Changes in the gene expression of sucrose synthases and invertases affected the local and systemic plant metabolism and communication, source–sink relationships, thus nutrition and development of sedentary endo-parasitic nematodes
Key words: Cytosolic invertase, enzyme activity, Heterodera schachtii, Meloidogyne javanica, nematode, neutral invertase, plant pathogen, sucrose synthase.
Abstract
Sedentary endoparasitic nematodes of plants induce highly specific feeding cells in the root central cylinder. From these, the obligate parasites withdraw all required nutrients. The feeding cells were described as sink tissues in the plant’s circulation system that are supplied with phloem-derived solutes such as sugars. Currently, there are several publications describing mechanisms of sugar import into the feeding cells. However, sugar processing has not been studied so far. Thus, in the present work, the roles of the sucrose-cleaving enzymes sucrose synthases (SUS) and invertases (INV) in the development of Heterodera schachtii were studied. Gene expression analyses indicate that both enzymes are regulated transcriptionally. Nematode development was enhanced on multiple INV and SUS mutants. Syncytia of these mutants were characterized by altered enzyme activity and changing sugar pool sizes. Further, the analyses revealed systemically affected sugar levels and enzyme activities in the shoots of the tested mutants, suggesting changes in the source–sink relationship. Finally, the development of the root-knot nematode Meloidogyne javanica was studied in different INV and SUS mutants and wild-type Arabidopsis plants. Similar effects on the development of both sedentary endoparasitic nematode species (root-knot and cyst nematode) were observed, suggesting a more general role of sucrose-degrading enzymes during plant–nematode interactions.
Introduction
Sedentary endoparasitic nematodes such as cyst and root-knot nematodes induce highly specific feeding cell systems in plant roots. The cyst nematode Heterodera schachtii infects different Chenopodiaceae and Brassicaceae species. Subsequently, second stage juveniles (J2s) invade host roots and migrate intracellularly towards the vasculature where they pierce a single cell with their stylets to inject nematode saliva. This provokes a dramatic morphological and physiological re-organization, as well as cell wall openings along plasmodesmata, resulting in the formation of highly specialized syncytial feeding cells (Golinowski et al., 1996). J2s of the root-knot nematodes migrate intercellularly towards the root tip where they pass the endodermis eventually to reach the vasculature. The injection of nematode secretions leads to the formation of several giant cells that emerge by successive karyokinesis events without cytokinesis (Huang and Maggenti, 1969). In the following days, syncytia and giant cells emerge as strong sink tissues that serve the endoparasitic nematodes as the sole source of energy, nutrients, and water (McClure, 1977; Böckenhoff et al., 1996).
Sucrose can be assumed to be the major source of carbohydrate input into nematode-induced feeding sites (NFS) in Arabidopsis thaliana roots. First, sucrose was described as the main transported sugar in the phloem of this plant species (Haritatos et al., 2000). Secondly, metabolite analyses revealed significantly increased sucrose levels in syncytia and giant cells (Hofmann et al., 2007; Baldacci-Cresp et al., 2011). While sugar import mechanisms have been studied intensively in recent years (Juergensen et al., 2003; Hammes et al., 2005; Hoth et al., 2005; Hofmann et al., 2007, 2009, 2010b) there is little information about their processing in NFS.
In sink tissues, sucrose is cleaved to make glucose and fructose available for energy-gaining reactions, macromolecule and amino acid biosynthesis (Ehness et al., 1997). This reaction is performed by two enzyme families, invertases (INVs) and sucrose synthases (SUSs) (Koch, 2004; Vargas and Salerno, 2010). Both use sucrose as a substrate but deliver different products. INVs break sucrose irreversibly into glucose and fructose, and SUSs produce fructose and UDP-glucose, enabling reversible sucrose synthesis.
The A. thaliana genome encodes six SUS genes (Baud et al., 2004; Bieniawska et al., 2007; Barratt et al., 2009) with specific temporal and spatial expression patterns (Bieniawska et al., 2007; Angeles-Nunez and Tiessen, 2010). Proteins for the first four can be found in the soluble extract of mature plants, whereas the remaining isoforms are found in the non-soluble fraction of roots and stems (AtSUS5) and in the hypocotyl (AtSUS6) (Barratt et al., 2009). In A. thaliana, single SUS T-DNA insertion lines showed no changes in sugar composition, while multiple mutants did (Bieniawska et al., 2007). However, all lines showed no phenotypic effects. Thus, so far there are only speculations about the role of different SUS isoforms.
INVs are grouped based on their pH optimum. Acidic INVs (pH 4.5–5.0) are active along cell walls (CWINVs) and in vacuoles (VINVs). In A. thaliana, six and two genes code for CWINVs and VINVs, respectively. Neutral or alkaline INVs (A/N-INVs) that are also named cytosolic invertases (CINVs) are active between pH 6.5 and 8.0. They are coded for by nine different genes in A. thaliana that are expressed in the cytosol as well as in the plasma membrane, the nucleus, chloroplasts, and mitochondria (reviewed by Vargas and Salerno, 2010). The Atcinv1/Atcinv2 double T-DNA insertion line showed reduced root growth and abnormal cell division, indicating the importance of AtCINV1 and AtCINV2 for plant growth and development (Barratt et al., 2009; Vargas and Salerno, 2010).
In additrion to their importance for plant development, the expression of SUS and INV genes was reported to be regulated under abiotic and biotic stresses. INV genes were described to respond to high salinity, drought, or anaerobiosis, as well as to low temperature or hormone treatment (Marana et al., 1990; Zeng et al., 1999; Baud et al., 2004). Further, SUS transcript levels increased during Glomus intraradices colonization of Phaseolus vulgaris (Blee and Anderson, 2002), the formation of root nodules in Pisum sativum (Gordon et al., 1999), the interaction between the symbiont Sinorhizobium meliloti and its host Medicago truncatula (Ferrarini et al., 2008), and in different Vitis vinifera varieties undergoing phytoplasma infection (Hren et al., 2009). Currently, there is no information about the role of A/N-INVs during plant–pathogen interaction. Previous transcriptome analyses on NFS showed that the the currently described 17 INV and six SUS isoforms were either up-, down-, or not regulated as compared with the control (Szakasits et al., 2009; Barcala et al., 2010).
In order to study sucrose breakdown in cyst nematode-infected plants, gene expression, and metabolite analyses, enzyme activity assays and nematode infection and development tests were performed in single and multiple INV and SUS mutants compared with the wild type. Further, the effect of H. schachtii root infection on systemic sucrose processing was studied in selected mutants. Infection tests were also performed on the same T-DNA lines with Meloidogyne javanica. The results showed that nematode infection affects local and systemic sucrose processing in plants and that changes in plant sucrose processing affect nematode development.
