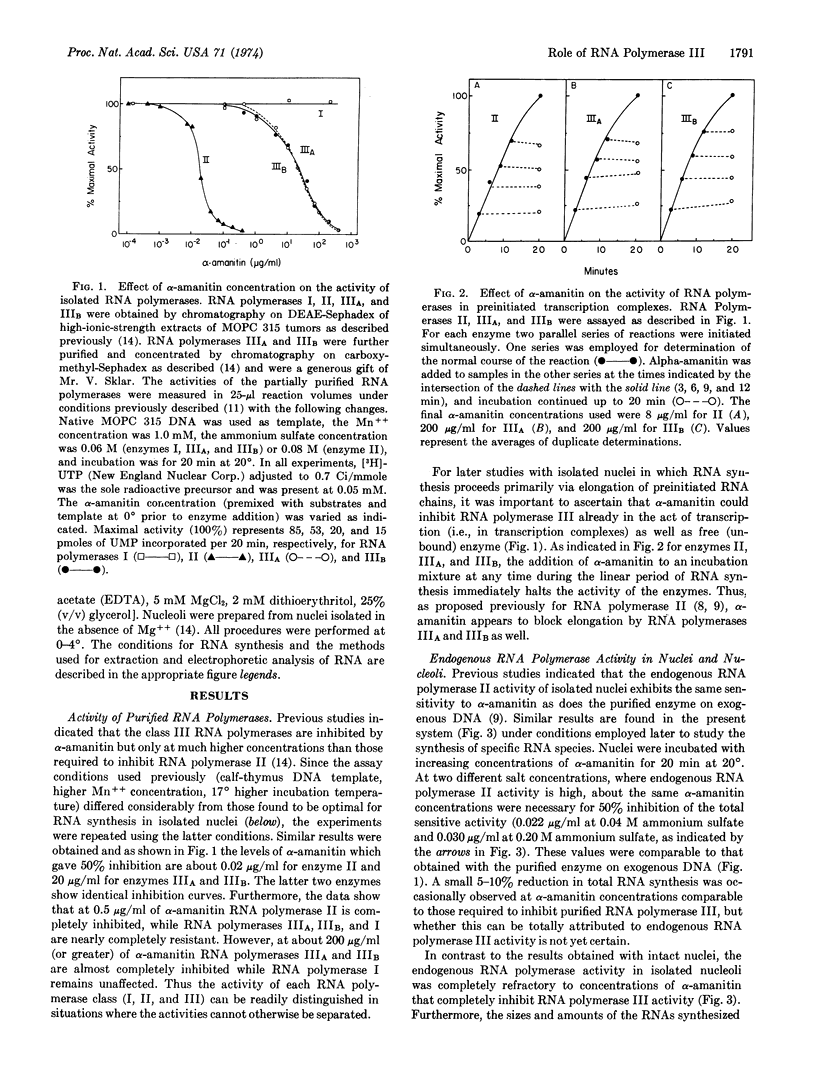
Abstract
Mouse myeloma cells have previously been shown (L. B. Schwartz, V. E. F. Sklar, J. A. Jaehning, R. Weinmann & R. G. Roeder, submitted for publication) to contain two chromatographically distinct forms of RNA polymerase III (designated IIIA and IIIB). The enzymes are unaffected by low α-amanitin concentrations which completely inhibit RNA polymerase II, but they exhibit characteristic inhibition curves (identical for IIIA and IIIB) at higher toxin concentrations. RNA polymerase I was unaffected at all α-amanitin concentrations tested. Myeloma RNA polymerases II, IIIA, and IIIB appear to be inhibited by the same mechanism, since the toxin rapidly blocks chain elongation by each enzyme. The characteristic α-amanitin sensitivity of RNA polymerase III has been employed in studies of the function(s) of the class III RNA polymerases.
