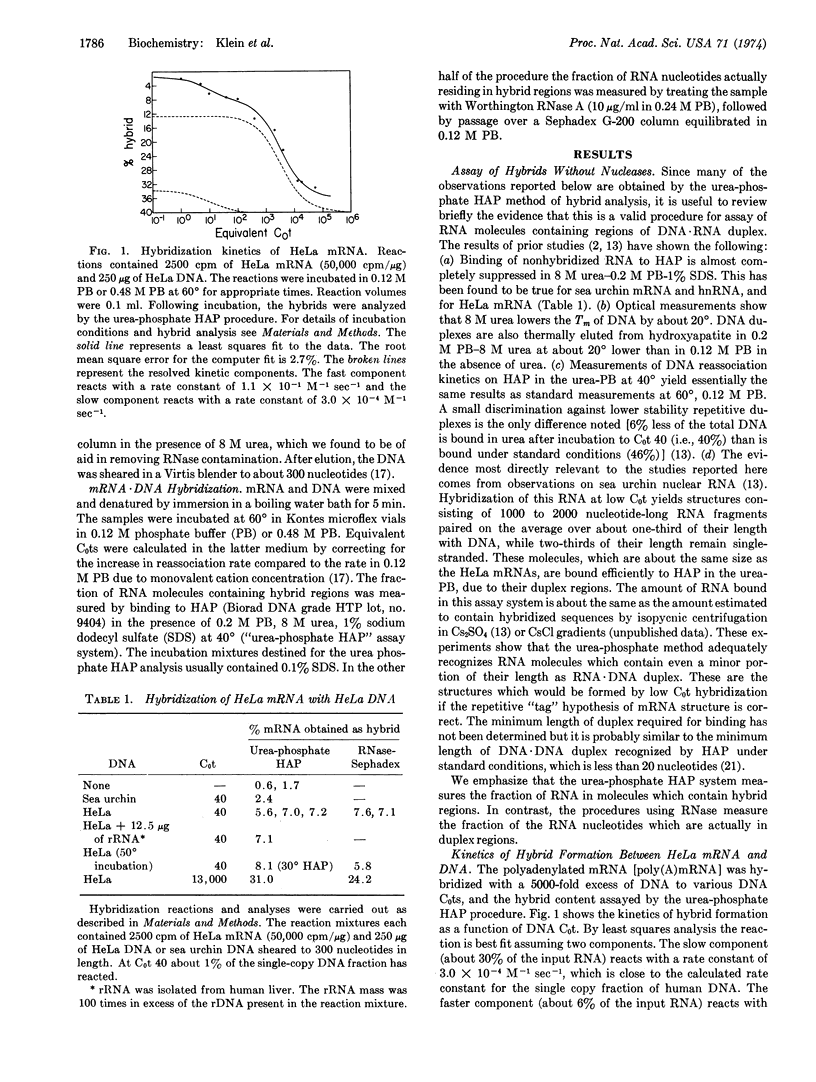
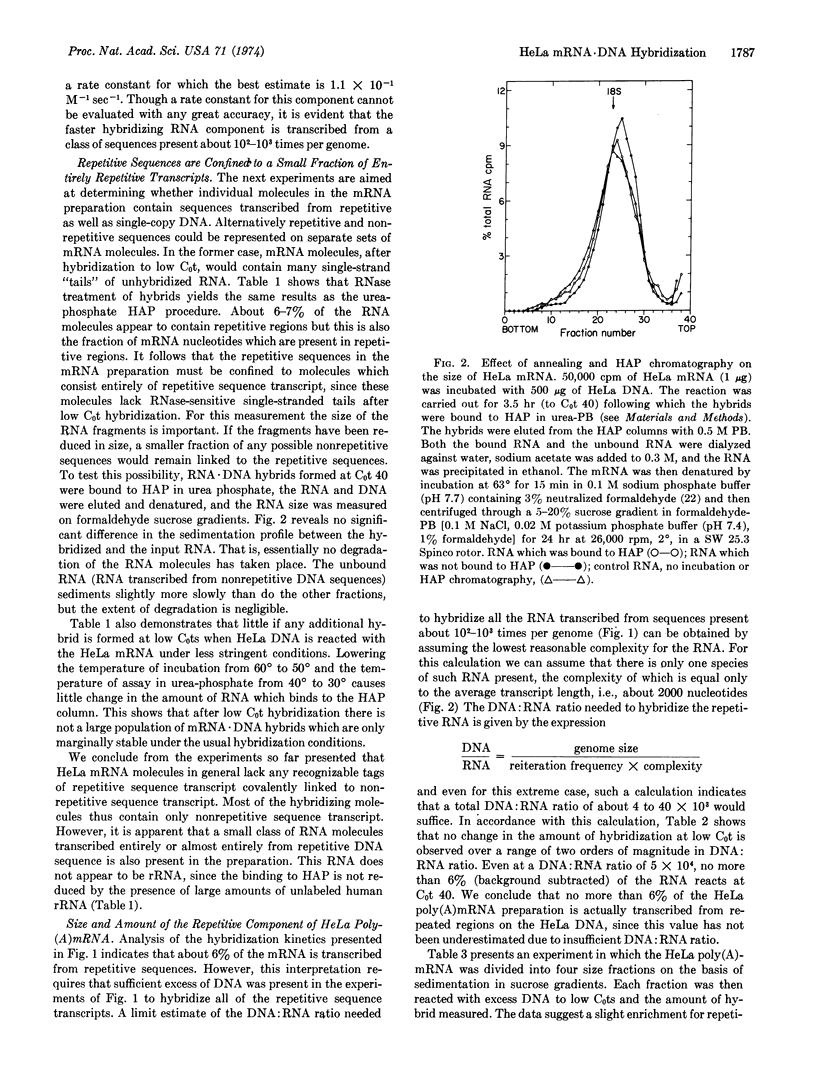
Polyadenylated messenger RNA extracted from HeLa cells was hybridized with a mass excess of HeLa DNA. The kinetics of the hybridization reaction demonstrated that most of the messenger RNA is transcribed from nonrepetitive DNA. The amount of messenger RNA hybridized to DNA was measured both with and without prior RNase treatment. Comparison of the results indicates that within the limits of detection, HeLa messenger RNA does not contain repetitive sequence elements covalently linked to nonrepetitive sequence transcripts. However, a small fraction of the HeLa messenger RNA preparation is transcribed entirely from repetitive DNA sequences. This fraction represents about 6% of the total polyadenylated messenger RNA preparation.
Keywords: polyadenylated mRNA, hydroxyapatite, RNA·DNA hybridization
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Reiteration frequency of duck haemoglobin genes. Nat New Biol. 1973 Feb 14;241(111):204–207. doi: 10.1038/newbio241204a0. [DOI] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Balint R. The distribution of rapidly hybridizing RNA sequences in heterogeneous nuclear RNA and mRNA from HeLa cells. J Cell Physiol. 1970 Dec;76(3):349–356. doi: 10.1002/jcp.1040760312. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Dina D., Crippa M., Beccari E. Hybridization properties and sequence arrangement in a population of mRNAs. Nat New Biol. 1973 Mar 28;242(117):101–105. doi: 10.1038/newbio242101a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Galau G. A., Britten R. J., Davidson E. H. Nonrepetitive DNA sequence representation in sea urchin embryo messenger RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3516–3520. doi: 10.1073/pnas.70.