Materials and methods
Plant growth and nematode inoculation
Sterile Arabidopsis (mutants and wild-type Col-0) seeds were sown on Knop medium-containing Petri dishes under axenic conditions. To avoid sucrose supplement on the roots that would falsify the results of the analyses, two types of Knop medium were prepared. Each Petri dish was one-third filled with Knop medium supplemented with sucrose. In this area, the seeds germinated so that the hypocotyl and the first millimetres of the main root were provided with sucrose, enabling successful plant growth. The other two-thirds of the dish contained Knop medium without any sugar added so that the collected root and syncytium samples were free of sucrose. For M. javanica (Portillo et al., 2009) infection, plants were grown as described but in Gamborg medium. All plants were cultivated under 16h light/8h dark, at 25 °C. Twelve-day-old plants were inoculated with ~50 freshly hatched sterile H. schachtii J2s per plant obtained from a sterile stock culture (Sijmons et al., 1991) or with 40 freshly hatched M. javanica J2s multiplied in vitro on cucumber roots (Cucumissativus cv. Hoffmanns Giganta).
Syncytia, shoots from infected plants (i-shoots), and roots and shoots from control plants were collected at 5, 10, and 15 days after inoculation (dai) in the middle of the photoperiod to avoid diurnal effects and were immediately shock-frozen in liquid nitrogen and stored at –80 °C until use. Samples were pooled from ~30 plants for each replicate and contained ~20mg fresh weight (FW) of material. Samples were collected in 3–5 biologically independent replicates (in total 90–150 plants).
Mutant selection
Nematode infection tests were performed using the T-DNA single and double mutants listed in Supplementary Table S1 available at JXB online. All these lines were used and described in previous publications (Bieniawska et al., 2007; Barratt et al., 2009), providing a solid basis for the current study. The single T-DNA lines were obtained from the Nottingham Arabidopsis Stock Centre (NASC; http://arabidopsis.info) and screened for homozygosity according to the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu). Multiple T-DNA insertion lines and the Atsus4 T-DNA insertion line were kindly donated by Dr A. Smith (John Innes Centre, Norwich, UK) (Supplementary Table S1). Primer sequences for testing the mutants are listed in Supplementary Table S2.
Nematode infection tests
All lines and the wild type were cultivated and inoculated as described above. For H. schachtii, females and males 15 dai were counted on 35–40 plants per replicate, resulting in between 140 and 330 plants in total in 4–8 replicates. Results were calculated as the number of nematodes per centimetre of root and finally expressed as the percentage of nematodes developing in the wild type. The female size was measured using an inverted microscope (Axiovert200M, Zeiss, Hallerbergmoos, Germany), studying between five and six female nematodes from five different Petri dishes for each line (25–30 females in total). Female sizes were obtained as μm2 and were expressed relative to the size of females that developed on the wild type. Eight weeks after inoculation, 10 brown cysts were collected randomly, crushed open, and suspended in 1ml of gelrite. The number of eggs was counted in 20 aliquots of 10 μl using an Axiovert 200M microscope. All experiments were performed as three biologically independent replicates. For M. javanica, the infection tests were performed with 4–5 plates with 10 seeds per plate in two sets of independent experiments per line (a minimum of 80 individual plants per line). Plates were thoroughly examined under the stereomicroscope at 10 dai to determine the number of galls per plant.
Quantitative reverse transcription–PCR
RNA was extracted from syncytium and control root samples 5, 10, and 15 dai using the RNAeasy QIAGEN extraction kit (Qiagen, Hilden, Germany) including a DNase digest (Qiagen) according to the manufacturer’s protocols. The quality and quantity of RNA were tested with a NanoDrop 2000c (Thermo Scientific, Bremen, Germany) and it was thereafter transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The quantitative PCR (qPCR) was performed using an ABI PRISM 7300 (Applied Biosystems, Foster City, CA, USA). Each reaction contained 12.5 μl of Platinium SYBER Green qPCR Super Mix (Invitrogen), 2 μl of cDNA, MgCl2, and primer according to Supplementary Table S3 at JXB online, and water was added to reach a total reaction volume of 25 μl. As internal references, genes coding for 18S and UBP22 were used (Hofmann and Grundler, 2007). Samples were tested in three biological replicates, each tested as technical triplicates. Blank samples and dissociation runs were made in order to rule out non-specific amplifications. Results were analysed using the SDS 2.0 software (Applied BioSytems) and were determined by the 2–ΔΔCt method (Schmittgen and Livak, 2008).
Metabolic analysis
Metabolites from 6–21mg FW of infected tissues of wild-type and T-DNA insertion lines at 15 dai were extracted by methanol/chloroform (Kaplan et al., 2004). From the remaining pellet, starch was digested by α-amylase from Bacillus licheniformis (Sigma-Aldrich) and amyloglucosidase (Sigma-Aldrich) to obtain glucose equivalents (Wanek et al., 2001). The polar phase and the glucose equivalents were dried in vacuo and stored at –80 °C. Carbohydrates were analysed by high-performance liquid chromatography (HPLC) coupled with pulsed amperometric detection on a Dionex DX500 system using an ED40 electrochemical detector with a gold electrode. Eluents were degassed by flushing with helium. An anion exchange 4×250mm CarboPac PA1 column connected to a 4×50mm guard column was used at 30 °C. The flow rate of the mobile phase was 1ml min–1. The initial mobile phase was 20mM NaOH for 20min; then a gradient from 20mM to 200mM NaOH was applied for 10min and the final 200mM NaOH concentration was maintained for a further 10min. Finally, 20mM NaOH was applied for 20min to equilibrate the columns for the next sample (20 μl injection volume). The identification of different carbohydrates was based on commercially available standards (Sigma-Aldrich, Roth, and VWR).
Enzymatic analysis
Assays for A/N-INVs were modified according to Gibon et al. (2004). Proteins were extracted from ~20mg FW of syncytia, i-shoots, and roots and shoots of non-infected plants at 15 dai. Plant material was ground using liquid nitrogen and incubated in extraction buffer according to Gibon et al. (2004). The supernatant and pellet from the extract were used to assay the acidic vacuolar and cell wall INV activity, respectively, in acetate/KOH pH 4.5. Neutral INV activity was assayed in the supernate in 50mM HEPES pH 7.0. Both types of reaction were started by adding sucrose to a final concentration of 20mM. The reaction was stopped after 20min by heating for 3min at 95 °C. After stopping the reaction, 40 μl of 0.2M HEPES buffer pH 7 were added to neutralize the pH. Glucose production was detected according to Gibon et al. (2004) using a fluorimeter, with an excitation of 571nm and an emission of 585nm (Microplate reader FLUOstar Omega, BMG LABTECH, Offenburg, Germany). Blanks were boiled for 3min before adding the sucrose.
Statistical analysis
Results are expressed as means± SE, n=3–33, all tested in at least three independent biological replicates. Significant differences were calculated using Student’s t-test.