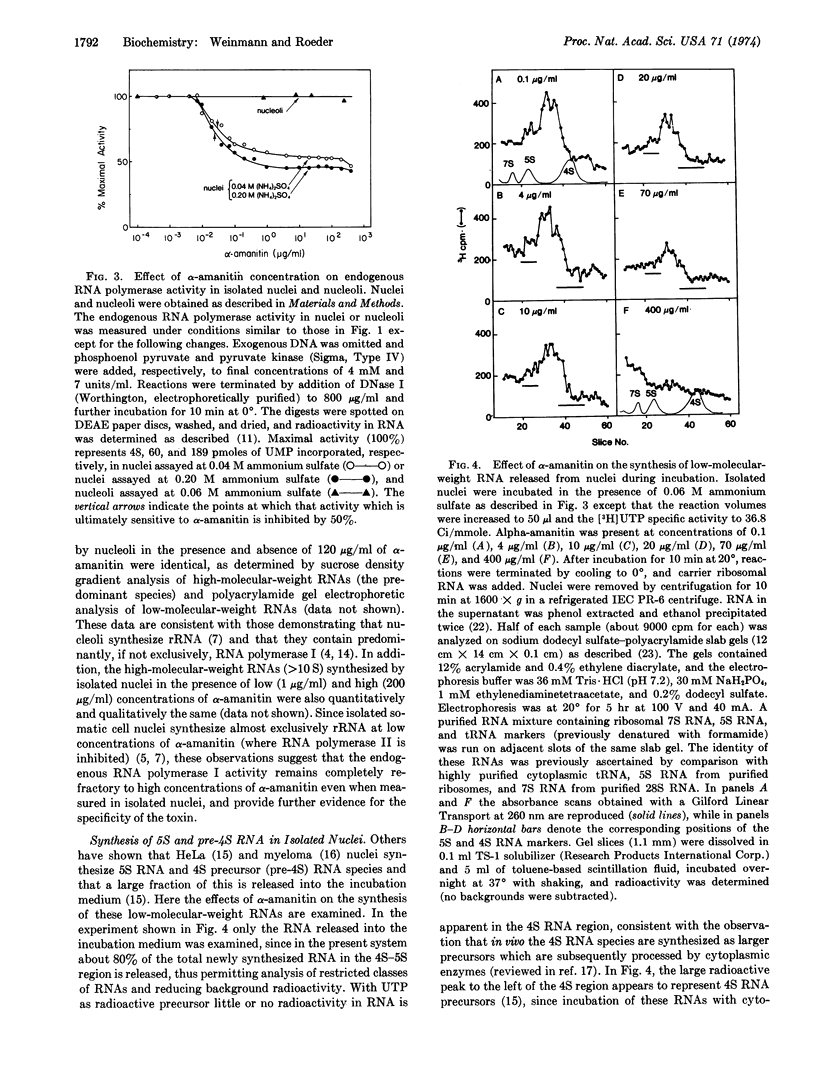
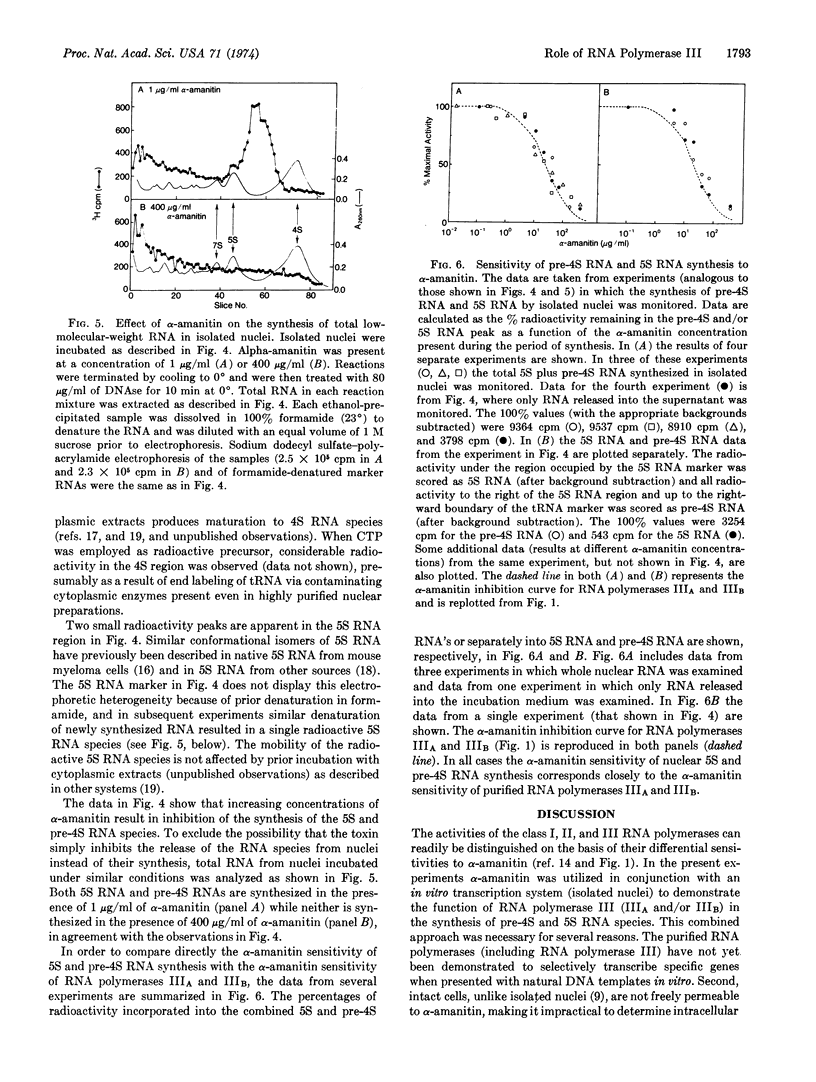
Isolated myeloma nuclei and nucleoli contińue to synthesize RNA via the endogenous RNA polymerases when incubated in vitro. With nuclei, newly synthesized 4S precursor (pre-4S) and 5S RNA species were detected by electrophoretic analysis either of the total nuclear RNA or of the RNA released into the supernatant during incubation. The synthesis of both pre-4S and 5S RNA species was inhibited by α-amanitin, but only at high concentrations; and the α-amanitin inhibition curves for these RNAs were identical to those obtained for solubilized RNA polymerases IIIA and IIIB. In control experiments it was shown that the endogenous RNA polymerase II activity of isolated nuclei was inhibited by α-amanitin concentrations similar to those required to inhibit purified enzyme II. However, 40-50% of the endogenous activity of nuclei and 100% of the endogenous activity of purified nucleoli was completely resistant to the high α-amanitin concentrations necessary to inhibit the RNA polymerase III activities. These experiments rule out nonspecific inhibitory effects in the endogenous systems.
These results unequivocally demonstrate the role of RNA polymerase III (IIIA and/or IIIB) in the synthesis of (pre) 4S RNAs and a 5S RNA species.
