SAG encodes a MDN1 domain containing protein in Arabidopsis. Seeds of a T-DNA insertion line of this gene exhibited hypersensitivity to ABA, mannitol and NaCl during seed germination and early seedling development. SAG was genetically epistatic to ABI3 and ABI5
Key words: Abscisic acid, AtSAG, drought, MDN1 domain, salt, seed germination
Abstract
Three proteins containing a midasin homologue 1 (MDN1) domain from the yeast Solanum chacoense and Arabidopsis thaliana have important functions in yeast survival, seed development, and female gametogenesis. In this study, a novel protein containing the MDN1 domain from Arabidopsis negatively regulated abscisic acid (ABA) signalling during seed germination. Seeds of a T-DNA insertion line of this gene exhibited increased sensitivity to ABA during seed germination and seedling development (named sag). By contrast, seeds with overexpressed AtSAG (OX2) were less sensitive to ABA. The seeds of the sag mutant showed similar sensitivity to high concentrations of mannitol and NaCl during these stages. AtSAG was also highly expressed in germinating seeds. However, ABA-induced AtSAG expression remained almost unchanged. ABA-responsive marker genes, including ABI3, ABI5, Em1, Em6, RD29A, and RAB18, were upregulated in sag mutants but were downregulated in OX2. Genetic analyses indicated that the function of AtSAG in ABA signalling depended on ABI3 and ABI5. The expression of some target genes of ABI3 and ABI5, such as seed storage protein and oleosin genes, was induced higher by ABA in sag mutants than in wild-type germinated seeds, even higher than in abi5 mutants. This finding indicated that other regulators similar to ABI3 or ABI5 played a role during these stages. Taken together, these results indicate that AtSAG is an important negative regulator of ABA signalling during seed germination and seedling development.
Introduction
Seed germination marks the beginning of a new growth cycle in higher plants and is subject to complex mechanisms of regulation by both internal and environmental signals (Bewley, 1997; Finch-Savage and Leubner-Metzger, 2006). For instance, abscisic acid (ABA) is a phytohormone that functions in plant seed germination, seedling growth, and stress tolerance (Koornneef et al., 1989; Leung and Giraudat, 1998; Finkelstein et al., 2002). Molecular genetics approaches have revealed several proteins that function in ABA signalling during seed germination (Finch-Savage and Leubner-Metzger, 2006; Holdsworth et al., 2008; Penfield and King, 2009). For example, ABA-insensitive1 (ABI1) (Leung et al., 1994) and ABI2 (Rodriguez et al., 1998) are protein phosphatases that negatively regulate ABA signalling during seed dormancy and germination. By contrast, ABI transcription factors, such as ABI3 and ABI5, can positively regulate ABA signalling during seed development and germination (Finkelstein et al., 2002). In particular, ABI5 is a basic leucine zipper (bZIP) transcription factor (Finkelstein and Lynch, 2000) that elicits enhanced response to exogenous ABA during germination, seedling development, and subsequent vegetative growth (Lopez-Molina et al., 2001; Finkelstein et al., 2002). ABI5 physically interacts with ABI3 (Nakamura et al., 2001) and is genetically epistatic to ABI3 (Lopez-Molina et al., 2002; Nakashima et al., 2009). ABI5 is required in germination and post-germination growth arrest checkpoint (Lopez-Molina et al., 2001). Some regulators that control ABA sensitivity are mediated by ABI5. For instance, three Arabidopsis SnRK2 protein kinases, namely SRK2D, SRK2E, and SRK2I, are involved in ABA signalling through ABI5 phosphorylation (Nakashima et al., 2009). Overexpression of two coupled components of the mitogen-activated protein kinase cascade, MdMPK1 and MdMKK1. from apple leads to ABA sensitivity by ABI5 phosphorylation during transgenic Arabidopsis seed germination (Wang et al., 2010).
The midasin homologue 1 (MDN1) domain contains a von Willebrand A (VWA) domain and an adenosine triphosphatases associated with diverse cellular activities (AAA) domain. Proteins containing those domains have important functions during development. For instance, the yeast MDN1 domain-containing protein Rea1 functions in ribosome maturation (Talkish and Woolford, 2009; Bassler et al., 2010). ScMDN1, a plant homologue of Rea1, is involved in seed and shoot development in Solanum chacoense (Chantha and Matton, 2007). AtMDN1 from Arabidopsis is essential for female gametophyte development (Chantha et al., 2010). Furthermore, VWA and AAA domain-containing proteins mediate protein–protein interactions involved in the assembly of complexes, such as ribosomes, proteasomes, and chloroplasts (Snider et al., 2008). For example, Arabidopsis Rpn10 contains the VWA domain at the N-terminal and functions as a component of 26S proteasome by recognition of multiubiquitin of specific proteins, such as ABI5 (Voges et al., 1999). The chloroplast Mg chelatase subunit D, which contains one AAA domain and one VWA domain (Whittaker and Hynes, 2002), plays a role in plant responses to ABA (Du et al., 2012). However, MDN1 domain-containing proteins related to ABI3 and ABI5 have not yet been reported.
The current study reports another MDN1 domain-containing AtSAG protein from Arabidopsis that negatively regulated ABA sensitivity during seed germination and seedling development. The possible regulatory mechanisms are discussed according to present data, bioinformatics, and related literature.
Materials and methods
Plant materials and growth conditions
Seeds of each genotype Arabidopsis thaliana from Columbia (Col-0) background were harvested at the same time from plants grown under the same conditions. Seeds were surface sterilized with 70% ethanol for 5min, incubated in 2.6% sodium hypochlorite for 10min, and washed five times with sterile water. Germination assays were carried out with three replicates of 100 seeds. The sterile seeds were plated on half-strength Murashige and Skoog (MS) medium containing 1% (w/v) sucrose and 0.8% agar (control). The seed-dotted plates were maintained in the dark at 4 °C for 3 d to break dormancy (stratification) and then transferred to a growth chamber under 16/8 light/dark conditions at 22 °C.
Verification of the single and double mutants
The sag mutant (Salk_013481), containing a T-DNA insertion in the exon of AtSAG, was bought from Arabidopsis Biological Resource Center (ABRC). The abi5 mutant (Salk_013163C) was a gift from Dr Yinggao Liu (Shandong Agricultural University, China). To determine whether the mutant line was homozygous, PCR was performed using genomic DNA with the following gene-specific primers: for sag, abi5 LP and RP and one specific primer LBb1.3 (Supplementary Table S1, available at JXB online).
The sag/abi5 double mutant was constructed by crossing the two single mutants. The double mutant was identified by PCR based on the genotype of the AtSAG locus and the confirmed sequence of the ABI5 locus.