Results
Heterodera schachtii root infection triggers changes in sucrose degradation
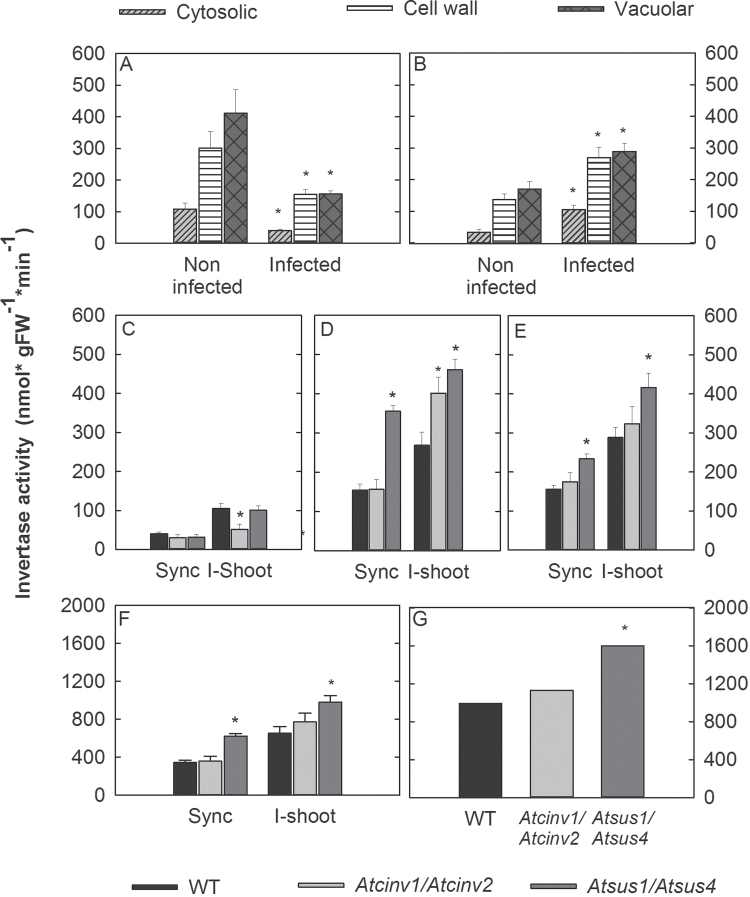
Expression levels of 10 INV and six SUS gene were studied in syncytia at 15 dai compared with control roots using qRT-PCR (Table 1). The analysis showed that AtVINV1, AtCINV1, AtCWIN1, and AtCWINV6 were significantly down-regulated, and, amongst those, AtCINV1 showed the strongest changes; AtSUS1, AtSUS4, and AtSUS6 were significantly up-regulated. Other members of the SUS and INV gene families were not differentially expressed (Table 1). The down-regulation of the different INV genes was reflected by a decrease in neutral (cytosolic) and acidic (vacuolar and cell wall) INV activity in H. schachtii-infected syncytia as compared with the control (Fig. 1A). Further, high levels of glucose and fructose but also sucrose and 1-kestose in nematode-induced syncytia were found (Fig. 2A).
Table 1.
Fold change (log 2 ) expression levels of members of the sucrose synthase (SUS) and invertase (INV) gene families in H. schachtii-induced syncytia (15 dai) analysed by qPCR, and M. javanica-induced giant cells and galls (3 dai) analysed by Superamine TeleChem gene chip by Barcala et al. (2010 ) compared with non-infected A. thaliana roots
| Locus | Name | H. schachtii | M. javanica a | |
|---|---|---|---|---|
| Fold change | Fold change | |||
| Syncytia | Giant cells | Galls | ||
| At1g12240 | AtVINV1 | –1.7±0.6* | 0.1 | –0.3 |
| At1g62660 | AtVINV2 | –1.0±0.9 | –0.5 | –0.4 |
| At1g35580 | AtCINV1 | –1.9±0.1*** | –0.8 | –0.3 |
| At4g09510 | AtCINV2 | 0.0±0.7 | 1.3* | 0.5 |
| At3g13790 | AtCWINV1 | –1.8±0.6* | –0.3 | 0.1 |
| At3g52600 | AtCWINV2 | –0.1±0.4 | 0.0 | –0.1 |
| At1g55120 | AtCWINV3 | 0.5±0.2 | –0.7 | 0.9 |
| At2g36190 | AtCWINV4 | –0.6±0.5 | –0.4 | –0.2 |
| At3g13784 | AtCWINV5 | 0.4±0.6 | 0.0 | –0.1 |
| At5g11920 | AtCWINV6 | –1.4±0.3*** | –0.3 | 0.2 |
| At5g20830 | AtSUS1 | 2.9±0.3** | 2.7* | 2.4** |
| At5g49190 | AtSUS2 | 0.5±0.6 | –0.1 | 0.1 |
| At4g02280 | AtSUS3 | 0.5±1.6 | –1.9 | –0.6 |
| At3g43190 | AtSUS4 | 3.3±0.1*** | –0.1 | 1.1* |
| At5g37180 | AtSUS5 | 0.7±0.8 | –0.3 | –0.1 |
| At1g73370 | AtSUS 6 | 1.8±0.3* | –0.1 | 0.1 |
The neutral invertases At4G34860, At5G22510, At3G06500, At3G05820, At1G56560, At1G72000, and At1G22650, were not analysed during this study.
Values are means±SE, n=3, (Student’s t-test, *P<0.05; **P<0.01; ***P<0.001).
a Data published in Barcala et al. (2010).
Fig. 1.
Invertase activity of cytosolic (CINV), cell wall (CWINV), and vacuolar (VINV) invertases in (A) roots and (B) shoots of non-infected and infected plants (15 dai). (C) CINV, (D) CWINV, and (E) VINV activity in syncytia (sync) and shoots of infected plants (i-shoot) of the wild type and Atcinv1/Atcinv2, and Atsus1/Atsus4 T-DNA double mutant lines. Total invertase activity was equivalent to the sum of cytosolic, cell wall, and vacuolar invertases in (F) syncytia and i-shoots, and (G) in whole infected plants. Values are means±SE, n=9. * indicates significant differences compared with the non-infected control (Student’s t-test, P<0.05).
Fig. 2.
(A) Sugar levels in H. schachtii-induced syncytia and (B) syncytia:shoot ratio of sugars in the wild type and cinv1/cinv2 and sus1/sus4 T-DNA insertion lines (15 dai). Values are means±SE, n=3, * indicates significant differences compared with the wild type (Student’s t-test, P≤0.05).