Keywords: mouse myeloma cells, isolated nuclei, α-amanitin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P., Gissinger F., Kedinger C., Mandel J. L., Meilhac M., Nuret P. Structural and functional properties of three mammalian nuclear DNA-dependent RNA polymerases. Acta Endocrinol Suppl (Copenh) 1972;168:222–246. doi: 10.1530/acta.0.071s222. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of ribonucleic acid in isolated mouse myeloma nuclei. Biochemistry. 1973 Aug 28;12(18):3440–3446. doi: 10.1021/bi00742a013. [DOI] [PubMed] [Google Scholar]
- Mowshowitz D. B. Transfer RNA synthesis in HeLa cells. II. Formation of tRNA from a precursor in vitro and formation of pseudouridine. J Mol Biol. 1970 May 28;50(1):143–151. doi: 10.1016/0022-2836(70)90111-7. [DOI] [PubMed] [Google Scholar]
- Niessing J., Schnieders B., Kunz W., Seifart K. H., Sekeris C. E. Inhibition of RNA synthesis by alpha-amanitin in vivo. Z Naturforsch B. 1970 Oct;25(10):1119–1125. doi: 10.1515/znb-1970-1011. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Isolation and partial characterization. J Biol Chem. 1974 Jan 10;249(1):241–248. [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Levels of activity during oocyte and embryonic development. J Biol Chem. 1974 Jan 10;249(1):249–256. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Krsmanovic V. KB cell RNA polymerases: occurrence of nucleoplasmic enzyme 3. FEBS Lett. 1973 Sep 15;35(2):331–335. doi: 10.1016/0014-5793(73)80316-3. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