Construction and generation of transgenic plants
To construct 35S:AtSAG, the AtSAG coding sequence was amplified using Col-0 cDNA by PCR with gene-specific primers (Supplementary Table S1). The resulting PCR product was cloned into the SalI and KpnI sites of binary vector pBI121 under the control of the cauliflower mosaic virus 35S promoter.
To generate a AtSAG-RNAi construct, Arabidopsis pFGC5941 vector (ABRC) for dsRNA production was used. A 360-bp fragment of AtSAG cDNA was amplified by PCR using gene-specific primers RNAi-F and RNAi-R (Supplementary Table S1). The fragment was initially cloned between AscI and SwaI sites before an inverted repeat of the same fragment was inserted between BamHI and XbaI sites of pFGC5941.
A 1550-bp promoter sequence was amplified from genomic DNA by PCR and verified by sequencing to construct pSAG:GUS. The PCR fragment was cloned into the HindIII and BamHI sites of PBI121 to obtain the construct containing the AtSAG native promoter fused in the β-glucuronidase (GUS) coding region. The primers used were GUS-F and GUS-R (Supplementary Table S1).
The transformation of Arabidopsis plants was performed by a floral dip infiltration method using Agrobacterium tumefaciens GV3101 (EHA105). T2 seeds from each selected transgenic plant were plated on half-strength MS medium containing 50mg l–1 kanamycin (for PBI121) or 10mg l–1 phosphinothricin (for pFGC5941) as selective antibiotics to select the homozygous lines.
Histochemical GUS staining
Histochemical localization of GUS activities in the transgenic seedlings or germinated seeds were analysed after the transgenic plants had been incubated overnight at 37 °C in 1mg ml–1 5-bromo-4-chloro-3-indolyl-glucuronic acid, 5mM potassium ferrocyanide, 0.03% Triton X-100 and 0.1M sodium phosphate buffer, pH 7.0. Then the tissues were cleaned with 70% ethanol. The cleaned tissues were then observed and pictures were taken by stereoscope. To examine the detailed GUS staining, the tissues were observed with a bright-field microscope and photographed. The GUS staining data were representative of at least five independent transgenic lines for each construct.
Seed germination and cotyledon greening
Plants of different genotypes were grown in the same conditions, and seeds were collected at the same time. For each comparison, seeds were planted on the same plate containing half-strength MS medium without or with different concentrations of ABA, 200mM NaCl, and 500mM mannitol. Plates were chilled at 4 °C in the dark for 3 d (stratified) and moved to 16/8 light/dark conditions at 22 °C. Seed germination and cotyledon greening were scored at the indicated times. Germination was defined as an obvious emergence of the radicle through the seed coat. Cotyledon greening is defined as obvious cotyledon expansion and turning green.
RNA extraction
For RNA isolation, the plant tissues grown after 3-d stratification for the indicated times in a growth chamber under 16/8 light/dark conditions at 22 °C were separately harvested, frozen in liquid nitrogen, and stored at –80 °C until use. Total RNA was isolated from different A. thaliana seedlings using a universal plant total RNA extraction kit (spin-column)-I (BioTeke, Beijing, China).
Real-time PCR analysis
cDNA was synthesized using PrimeScript RT (reverse transcriptase) with oligo-dT primer using the PrimeScript RT master mix kit (Takara). All samples were prepared to a final volume of 10 µl. A SYBR green real-time PCR master mix (Takara) and a Chromo 4 real-time PCR detector (Bio-Rad) were used. The primers used to amplify AtSAG and the other genes were designed based on sequences downloaded from the TAIR database (http://www.arabidopsis.org/). Real-time PCR experiments were performed at least thrice under similar conditions with EF1-α as an internal control. The primers are shown in Supplementary Table S1. Although EF1-α was reported as ABA-inducible in the micropylar endosperm or/and radicle (Graeber et al., 2011), the data showed that, during seed germination, it was induced less than 1.5-times in 1-d-old germinating seedlings, and its expression pattern was similar in the presence or absence of ABA (data not shown). Furthermore, when actin2 was used as the internal control for qRT-PCR, a similar pattern of gene expression was obtained between the wild type (WT) and the sag mutant with or without ABA.
Results
Isolation of the sag mutant
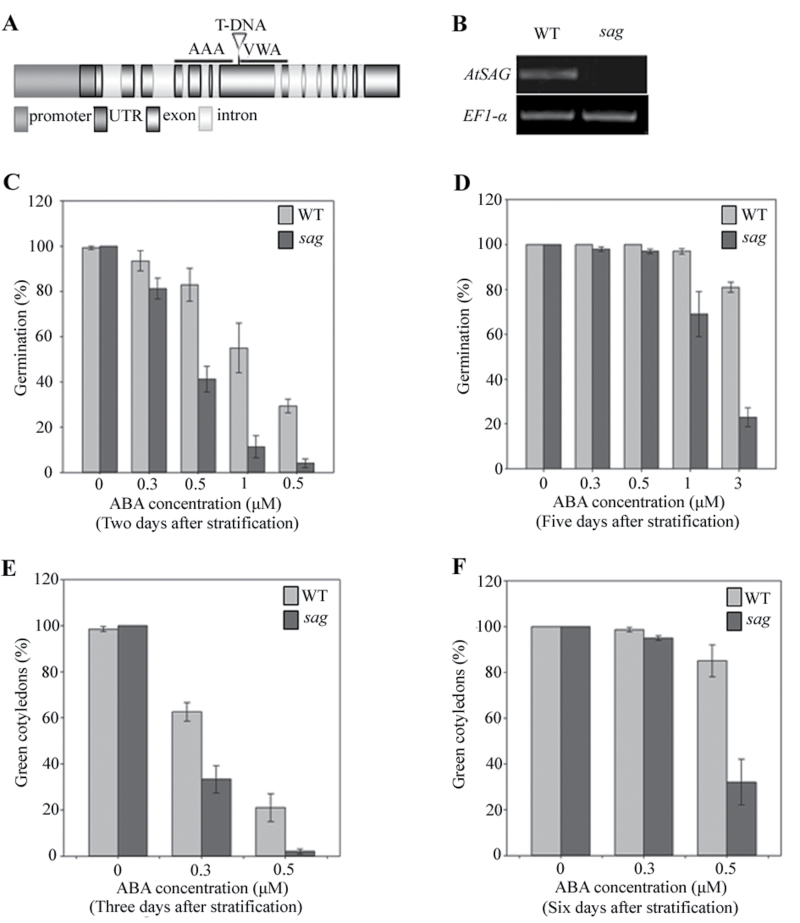
To find other novel regulators and to expand ABA signalling networks during seed germination and abiotic stresses, various T-DNA insertion mutants purchased from the ABRC were screened on half-strength MS medium containing 0.5 µM ABA during seed germination. The mutant sensitive to ABA during seed germination is called sag. A sag mutant was produced by knocking out a MDN1 domain-containing protein (Fig. 1A, B, Supplementary Fig. S1). T-DNA was inserted after nucleotide 938 (Fig. 1A). This procedure may result in VWA domain deletion (Supplementary Fig. S1: asterisks for VWA domain and a arrowhead for T-DNA insertion site).