Lack of INV and SUS expression affects H. schachtii development and offspring production
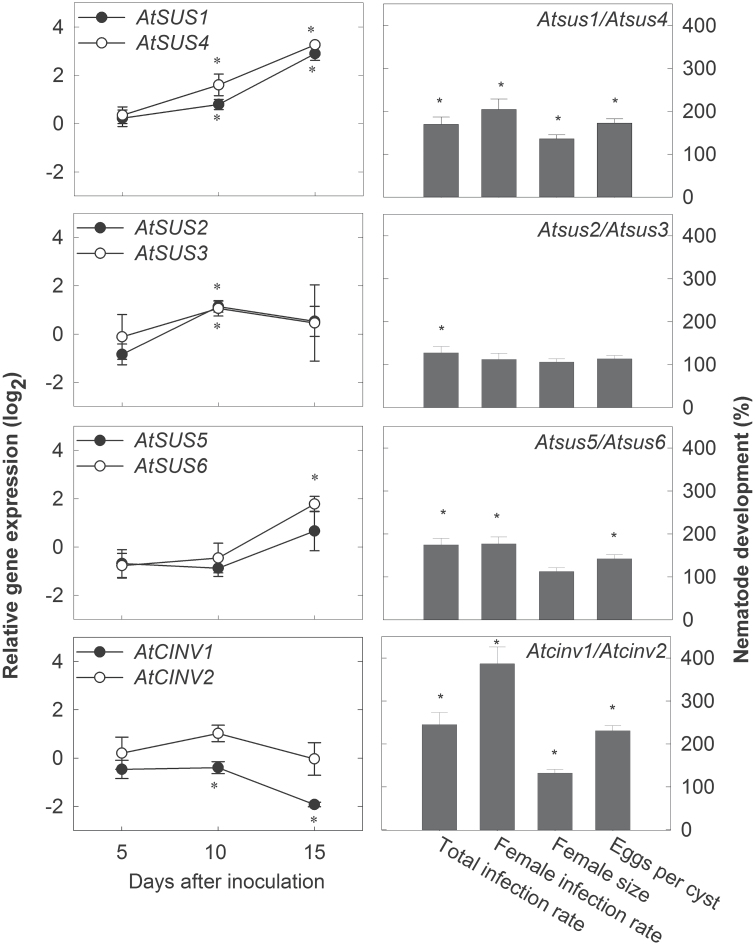
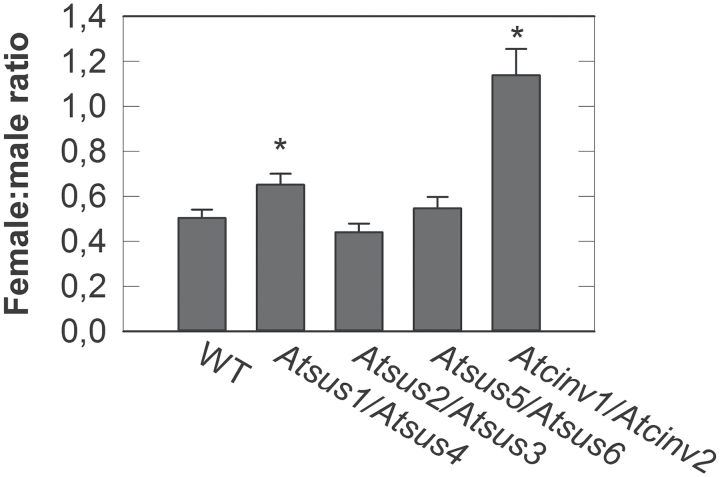
In order to study the role of INV and SUS in nematode development and offspring production, infection tests were performed on single and double mutants. T-DNA insertion lines of all six SUS genes and of the A/N-INV genes CINV1 and CINV2 that were studied most intensively, showing clear effects on plant development (Barratt et al., 2009), were selected. The tests showed that SUS single mutants and the Atcinv2 mutant did not have an effect on H. schachtii development or reproduction. In the Atcinv1 mutant line, significantly more females developed (Supplementary Fig. S1 at JXB online). When two SUS genes were silenced simultaneously, positive effects on nematode development were observed. In the Atsus1/Atsus4 and the Atsus5/Atsus6 lines, female infections doubled compared with the wild type, and eggs per cyst rose to almost 150–200% (Fig. 3). Further, in the Atsus1/Atsus4 line, female size was significantly increased. The Atsus2/Atsus3 double mutant line presented an increase in total infection rate, while none of the developmental or reproductive aspects showed any significant deviation compared with the wild type. The double Atcinv1/Atcinv2 mutant showed the most notable results (Fig. 3). Female development reached up to 400%, the total infection increased up to 300%, the females were 50% bigger, and the number of eggs per cyst was 120% higher than in the wild type. Thus, these data suggest that silencing SUS or CINV genes in double mutants is beneficial for nematode development. Further, the female to male ratio was calculated as a marker for H. schachtii living conditions. There is already evidence to show that sucrose addition into media of axenically cultivated plants enhanced female development, while the absence of sucrose promoted male nematode development (Grundler et al., 1991). Especially in the Atcinv1/Atcinv2 but also in the Atsus1/Atsus4 line, the sex ratio significantly shifted towards the occurrence of females compared with the wild type (Fig. 4).
Fig. 3.
Fold change (log2) expression levels of SUS and CINV genes in H. schachtii-induced syncytia compared with non-infected A. thaliana roots. Values are means±SE, n=3 (left-hand side). Total and female nematode infection rates, female size, and eggs per cyst of H. schachtii on multiple A. thaliana sus and cinv T-DNA double mutant lines relative to the wild type. Values are means±SE, n=14–33 (right-hand side). * indicates significant differences (Student’s t-test, P≤0.05).
Fig. 4.
Female:male ratio of H. schachtii developing on the wild type and on multiple A. thaliana SUS and CINV T-DNA insertion lines. Values are means±SE, n=14–33. * indicates significant differences compared with the wild type (Student’s t-test, P≤0.05).
In order to study the results obtained from the infection assays in depth, detailed gene expression analyses were performed. The expression of AtCINV1, AtCINV2, and all six SUS genes was analysed in wild-type syncytia compared with non-infected control roots during nematode pathogenesis (5, 10, and 15 dai) (Fig. 3). At 5 dai, none of the studied genes was differentially expressed in syncytia compared with non-infected roots. AtSUS1 and AtSUS4 were significantly up-regulated at 10 dai, reaching the highest levels at 15 dai. Similarly, AtSUS5 and AtSUS6 expression increased significantly at 15 dai, but was steady between 5 and 10 dai. Fold change levels of AtSUS2 and AtSUS3 increased significantly at 10 dai (Fig. 3), but was not maintained over time. AtCINV2 was not differentially expressed, but AtCINV1 was significantly down-regulated at 10 and 15 dai. These data show that the SUS genes AtSUS1 and AtSUS4, AtSUS2 and AtSUS3, and AtSUS5 and AtSUS6 as well as AtCINV1 and AtCINV2 have a similar expression pattern during nematode development (Fig. 3). A Pearson’s correlation analysis for the aforementioned genes showed that AtSUS1, AtSUS4, and AtSUS6 expression correlated strongly. Further, AtSUS1 and AtSUS4 showed the highest number of correlations. The CINV genes did not show any significant correlation (Table 2.)
Table 2.
Pearson’s correlation analysis of fold change (log 2 ) expression levels of SUS and CINV genes in H. schachtii-induced syncytia compared with non-infected A. thaliana roots 5, 10, and 15 dai
| AtCINV1 | AtCINV2 | AtSUS1 | AtSUS2 | AtSUS3 | AtSUS4 | AtSUS5 | AtSUS6 | |
|---|---|---|---|---|---|---|---|---|
| AtCINV1 | 1 | |||||||
| AtCINV2 | 0.507 | 1 | ||||||
| AtSUS1 | 0.122 | 0.297 | 1 | |||||
| AtSUS2 | 0.441 | 0.401 | 0.724 | 1 | ||||
| AtSUS3 | 0.189 | 0.593 | 0.392 | 0.615 | 1 | |||
| AtSUS4 | 0.203 | 0.493 | 0.937 | 0.747 | 0.449 | 1 | ||
| AtSUS5 | 0.294 | 0.158 | 0.851 | 0.738 | 0.497 | 0.753 | 1 | |
| AtSUS6 | 0.195 | 0.307 | 0.912 | 0.678 | 0.426 | 0.923 | 0.888 | 1 |
Values in bold represent significant correlations (P≤0.05, Student’s t-test, –0.7 <r> 0.7). Note that the correlation coefficient r is displayed.