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Hybridization properties of DNA sequences directing the synthesis of messenger RNA and heterogeneous nuclear RNA. J Cell Biol. 1971 Sep;50(3):774–786. doi: 10.1083/jcb.50.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Hell A., Birnie G. D., Paul J. Evidence for single copies of globin genes in the mouse genome. Nature. 1972 Sep 22;239(5369):219–221. doi: 10.1038/239219a0. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Renaturation of bacteriophage phiX174 DNA-RNA hybrid: RNA length effect and nucleation rate constant. J Mol Biol. 1973 Jul 15;77(4):495–500. doi: 10.1016/0022-2836(73)90218-0. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L., DARNELL J. E., Jr A simplified procedure for purification of large amounts of poliovirus: characterization and amino acid analysis of Type 1 poliovirus. J Biol Chem. 1960 Jan;235:70–73. [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Murphy W., Attardi G. Stability of cytoplasmic messenger RNA in HeLa cels. Proc Natl Acad Sci U S A. 1973 Jan;70(1):115–119. doi: 10.1073/pnas.70.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Birnstiel M. L., Purdom I. F., Williamson R. Genes coding for polysomal 9S RNA of sea urchins: conservation and divergence. Nature. 1972 Nov 24;240(5378):225–228. doi: 10.1038/240225a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]