Fig. 1.
Characterization and ABA-responsive analysis of the T-DNA insertion mutant of sag plants. (A) T-DNA insertion site in sag; black boxes represent exons; white boxes represent introns; AAA and VWA represent the AAA and VWA domains in the putative peptide. (B) Reverse-transcription PCR analysis to confirm the knockout status of sag; upper panel shows AtSAG expression (35 cycles) in wild type (WT) and mutant line; lower panel shows EF1-α expression (25 cycles) as a control. (C and D) Seed germination records of WT and sag mutants treated with 0, 0.3, 0.5, 1.0, and 3.0 μM abscisic acid (ABA) at 2 and 5 d after stratification, respectively. (E and F) Cotyledon greening rates of the germinated seeds described in C and D with 0, 0.3, and 0.5 μM ABA at 3 and 6 d after stratification, respectively. Data are mean ± SD of at least three replicates; at least 100 seeds per genotype were counted in each replicate.
sag seeds are hypersensitive to ABA during seed germination and seedling establishment
In the absence of ABA, no obvious differences were observed in germination rates between WT and sag seeds (Fig. 1C, D). At 0.3 and 0.5 µM ABA, while the germination rates of WT seeds were 94 and 83% at 2 d of germination, respectively, the germination rates of sag seeds were 81 and 41%, respectively (Fig. 1C). At 1.0 and 3.0 µM ABA, while the germination rates of WT seeds were 97 and 81% at 5 d of germination, respectively, the germination rates of sag seeds were 69 and 23%, respectively (Fig. 1D).
The early seedling growth of sag mutants was also slower than that of WT (Fig. 1E, F). A maximum of 100% green cotyledons were observed in both WT and sag seedlings after 3 d of germination in the absence of ABA. At 0.3 µM ABA, 62% of WT but only 33% of sag mutants had green cotyledons after 3 d of germination. At 0.5 µM ABA, 21% of WT but only a few sag seedlings had green cotyledons after 3 d of germination. After 6 d of germination, 32% of sag seedlings and 85% of WT seedlings had green cotyledons. These results indicated that sag mutants were hypersensitive to ABA during seed germination and seedling development.
Higher expression levels of AtSAG during germination were not induced by ABA in Arabidopsis
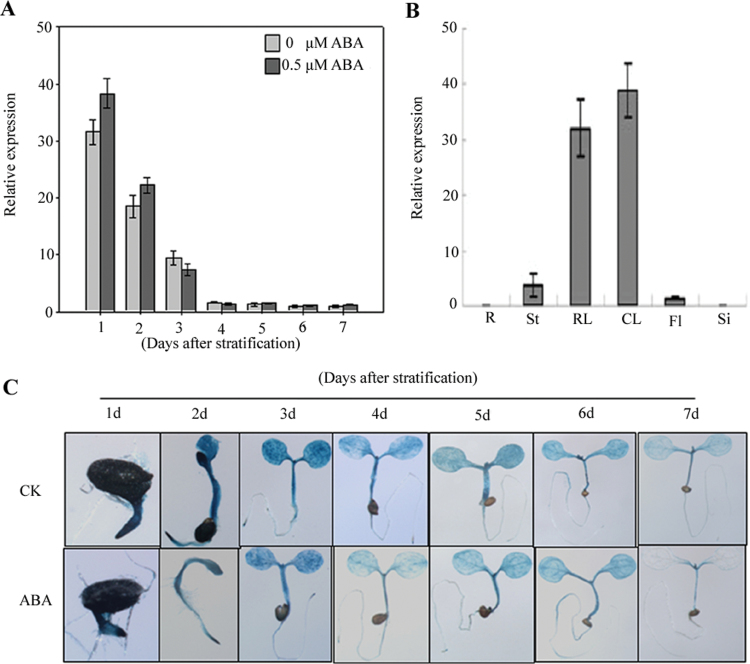
To determine the expression pattern of AtSAG during germination in Arabidopsis, real-time RT-PCR was carried out. Fig. 2A shows that the expression levels of AtSAG were very high at 1–3 d of germination. As germination continued, the expression levels of AtSAG decreased. After 4 d of germination, the expression levels of AtSAG became much lower. The expression levels of AtSAG were not evidently changed by ABA. To further analyse the AtSAG expression pattern, GUS activity driven by native promoter of AtSAG was detected in pSAG:GUS transgenic plants. Strong GUS staining was observed in seeds germinated at 1, 2, and 3 d, but weak staining was observed in 4- to 7-d-old seedlings (Fig. 2C). ABA treatment did not change the GUS staining pattern during the investigated time points (Fig. 2C), although a ABA-responsive element (ABRE-like) was observed in the promoter region of AtSAG (data not shown). Real-time RT-PCR analysis revealed AtSAG expression in multiple organs of more mature plants (Fig. 2B). These results suggested that AtSAG was not induced by ABA during seed germination and may function in other developmental stages under specific conditions.
Fig. 2.
Expression pattern of AtSAG. (A) Relative expression of AtSAG during and after germination after the end of stratification in WT plants treated with 0 or 0.5 μM ABA. (B) Relative expression of AtSAG at differential tissues from the same growth stage in the WT plants. R, root; St, stem; RL, rosette leaf; CL, cauline leaf; F, flower; Si, silique. (C) GUS staining of the pAtASG::GUS transgenic germinating and germinated seedlings grown for 1–7 d on half-strength MS medium containing 1 or 0.5 μM ABA (this figure is available in colour at JXB online).