Effects of INV and SUS mutation on sucrose processing in H. schachtii-infected roots
Since the Atsus1/Atsus4 and Atcinv1/Atcinv2 double mutants showed the greatest effects on nematode development and reproduction, sucrose breakdown in these mutants was studied in detail in nematode-infected plants. Enzyme activity assays revealed that the Atcinv1/Atcinv2 double mutant showed no change in cytosolic, cell wall, or vacuolar INV activity in syncytia at 15 dai. Cytosolic INV activities were also not affected in syncytia of the Atsus1/Atsus4 line, but vacuolar and cell wall INV activity was significantly increased (Fig. 1C–E). Thus, the total analysed INV activity was significantly increased in syncytia in the Atsus1/Atsus4 line (Fig. 1F). Potential changes in sucrose synthase in the Atcinv1/Atcinv2 line were studied transcriptionally by qPCR. None of the six SUS genes showed differential expression in the Atcinv1/Atcinv2 double mutant compared with the wild type (Supplementary Fig. S2 at JXB online).
Finally, sugar levels of syncytia in the studied double mutants were analysed (Fig. 2A). As in the wild type, glucose, fructose, sucrose, and 1-kestose were the most abundant sugars. Syncytia in the Atcinv1/Atcinv2 lines showed significantly increased sucrose, glucose, and fructose levels, while syncytia in the Atsus1/Atsus4 line revealed increased trehalose and 1-kestose levels.
Heterodera schachtii root infection affects systemic sucrose processing
Changes in syncytial sugar levels may indicate altered sink strength and thus changes in systemic sugar partitioning. Therefore, in the present study, sugar levels in shoots of nematode-infected plants were analysed (Supplementary Fig. S3 at JXB online). The most abundant sugars were, similarly to syncytia, glucose, fructose, and sucrose. In contrast to syncytia, in shoots nematode root infection triggered increased cytosolic, cell wall, and vacuolar INV activities (Fig. 1B). The Atcinv1/Atcinv2 double mutant showed reduced CINV and increased CWINV activity compared with the control. The Atsus1/Atsus4 double mutant had higher CWINV and VINV activity (Fig. 1C–E). This resulted in the highest INV activity levels in shoots and entire nematode-infected plants as compared with the other lines (Fig. 1F, G). However, changed enzyme activities were not reflected in altered sugar levels (Supplementary Fig. S3). In order to verify syncytial sink strength and sugar partitioning, the syncytia:i-shoot ratio of the single analysed sugars was calculated (Fig. 2B). The data revealed striking differences for 1-kestose and starch that were most enriched in syncytia or shoots, respectively (Fig. 2B). However, the syncytia:i-shoot ratios remained unaffected when comparing the Atcinv1/Atcinv2 and the Atsus1/Atsus4 double mutants with the wild type. In the Atcinv1/Atcinv2 line, the sucrose, glucose, and fructose syncytia:i-shoot ratio was significantly changed.
Effect on Meloidogyne javanica
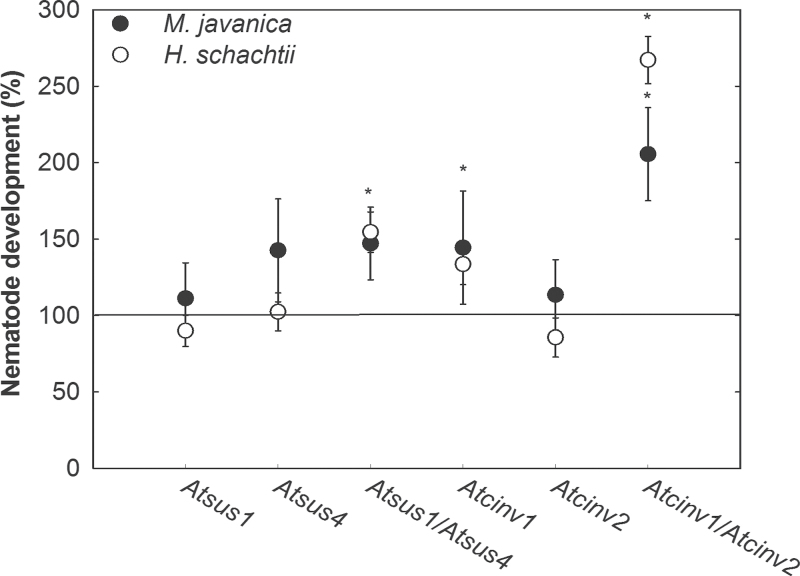
Next, it was examined whether the beneficial effects of lowered SUS and INV expression during development and reproduction of H. schachtii were specific for cyst nematodes or were a more common phenomenon during plant–nematode interactions. A previous transcript profiling showed that gene expression of AtCINV2, AtSUS1, and AtSUS4 was up-regulated in 3-day-old galls and laser-microdissected giant cells of M. javanica (Barcala et al., 2010) (Table 1). Thus, the role of sucrose degradation in the development of the root-knot nematode M. javanica was studied. The number of nematode-induced galls was assayed in the wild type as compared with the Atsus4, Atsus1, Atsus1/Atsus4, Atcinv1, Atcinv2, and Atcinv1/Atcinv2 mutant lines. Figure 5 shows a significantly increased gall formation in the Atsus1/Atsus4 and Atcinv1 lines, and especially in the Atcinv1/Atcinv2 lines. These increased nematode development rates closely match those observed for H. schachtii.
Fig. 5.
Nematode development in single and multiple A. thaliana sus and cinv T-DNA insertion lines compared with the wild type. To determine nematode development, for H. schachtii the number of females, and for M. javanica the number of galls was counted. Values are means±SE, n=10–18. * indicates significant differences compared with the wild type (Student’s t-test, P<0.05).
Discussion
Pathogen-triggered changes in metabolite levels of their hosts may reflect a plant’s responses on the one hand and pathogen requirements on the other hand. In syncytial and giant cells, as well as for the feeding nematodes, sucrose cleavage by INV and SUS can be expected to play a significant role; however, to date, no studies had been performed.
Sucrose cleaving efficiencies affect nematode development
In A. thaliana, 17 INV isoforms were described showing unique expression across plant organs and developmental stages (Vargas and Salerno, 2010); however, in NFS these genes were either down-regulated or not changed (Szakasits et al., 2009; Barcala et al., 2010). INVs are regulated at the post-transcriptional level by proteinase inhibitors, kinases, or compartmentalization (Rausch and Greiner, 2004; Huang et al., 2007), so that transcriptional analyses do not necessarily demonstrate the real extent of enzymatic activity changes. In syncytia, enzyme activities of all three INV groups were reduced, which matched the reduced expression of VINV1, CINV1, CWINV1, and CWINV6 which have been described as defective INVs (Le Roy et al., 2013) (Table 1). This indicates that in syncytia these genes are predominantly responsible for INV activity and that they are transcriptionally regulated.