Response of sag mutant to ABA defined a limited developmental period
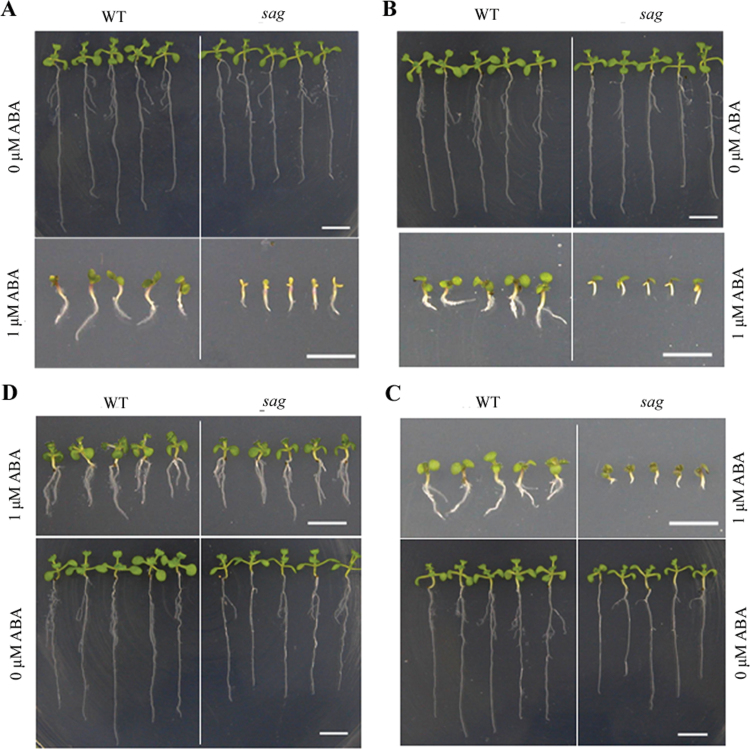
Considering the ABA sensitivity of sag mutant seeds, the effect of ABA on seed germination and seedling development was investigated. When sag seeds were transferred to ABA-containing medium immediately after stratification without ABA, they also showed sensitivity to ABA compared with WT seeds (Fig. 3B). By contrast, the sag seeds showed less sensitivity than those directly stratified and germinated seeds on ABA-containing medium (Fig. 3A). When sag seeds were transferred to ABA-containing medium after 1 d of germination without ABA, they also showed sensitivity to ABA (Fig. 3C). However, when seeds were transferred to ABA-containing medium after 2 d of germination without ABA, no obvious difference was observed between WT and sag seedlings (Fig. 3D). The mutant displayed similar phenotypes in terms of morphology, growth, or development (data not shown). Therefore, AtSAG was involved in ABA responses in seed germination and early seedling development.
Fig. 3.
Response of sag plants to abscisic acid (ABA) defines a limited developmental period. (A) Wild-type (WT) and sag seedlings stratified and germinated on medium containing 0 or 1.0 μM ABA. (B) WT and sag seedlings transferred immediately to medium containing 0 or 1.0 μM ABA after stratification on control medium. (C) WT and sag seedlings transferred to the medium containing 0 or 1.0 μM ABA at 1 d of germination after stratification on control medium. (D) WT and sag seedlings that were transferred to the medium containing 0 or 1.0 μM ABA at 2 d of germination after stratification on control medium (this figure is available in colour at JXB online).
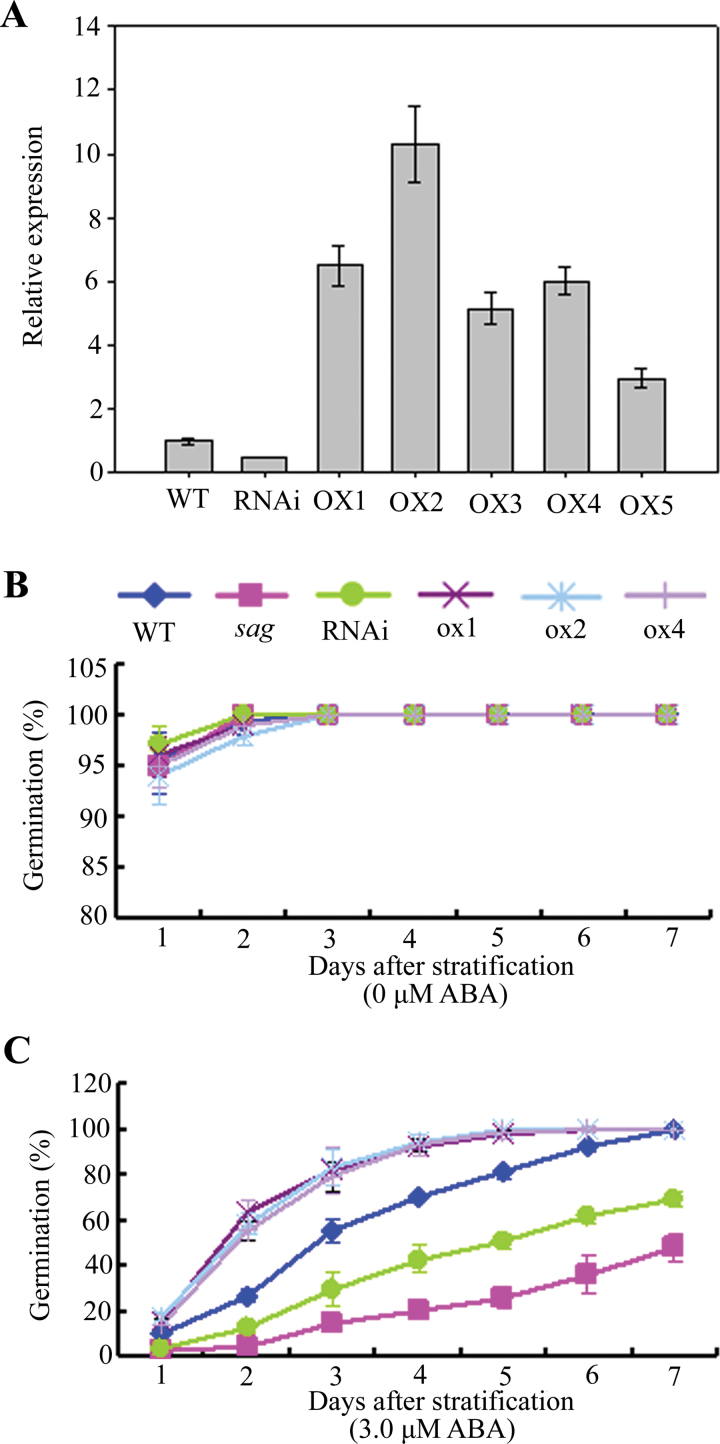
To confirm the function of AtSAG in ABA responses, AtSAG-RNAi transgenic plants (RNAi) and AtSAG-overexpressing plants (OX) were generated. AtSAG expression in these lines was assessed by real-time RT-PCR (Fig. 4A). The AtSAG-OX lines showed decreased sensitivity to ABA, whereas the RNAi line showed less sensitivity to ABA than sag mutant (Fig. 4C), demonstrating that AtSAG functioned as a negative regulator in ABA response during seed germination and early seedling development.
Fig. 4.