Silencing AtCINV1 and AtCINV2, predicted to be most abundant in root cells (Barratt et al., 2009), in a double mutant had beneficial effects for both of the studied nematode species (Fig. 5). Since the double mutation had no effect on syncytial INV activity compared with the wild type (Fig. 1), it can be suggested that other INV isoforms have become activated post-transcriptionally. This is supported by elevated glucose and fructose levels in syncytia of the mutant compared with syncytia of wild-type roots (Fig. 2A). The increased sucrose levels may be related to altered import efficiencies and sink–source relationships, as suggested before (Trouverie et al., 2003). Elevated sugar pools were shown to contribute substantially to enhanced nematode development (Grundler et al., 1991) and may thus have major nutritional value for the obligate parasites that may cleave sucrose with their own INVs. In agreement with this, INV activity has been described in Pratylenchus penetrans, Panagrellus redivivus, and M. javanica (Myers, 1965; Claussen and Bird, 1984), and in M. incognita two INV gene were identified (Abad et al., 2008).
In addition to INVs, SUSs may play an essential role in sucrose cleavage in NFS. In plants, the different SUS isoforms show specific temporal and spatial expression patterns and have different roles during plant development and stress response (Angeles-Nunez and Tiessen, 2010). In contrast to INV genes, SUS genes were either up-regulated or not changed in NFS (Fig. 3; Szakasits et al., 2009; Barcala et al., 2010). Nematodes may favour SUS activity that is generating UDP-glucose. UDP-glucose can be directly used for starch, cell wall, and callose synthesis (reviewed by Koch, 2004), which were described to play significant roles during syncytium expansion and nematode development (Hofmann et al., 2008, 2010b; Wieczorek et al., 2008). In agreement with this, AtSUS2 and AtSUS3 were shown to be involved in starch synthesis (Baud et al., 2004; Angeles-Nunez and Tiessen, 2010), and AtSUS5 and AtSUS6 were found to be associated with callose deposition in phloem elements (Barratt et al., 2009).
Consistent with the results obtained for the cinv1/cinv2 line, silencing SUS genes in double mutants had significantly beneficial effects on nematode development. Amongst these, the Atsus1/Atsus4 line offered the best living conditions for both assayed nematode species. The two genes showed 95% similarity (Baud et al., 2004) and exhibit overlapping but also distinct expression profiles. AtSUS1 is expressed throughout the plant and AtSUS4 is mainly confined to roots (Bieniawska et al., 2007); both genes were up-regulated in syncytia (Fig. 3, Table 2).
Invertases and sucrose synthases play particular roles in nematode-induced feeding sites
Even though INVs and SUS both cleave sucrose, the enzymes showed different involvement in the plant’s metabolism, although their impact has so far not been fully uncovered (Bieniawska et al., 2007; Barratt et al., 2009). In nematode-induced syncytia, transcripts of the enzymes are differentially regulated. INVs were described as post-transcriptionally regulated when plants face biotic stressors (Bonfig et al., 2010); and while SUSs were also regulated on the transcriptional level, the induction of AtSUS1 and AtSUS4 gene expression has been reported during plant–pathogen interactions (Deeken et al., 2006). Further, the Atsus1/Atsus4 line presented no altered sucrose, glucose, and fructose levels in roots (Bieniawska et al., 2007) and syncytia (this study). The observed increase in CWINV and VINV activity of nematode-infected Atsus1/Atsus4 plants (Fig. 1D, E) may compensate for the lack of AtSUS4 that is localized not only in the cytosol but also in membranes and vacuoles (Szponarski et al., 2004). The strongly reduced SUS activity in the Atsus1/Atsus4 line (Bieniawska et al., 2007) does not indicate that other SUS isoforms may have taken over sucrose cleavage. The increased sugar pools in the Atsus1/Atsus4 line were trehalose and 1-kestose (Fig. 2A), which may enhance nematode development. Trehalose is a disaccharide that is synthesized by trehalose-6-phosphate synthase from UDP-glucose, a product of sucrose breakdown by SUS (Wingler, 2002). It accumulates under drought, high salinity, and cold stress (Wingler, 2002), it is fundamental for the infection by several pathogens (reviewed by Fernandez et al., 2010), and is enriched during nematode infection (Hofmann et al., 2010a). Even though its role is not yet fully understood, it has been proposed as a sugar signal (Lunn et al., 2006). This may also apply to syncytia since trehalose levels were comparably low. Similarly to trehalose, 1-kestose accumulated during cold stress, stabilizing cell membranes in plant species such as chicory and Jerusalem artichoke (Livingston et al., 2009). Even though 1-kestose enrichment during the A. thaliana–H. schachtii interaction was reported before (Hofmann et al., 2010a), there is currently no information about its metabolism and its role in the non-fructan-accumulating A. thaliana. Fructan biosynthesis was shown to be stimulated by high sucrose and trehalose levels (Cairns and Ashton, 1991; Müller et al., 2000; De Coninck et al., 2005). In the current work its levels also correlated with trehalose, as well as with VINV activity in syncytia of the Atsus1/Atsus4 line.
Changes in INV and SUS expression may affect nematode-triggered plant stress response
The activity of INVs and SUSs may not only be responsible for providing fructose and glucose for the plant cell’s catabolism and anabolism, but were suggested to be involved in plant stress responses and signalling cascades (reviewed by Bolouri-Moghaddam et al., 2010). In fact, the expression of the different INVs was found to be related to plant defence, PR-gene expression, antioxidative enzymes, and changing salicylic acid levels (Herbers et al., 1996; Bonfig et al., 2008, 2010; Bolouri-Moghaddam et al., 2010). Further, changes in free hexose levels may act as signalling compounds regulating plant cell gene expression and activating Snf1-related kinase1, the kinase AtPIP5K9, and hexokinases (Koch, 1996; Tiessen et al., 2003; Lou et al., 2007). These kinases are also described as involved in hormone signalling and balancing reactive oxygen species (Koch, 1996, 2004; Rolland et al., 2001; Camacho-Pereira et al., 2009). The exact molecular mechanisms involved in sugar signalling are still largely unknown and thus also so are their role during plant–nematode interactions. In the study of Bieniewska et al. (2007), SUS1 and SUS4 were further shown to be up-regulated in roots facing hypoxia. Nematode-induced syncytia are described as metabolically highly active (Hofmann et al., 2010a) which may increase oxygen demand and potentially lead to hypoxia. Transcriptome analyses showed that the alcohol dehydrogenase gene At1g77120, and At2G16060 coding for a non-symbiotic leghaemoglobin are significantly up-regulated in syncytia and in giant cells, indicating hypoxia (Szakasits et al., 2009; Barcala et al., 2010).