Expression analysis and abscisic acid (ABA) responses of the RNAi and OX lines of AtSAG. (A) Real-time PCR analysis of one RNAi line and five independent OX lines of AtSAG; cDNA was obtained from total RNA of 10-d-old seedlings of each phenotype; gene expression was normalized to the WT expression level, which was assigned as a value of 1; standard errors are shown as bars above the columns. (B and C) Seed germination of WT, RNAi, and three OX lines during the time course with 0 and 3.0 μM ABA respectively. Data are mean ± SD of at least three replicates; at least 100 seeds per genotype were counted in each replicate (this figure is available in colour at JXB online).
AtSAG functioned upstream of ABI5 during germination and seedling development
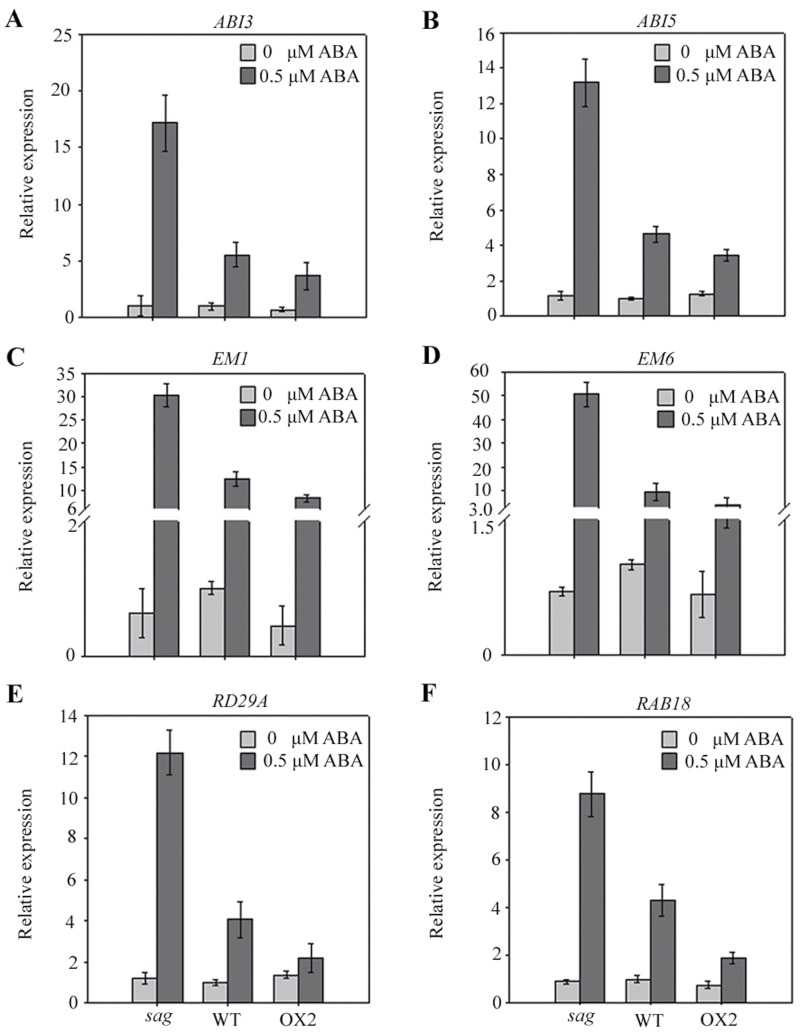
ABI3, ABI5, and late embryogenesis genes were reactivated by ABA within a short development period. To determine whether ABI3 and ABI5 mediated this process, the expression of these genes and their target genes were detected in WT, OX2, and sag seeds germinated for 2 d with or without 0.5 µM ABA by real-time RT-PCR. Fig. 5A–F shows that the expression of ABI3, ABI5, Em1, Em6, RD29A, and RAB18 were very low and showed no obvious differences among WT, OX2, and sag mutants after the seeds were germinated for 2 d without ABA. By contrast, the expression levels of the detected genes, in the presence of 0.5 µM ABA, remarkably increased in sag mutants but decreased in OX2 lines. Western blot analysis also showed that ABI3 and ABI5 proteins accumulated at a higher extent in the presence of 0.5 µM ABA in sag mutant seeds than in WT seeds (Supplementary Fig. S2). These results suggested that AtSAG may function upstream of ABI3 and ABI5 in ABA signalling during seed germination and seedling development.
Fig. 5.
Expression analysis of downstream genes in abscisic acid (ABA) signalling: relative expression of ABI3 (A), ABI5 (B), Em1 (C), Em6 (D), RD29A (E), and RAB18 (F) in the sag, WT, and OX2 seeds germinated for 2.5 d on medium containing 0 or 0.5 μM ABA.
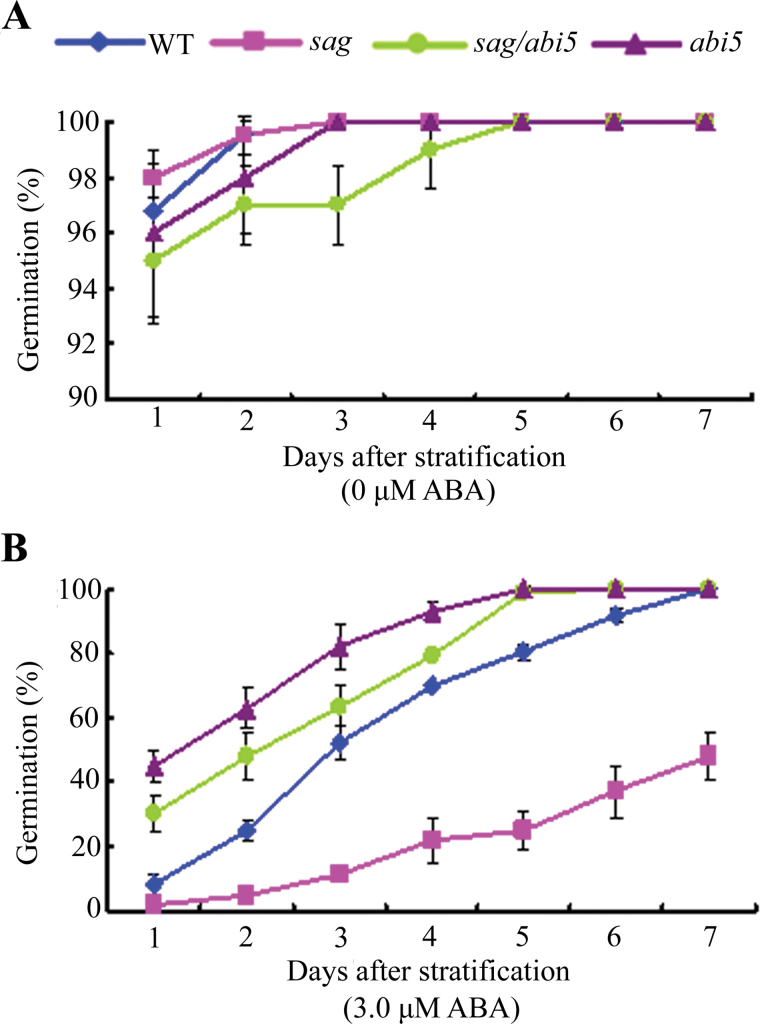
To determine whether AtSAG functioned upstream of ABI5, the sag/abi5 double mutant was generated. In general, the double mutant responded similarly to ABA to abi5 as if AtSAG functioned upstream of ABI5. As expected, the ABA response assays (Fig. 6B) indicated that the sag/abi5 double mutant was more insensitive to ABA than the sag mutant, but exhibited similar sensitivity to abi5 in the presence of 3 µM ABA. These results suggested that AtSAG functioned upstream of ABI5 in ABA signalling.
Fig. 6.
Abscisic acid (ABA) responses of WT, sag, sag/abi5, and abi5 germinated seeds: germination rates on half-strength MS medium containing 0 (A) or 3.0 (B) μM ABA during the time course, respectively. Data show the mean ± SD of at least three replicates; at least 100 seeds per genotype were counted in each replicate (this figure is available in colour at JXB online).
AtSAG participated in the regulation of seed storage protein and oleosin genes during germination and seedling development
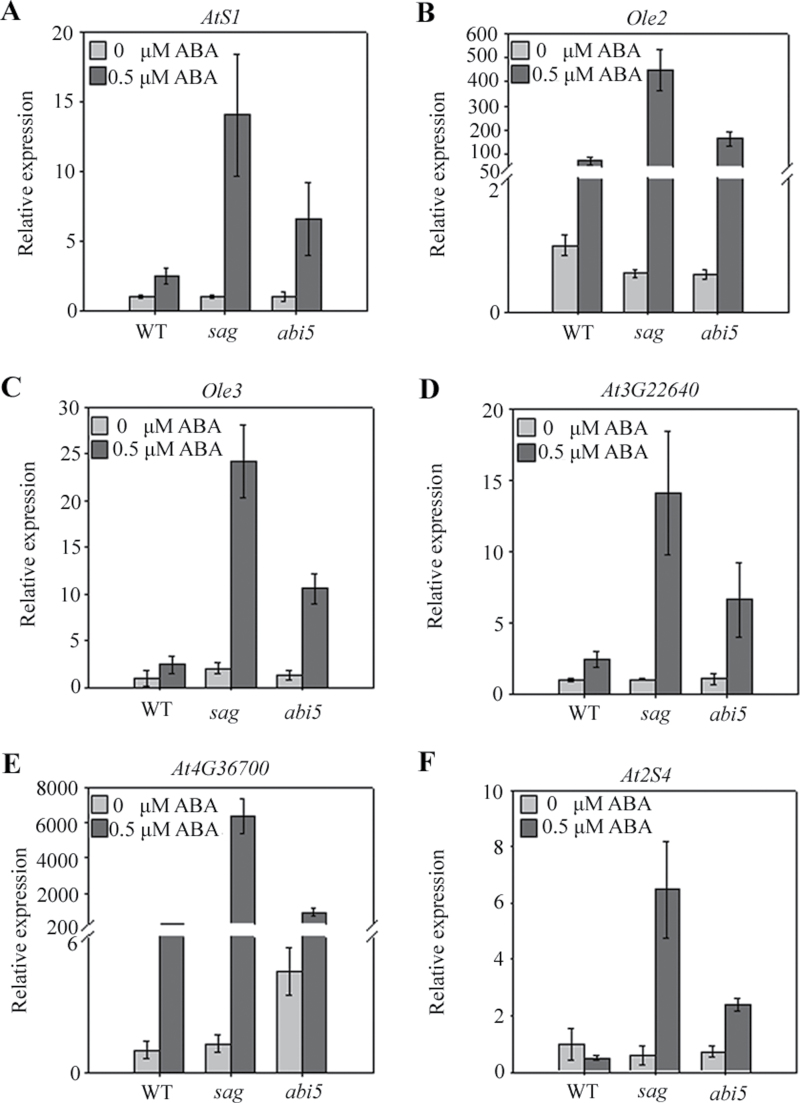
The expression of genes encoding seed storage proteins and oleosins are induced by ABI3 during seed development (Crowe et al., 2000; Lara et al., 2003). They may be induced in sag mutants. To illustrate this phenomenon, three genes encoding for oleosins and three genes encoding for seed storage proteins were selected for real-time RT-PCR. The expression of these selected genes showed no evident change in sag and WT plants without ABA treatment, but their expression was induced more in the sag mutants than in the WT after ABA treatment (Fig. 7). Unlike the induced expression of these genes by ABI3, the expression of these genes was induced in abi5 mutants. Expression of the selected genes was higher in the sag mutant than in the abi5 mutant (Fig. 7). These results demonstrated that the selected genes can be suppressed by AtSAG partially through ABI5.
Fig. 7.
Expression analysis of genes encoding for oleosin and seed storage proteins: relative expression of AtS1 (A), Ole2 (B), Ole3 (C), At3G22640 (D), At4G36700 (E), and At2S4 (F) in WT, sag, and abi5 seeds germinated for 2.5 d on half-strength MS medium with 0 or 0.5 μM abscisic acid (ABA).