The altered sink character of syncytia affects systemic sucrose processing
Sugars further act as systemic signalling compounds in plants, facilitating systemic communication between source and sink tissues. NFS have previously been described as metabolic sinks in the plant’s circulation system (Böckenhoff et al., 1996; Hofmann et al., 2007, 2010a; Baldacci-Cresp et al., 2011). This was also observed in the present work showing that most sugar pools were elevated in syncytia; only starch levels were higher in the shoots. The data further indicate that the sink character of syncytia in the Atcinv1/Atcinv2 double mutant was increased as, for example, the syncytia:shoot ratios of sucrose, glucose, and fructose, but also of 1-kestose were increased compared with the wild type (Fig. 2B). Changes in INV or SUS activity in source tissues may affect phloem loading and thus local and systemic sugar levels (Sonnewald et al., 1991) as well as systemic sugar signalling (Koch, 1996, 2004). In agreement with this, silencing Atcinv1/Atcinv2 or Atsus1/Atsus4 triggered increased VINV and CWINV activity in shoots of nematode-infected plants. Further, INV and SUS may affect phloem unloading determining sink strength (Martin et al., 1993; Koch, 1996; Ehness et al., 1997; Fotopoulos et al., 2003). Systemic changes in INV and SUS activity have frequently been described during plant–pathogen interactions. Root CWINV activity in Vicia faba was affected by the shoot pathogen Uromyces fabae (Voegele et al., 2006). In Solanum lycopersicum (formerly Lycopersicon esculentum), the shoot pathogen Botrytis cinerea induced the transcription of two CWINV genes in fruits (Hyun et al., 2011). VINV transcripts increased in shoots when roots were treated with hexanoyl homoserine lactones, a signal molecule of Gram-negative bacteria (Schuhegger et al., 2006).
Summarizing, the present results revealed that SUS and INV play distinct and significant roles during plant–nematode interactions. This was reflected by different transcript and sugar levels that were beneficial for both tested nematode species. Changes in INV and SUS expression led to alterations in the delicate balance of local and systemic sugar processing and signalling. This affects the metabolism of the plant cells as well as systemic plant communication, source–sink relationships, and also nutrition of the parasitic nematodes.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Expression of single SUS and CINV genes in syncytia of wild-type roots and impact of single SUS and CINV mutants in nematode development
Figure S2. Fold change of SUS gene expression in syncytia of the Atcinv1/Atcinv2 line.
Figure S3. Sugar levels of shoots of nematode-infected plants.
Table S1. List of applied T-DNA lines.
Table S2. Primer sequence used to identified the correct T-DNA insertion in SUS and CINV genes.
Table S3. Primers sequences and concentrations, and MgCl2 concentrations for q-PCR
Acknowledgements
We acknowledge the Austrian Science Fund (FWF) for providing a grant for the present work (project P21717-B16) and the Spanish Government for grant AGL2010-17388 to CE and an FPI fellowship to JC. We also thank Dr Alison Smith, John Innes Centre, Norwich, UK, for kindly donating T-DNA lines used in this work.
References
- Abad P, Gouzy J, Aury J-M, et al. 2008. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita . Nature 26, 909–915 [DOI] [PubMed] [Google Scholar]
- Angeles-Nunez J, Tiessen A. 2010. Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta 232, 701–718 [DOI] [PubMed] [Google Scholar]
- Baldacci-Cresp F, Chang C, Maucourt M, et al. 2011. (Homo)glutathione deficiency impairs root-knot nematode development in Medicago truncatula . PLoS Pathogens 8, e1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcala M, Garcia A, Cabrera J, et al. 2010. Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. The Plant Journal 61, 698–712 [DOI] [PubMed] [Google Scholar]
- Barratt DHP, Derbyshire P, Findlay K, et al. 2009. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proceedings of the National Academy of Sciences, USA 106, 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Vaultier M-N, Rochat C. 2004. Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. Journal of Experimental Botany 55, 397–409 [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM. 2007. Analysis of the sucrose synthase gene family in Arabidopsis. The Plant Journal 49, 810–828 [DOI] [PubMed] [Google Scholar]
- Blee K, Anderson A. 2002. Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Molecular Biology 50, 197–211 [DOI] [PubMed] [Google Scholar]
- Böckenhoff A, Prior DAM, Grundler FMW, Oparka KJ. 1996. Induction of phloem unloading in Arabidopsis thaliana roots by the parasitic nematode Heterodera schachtii . Plant Physiology 112, 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W. 2010. Sugar signalling and antioxidant network connections in plant cells. FEBS Journal 277, 2022–2037 [DOI] [PubMed] [Google Scholar]
- Bonfig KB, Gabler A, Simon UK, et al. 2010. Post-translational derepression of invertase activity in source leaves via down-regulation of invertase inhibitor expression is part of the plant defense response. Molecular Plant 3, 1037–1048 [DOI] [PubMed] [Google Scholar]
- Cairns AJ, Ashton JE. 1991. The interpretation of in vitro measurements of fructosyl transferase activity: an analysis of patterns of fructosyl transfer by fungal invertase. New Phytologist 118, 23–34 [Google Scholar]
- Camacho-Pereira J, Meyer LE, Machado LB, Oliveira MF, Galina A. 2009. Reactive oxygen species production by potato tuber mitochondria is modulated by mitochondrially bound hexokinase activity. Plant Physiology 149, 1099–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen W, Bird AF. 1984. The influence of root-knot nematodes (Meloidogyne javanica) on yield and on activity of sucrose synthase and invertase in roots of eggplants (Solanum melongena). Physiological Plant Pathology 25, 209–217 [Google Scholar]
- De Coninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday AM, Smith SM, Laere A, Van den Ende W. 2005. Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant, Cell and Environment 28, 432–443 [Google Scholar]
- Deeken R, Engelmann JC, Efetova M, et al. 2006. An integrated view of gene expression and solute profiles of Arabidopsis tumors: a genome-wide approach. The Plant Cell 18, 3617–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T. 1997. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. The Plant Cell 9, 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C. 2010. Trehalose and plant stress responses: friend or foe? Trends in Plant Science 15, 409–417 [DOI] [PubMed] [Google Scholar]
- Ferrarini A, De Stefano M, Baudouin E, Pucciariello C, Polverari A, Puppo A, Delledonne M. 2008. Expression of Medicago truncatula genes responsive to nitric oxide in pathogenic and symbiotic conditions. Molecular Plant-Microbe Interactions 21, 781–790 [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE. 2003. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum . Plant Physiology 132, 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, et al. 2004. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. The Plant Cell 16, 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinowski W, Grundler FMW, Sobczak M. 1996. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii . Protoplasma 194, 103–116 [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O. 1999. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiology 120, 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundler FMW, Betka M, Wyss U. 1991. Influence of changes in the nurse cell system (syncytium) on sex determination and development of the cyst nematode Heterodera schachtii: total amounts of proteins and amino acids. Phytopathology 81, 70–74 [Google Scholar]