Salt and osmotic responses of sag and OX2 plants during seed germination and seedling development
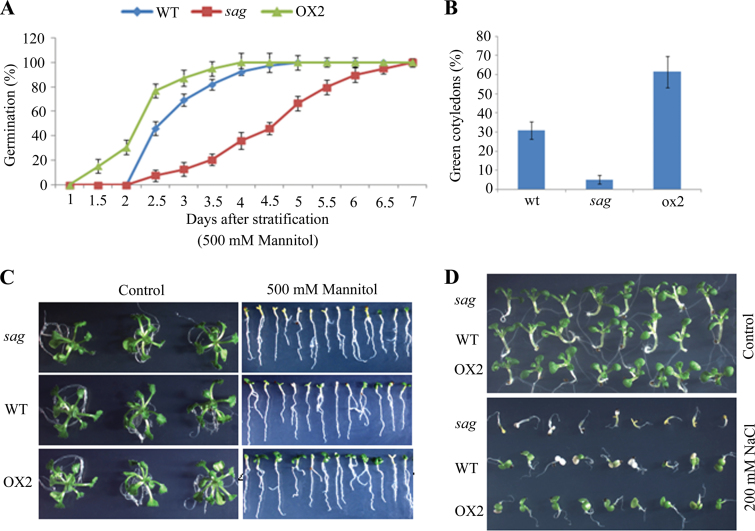
Plants respond to abiotic stresses, such as drought, salt, and dehydration, and such responses are mediated by ABA signalling. To determine whether AtSAG was regulated in response to abiotic stresses, seeds of sag, OX2, and WT plants were sown on a medium containing 500mM mannitol or 200mM NaCl. Germination and green cotyledon rates were scored. The germination and green cotyledon rates of the OX2 lines were higher than those of WT seeds in medium supplemented with 500mM mannitol and the germination and green cotyledon rates of the sag mutants were much lower than those of WT seeds (Fig. 8A–C). The seed germination and green cotyledon rates of the sag mutant and the OX2 lines in medium supplemented with 200mM NaCl showed similar results to those in the mannitol-containing medium (Fig. 8D). These results suggested that AtSAG can regulate abiotic stresses during seed germination and seedling development.
Fig. 8.
Osmotic and salt responses of the sag and OX2 plants during seed germination. (A and B) Germination and cotyledon greening rates of the WT, sag, and OX2 plants with 500mM mannitol during the time course. Data show the mean ± SD of at least three replicates; at least 100 seeds per genotype were counted in each replicate. (C and D) WT, sag and OX2 seedlings grown on half-strength MS medium containing 500mM mannitol or 200mM NaCl, respectively (this figure is available in colour at JXB online).
Discussion
This study demonstrated that AtSAG, encoding a deduced MDN1-containing protein, played an important role in ABA signalling. ABA response assays indicated that the sag mutant and AtSAG-RNAi (RNAi) plants were more sensitive to ABA, whereas 35S:AtSAG plants were less sensitive (Figs 1C–F, 3A–3C, and 4C), suggesting that AtSAG negatively regulated ABA signalling during seed germination and seedling development. However, downregulation of AtSAG did not obviously affect the ABA response of young seedlings (Fig. 3D), supporting the idea that AtSAG may be a ABA signalling component that was specifically effective during seed germination and early seedling development.
Studies have revealed that ABI5 is required for the germination and post-germination developmental arrest checkpoint (Lopez-Molina et al., 2001). Other studies have suggested that the action of AtSAG in ABA signalling may be upstream of ABI5. First, the expression of ABI5 and its target genes (Em1, Em6, RD29A, and RAB18) were upregulated in 2-d germinated seeds of the sag mutant, but were downregulated in OX2 (Fig. 5B–F). Second, the sag/abi5 double mutant showed ABA sensitivity similar to that of the abi5 single mutant, which was more insensitive to ABA than the sag single mutant and the WT seeds (Fig. 6). Finally, AtSAG expression was not largely changed by abi5 mutation (Supplementary Fig. S3).
The ABI3 protein has been shown to interact with ABI5 in a yeast two-hybrid assay (Nakamura et al., 2001). In this context, the current finding that ABI3 had similar upregulated expression patterns to ABI5 in 2-d germinated seeds of sag mutant but downregulated in OX2 (Fig. 5A) suggested that AtSAG may also function upstream of ABI3 in ABA signalling. Together, these data supported the idea that AtSAG was an important negative regulator of ABA signalling depending on ABI3 and ABI5 during seed germination and early seedling development.
The expressions of some seed storage protein and oleosin genes were induced by ABI3 during seed development (Crowe et al., 2000; Lara et al., 2003). In this study, the expression of each three selected seed storage protein and oleosin genes were induced higher by ABA in germinated seeds of abi5 than in WT (Fig. 7), suggesting that, unlike ABI3 during seed development, ABI5 can inhibit the expression of the selected seed storage protein and oleosin genes during seed germination. These genes also showed higher induced expression levels by ABA in sag germinated seeds, even higher than in abi5 (Fig. 7), suggesting that other regulators, downstream of AtSAG, functioned parallel to ABI5 or ABI3 in ABA signalling during seed germination.
Genevestigator analysis indicated that AtSAG was induced in the mapk4 (mitogen-activated protein kinase 4) mutant but repressed in 35S:MKS1 (MAPK4 substrate 1) plants (Supplementary Fig. S4A). This real-time RT-PCR analysis indicated that MAPK4 showed mRNA levels upon treatment with ABA in the sag mutant that were not obviously different from WT (Supplementary Fig. S4B). These data suggest that AtSAG functioned parallel to or downstream of MAPK4 and MKS1. A previous study has confirmed that MAPK4 and MKS1 were associated with WRKY33 in vivo; necrotrophic pathogen infection led to the activation of MAPK4 and phosphorylation of MKS1, and subsequently, MKS1 and WRKY33 were released from MAPK4, and WRKY33 was recruited to the promoter of PHYTOALEXIN DEFICIENT3 (PAD3) (Qiu et al., 2008). WRKY33 has been reported to be induced by 150mM NaCl treatment in Arabidopsis roots (Jiang and Deyholos, 2006), and wrky33 was slightly more sensitive to NaCl treatment in a root elongation assay (Jiang and Deyholos, 2009). Therefore, AtSAG may be regulated by MAPK4, MKS1 and WRKY33 by similar pattern of PAD3.
AtSAG encoded a deduced MDN1-containing protein containing one VWA domain located after the only AAA domain at the N-terminus and a divergent C-terminus (Fig. 1A and Supplementary Fig. S1). The proteins containing AAA and VWA domains often functioned in the assembly of multiprotein complexes. Overexpression of MdMAPK1 from apple led to ABA sensitivity by ABI5 phosphorylation during transgenic Arabidopsis seed germination (Wang et al., 2010). Thus, AtSAG may be involved in the assembly of protein kinase and ABI5 complexes to activate downstream gene expression. By contrast, oleosin deficiency retarded germination in Arabidopsis (Siloto et al., 2006; Shimada and Hara-Nishimura, 2010). Major depletion of seed storage proteins resulted in the completion of germination (Angelovici et al., 2011). Thus, AtSAG may be involved in post-transcriptional other than transcriptional regulation of these genes. Further analysis of one obviously different protein band that was coimmunoprecipitated by AtSAG:GFP (Supplementary Fig. S5) revealed that proteins encoded by Olesin2 (Ole2), At3g01570, and At4g36700 can be coimmunoprecipitated with AtSAG:GFP with molecular weight about 30kDa. However, the predicted molecular weight of these proteins is 21, 19, and 59kDa respectively. This result confirmed the post-transcriptional regulation of AtSAG to these genes.