- Hammes UZ, Schachtman DP, Berg RH, Nielsen E, Koch W, McIntyre LM, Taylor CG. 2005. Nematode-induced changes of transporter gene expression in Arabidopsis roots. Molecular Plant-Microbe Interactions 18, 1247–1257 [DOI] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R. 2000. Minor vein structure and sugar transport in Arabidopsis thaliana . Planta 211, 105–111 [DOI] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Metraux JP, Sonnewald U. 1996. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. The Plant Cell 8, 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, El Ashry AEN, Anwar S, Erban A, Kopka J, Grundler F. 2010a. Metabolic profiling reveals local and systemic responses of host plants to nematode parasitism. The Plant Journal 62, 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Grundler FMW. 2007. Identification of reference genes for qRT-PCR studies of gene expression in giant cells and syncytia induced in Arabidopsis thaliana by Meloidogyne incognita and Heterodera schachtii . Nematology 9, 317–323 [Google Scholar]
- Hofmann J, Hess PH, Szakasits D, Bloechl A, Wieczorek K, Daxboeck-Horvath S, Bohlmann H, van Bel AJE, Grundler FMW. 2009. Diversity and activity of sugar transporters in nematode-induced root syncytia. Journal of Experimental Botany 60, 3085–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Szakasits D, Blöchl A, Sobczak M, Daxböck-Horvath S, Golinowski W, Bohlmann H, Grundler FMW. 2008. Starch serves as carbohydrate storage in nematode-induced syncytia. Plant Physiology 146, 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Wieczorek K, Blöchl A, Grundler FMW. 2007. Sucrose supply to nematode-induced syncytia depends on the apoplasmic and symplasmic pathways. Journal of Experimental Botany 58, 1591–1601 [DOI] [PubMed] [Google Scholar]
- Hofmann J, Youssef-Banora M, De Almeida Engler J, Grundler FMW. 2010b. The role of callose deposition along plasmodesmata in nematode feeding sites. Molecular Plant-Microbe Interactions 23, 549–557 [DOI] [PubMed] [Google Scholar]
- Hoth S, Schneidereit A, Lauterbach C, Scholz-Starke J, Sauer N. 2005. Nematode infection triggers the de novo formation of unloading phloem that allows macromolecular trafficking of green fluorescent protein into syncytia. Plant Physiology 138, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hren M, Ravnikar M, Brzin J, et al. 2009. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathology 58, 170–180 [Google Scholar]
- Huang CS, Maggenti AR. 1969. Root-knot nematode induced mitotic aberrations and nuclear changes of developing giant cells in Vicia faba . Phytopathology 59, 447–455 [PubMed] [Google Scholar]
- Huang L-F, Bocock PN, Davis JM, Koch KE. 2007. Regulation of invertase: a suite of transcriptional and post-transcriptional mechanisms. Functional Plant Biology 34, 499–507 [DOI] [PubMed] [Google Scholar]
- Hyun TK, Eom SH, Rim Y, Kim J-S. 2011. Alteration of the expression and activation of tomato invertases during Botrytis cinerea infection. Plant Omics 4, 413–417 [Google Scholar]
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, van Bel AJE, Grundler FMW. 2003. The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiology 131, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. 2004. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology 136, 4159–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 509–540 [DOI] [PubMed] [Google Scholar]
- Koch K. 2004. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology 7, 235–246 [DOI] [PubMed] [Google Scholar]
- Le Roy K, Vergauwen R, Struyf T, Yuan S, Lammens W, Mátrai J, De Maeyer M, Van den Ende W. 2013. Understanding the role of defective invertases in plants: tobacco Nin88 fails to degrade sucrose. Plant Physiology 161, 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D, III, Hincha D, Heyer A. 2009. Fructan and its relationship to abiotic stress tolerance in plants. Cellular and Molecular Life Sciences 66, 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Gou JY, Xue HW. 2007. PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. The Plant Cell 19, 163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, et al. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana . Biochemistry Journal 397, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marana C, Garciaolmedo F, Carbonero P. 1990. Differential expression of 2 types of sucrose synthase-encoding genes in wheat in response to anaerobiosis, cold shock and light. Gene 88, 167–172 [DOI] [PubMed] [Google Scholar]
- Martin T, Frommer WB, Salanoubat M, Willmitzer L. 1993. Expression of an Arabidopsis sucrose synthase gene indicates a role in metabolization of sucrose both during phloem loading and in sink organs. The Plant Journal 4, 367–377 [DOI] [PubMed] [Google Scholar]
- McClure MA. 1977. Meloidogyne incognita: a metabolic sink. Journal of Nematology 9, 88. [PMC free article] [PubMed] [Google Scholar]
- Müller J, Aeschbacher RA, Sprenger N, Boller T, Wiemken A. 2000. Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiology 123, 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RF. 1965. Amylase, cellulase, invertase and pectinase in several free-living, mycophagus, and plant-parasitic nematodes. Nematologica 11, 441–448 [Google Scholar]
- Portillo M, Lindsey K, Casson S, Garc ÍA-Casado G, Solano R, Fenoll C, Escobar C. 2009. Isolation of RNA from laser-capture-microdissected giant cells at early differentiation stages suitable for differential transcriptome analysis. Molecular Plant Pathology 10, 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch T, Greiner S. 2004. Plant protein inhibitors of invertases. Biochimica et Biophysica Acta 1696, 253–261 [DOI] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein JM. 2001. Glucose-sensing mechanisms in eukaryotic cells. Trends in Biochemical Sciences 26, 310–317 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, et al. 2006. Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant, Cell and Environment 29, 909–918 [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, von Mende S, Burrows PR, Wyss U. 1991. Arabidopsis thaliana as a new model host for plant parasitic nematodes. The Plant Journal 1, 245–254 [Google Scholar]
- Sonnewald U, Brauer M, Vonschaewen A, Stitt M, Willmitzer L. 1991. Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast—a powerful tool for studying sucrose metabolism and sink source interactions. The Plant Journal 1, 95–106 [DOI] [PubMed] [Google Scholar]
- Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil DP, Sykacek P, Grundler FMW, Bohlmann H. 2009. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. The Plant Journal 57, 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szponarski W, Sommerer N, Boyer J-C, Rossignol M, Gibrat R. 2004. Large-scale characterization of integral proteins from Arabidopsis vacuolar membrane by two-dimensional liquid chromatography. Proteomics 4, 397–406 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. 2003. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. The Plant Journal 35, 490–500 [DOI] [PubMed] [Google Scholar]
- Trouverie J, Thevenot C, Rocher J, Sotta B, Prioul J. 2003. The role of abscisic acid in the response of a specific vacuolar invertase to water stress in the adult maize leaf. Journal of Experimental Botany 54, 2177–2186 [DOI] [PubMed] [Google Scholar]
- Vargas WA, Salerno GL. 2010. The Cinderella story of sucrose hydrolysis: alkaline/neutral invertases, from cyanobacteria to unforeseen roles in plant cytosol and organelles. Plant Science 178, 1–8 [Google Scholar]
- Voegele RT, Wirsel S, Moll U, Lechner M, Mendgen K. 2006. Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Molecular Plant-Microbe Interactions 19, 625–634 [DOI] [PubMed] [Google Scholar]
- Wanek W, Heintel S, Richter A. 2001. Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Communications in Mass Spectrometry 15, 1136–1140 [DOI] [PubMed] [Google Scholar]
- Wieczorek K, Hofmann J, Blöchl A, Szakasits D, Bohlmann H, Grundler FMW. 2008. Arabidopsis endo-1,4-β-glucanases are involved in the formation of root syncytia induced by Heterodera schachtii . The Plant Journal 53, 336–351 [DOI] [PubMed] [Google Scholar]
- Wingler A. 2002. The function of trehalose biosynthesis in plants. Phytochemistry 60, 437–440 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. 1999. Rapid repression of maize invertases by low oxygen. invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiology 121, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.