Two regulatory patterns for AtSAG are possible. First, the yeast MDN1-containing protein Rea1 had an important function in ribosome maturation (Bassler et al., 2010). ScMDN1 and AtMDN1, the plant homologues of Rea1, were predicted to function in similar pattern to Rea1 (Chantha and Matton, 2007). If AtSAG functioned in the similar pattern, it may regulate seed storage protein and oleosin genes at the translational level. Second, RPN10 was one of the subunits of the regulatory particle (RP) of 26S proteasome that contained a VWA domain (Voges et al., 1999) and was originally identified by its ability to bind polyubiquitin chains in vitro (van Nocker et al., 1996). The Arabidopsis rpn10-1 mutant exhibited highly sensitivity to ABA, salt, and sucrose stress by failure to specifically and rapidly degrade the ABA response protein ABI5 during early seedling development (Smalle et al., 2003). The larger molecular weight of the two proteins that were coimmunoparticipated by AtSAG:GFP might resulted from the binding of polyubiquitin chains. Therefore, AtSAG may alternatively function in the assembly of 26S proteasome to degrade seed storage proteins and oleosins. However, the exact molecular process of AtSAG in ABA signalling must be investigated by discovering the interacting proteins.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Primers used in this study.
Supplementary Fig. S1. BlastP alignment between AtSAG and other homologous proteins from Glycine max, Vitis vinifera, and Ricinus communis.
Supplementary Fig. S2. Accumulation of ABI3 and ABI5 in sag mutant germinated seeds with 0.5 μM ABA.
Supplementary Fig. S3. Expression analysis of AtSAG in abi5 germinated seeds.
Supplementary Fig. S4. Expression analysis of AtSAG in mapk4 and 35S:MKS1 plants by genevestigator analysis and expression of MAPK4.
Supplementary Fig. S5. SDS-PAGE of proteins coimmunoprecipitated by AtSAG:GFP.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31071339) and the National Basic Research Program (grant no. 2012CB114200) in China.
References
- Angelovici R, Fait A, Fernie AR, Galili G. 2011. A seed high-lysine trait is negatively associated with the TCA cycle and slows down Arabidopsis seed germination. New Phytologist 189, 148––159 [DOI] [PubMed] [Google Scholar]
- Bassler J, Kallas M, Pertschy B, Ulbrich C, Thoms M, Hurt E. 2010. The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Molecular Cell 38, 712––721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9, 1055––1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantha SC, Matton DP. 2007. Underexpression of the plant NOTCHLESS gene, encoding a WD-repeat protein, causes pleitropic phenotype during plant development. Planta 225, 1107––1120 [DOI] [PubMed] [Google Scholar]
- Crowe AJ, Abenes M, Plant A, Moloney MM. 2000. The seed-specific transactivator, ABI3, induces oleosin gene expression. Plant Science 151, 171––181 [DOI] [PubMed] [Google Scholar]
- Du SY, Zhang XF, Lu Z, Xin Q, Wu Z, Jiang T, Lu Y, Wang XF, Zhang DP. 2012. Roles of the different components of magnesium chelatase in abscisic acid signal transduction. Plant Molecular Biology 80, 519––537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171, 501––523 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14 Suppl, S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. 2000. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. The Plant Cell 12, 599––609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber K, Linkies A, Wood AT, Leubner-Metzger G. 2011. A guideline to family-wide comparative state-of-the-art quantitative RT-PCR analysis exemplified with a Brassicaceae cross-species seed germination case study. The Plant Cell 23, 2045––2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJ. 2008. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytologist 179, 33––54 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK. 2006. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biology 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK. 2009. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Molecular Biology 69, 91––105 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, Hilhorst HW, Karssen CM. 1989. In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana . Plant Physiology 90, 463––469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara P, Onate-Sanchez L, Abraham Z, Ferrandiz C, Diaz I, Carbonero P, Vicente-Carbajosa J. 2003. Synergistic activation of seed storage protein gene expression in Arabidopsis by ABI3 and two bZIPs related to OPAQUE2. Journal of Biological Chemistry 278, 21003––21011 [DOI] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. 1994. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264, 1448––1452 [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. 1998. Abscisic acid signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology 49, 199––222 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. 2001. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis . Proceedings of the National Academy of Sciences, USA 98, 4782––4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. 2002. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. The Plant Journal 32, 317––328 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Lynch TJ, Finkelstein RR. 2001. Physical interactions between ABA response loci of Arabidopsis . The Plant Journal 26, 627––635 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, et al. 2009. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiology 50, 1345––1363 [DOI] [PubMed] [Google Scholar]
- Penfield S, King J. 2009. Towards a systems biology approach to understanding seed dormancy and germination. Proceedings of the Royal Society B 276, 3561––3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Fiil BK, Petersen K, et al. 2008. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO Journal 27, 2214––2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PL, Benning G, Grill E. 1998. ABI2, a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis . FEBS Letters 421, 185––190 [DOI] [PubMed] [Google Scholar]
- Shimada TL, Hara-Nishimura I. 2010. Oil-body-membrane proteins and their physiological functions in plants. Biological and Pharmaceutical Bulletin 33, 360––363 [DOI] [PubMed] [Google Scholar]
- Chantha S-C, Gray-Mitsumune M, Houde J, Matton DP. 2010. The MIDASIN and NOTCHLESS genes are essential for female gametophyte development in Arabidopsis thaliana . Physiol Mol Biol Plants 16, 3––18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siloto RM, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM. 2006. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis . The Plant Cell 18, 1961––1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Kurepa J, Yang P, Emborg TJ, Babiychuk E, Kushnir S, Vierstra RD. 2003. The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. The Plant Cell 15, 965––980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J, Thibault G, Houry WA. 2008. The AAA+ superfamily of functionally diverse proteins. Genome Biology 9, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkish J, Woolford JL., Jr 2009. The Rea1 tadpole loses its tail. Cell 138, 832––834 [DOI] [PubMed] [Google Scholar]
- van Nocker S, Deveraux Q, Rechsteiner M, Vierstra RD. 1996. Arabidopsis MBP1 gene encodes a conserved ubiquitin recognition component of the 26S proteasome. Proceedings of the National Academy of Sciences, USA 93, 856––860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges D, Zwickl P, Baumeister W. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annual Review of Biochemistry 68, 1015––1068 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Zhu SY, Lu YF, Zhao R, Xin Q, Wang XF, Zhang DP. 2010. Two coupled components of the mitogen-activated protein kinase cascade MdMPK1 and MdMKK1 from apple function in ABA signal transduction. Plant Cell Physiology 51, 754––766 [DOI] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Molecular Biology of the Cell 13, 3369––3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.