This study evaluated if plant ageing can influence the production and composition of seeds in controlled and natural populations of Cistus albidus. Results indicate that reduced plant size in natural populations can help old individuals escape senescence in terms of seed viability loss
Key words: Ageing, Cistus albidus L., perennials, seed composition, seed production, senescence, vitamin E.
Abstract
The question of whether or not perennial plants senesce at the organism level remains unresolved. The aim of this study was to unravel whether or not plant age can influence the production and composition of seeds. Flower and seed production was examined in 3-, 8-, and 13-year-old Cistus albidus plants growing in experimental plots corresponding to the F2, F1, and F0 generations of the same population. Furthermore, the phytohormone, fatty acid, and vitamin E content of the seeds was evaluated, and their viability was examined. Whether or not age-related differences in seed quality were observed in a natural population in the Montserrat Mountains (NE Spain) was also tested. The results indicate that under controlled conditions, the oldest plants not only produced fewer flowers, but also had higher rates of embryo abortion in mature seeds. However, germination capacity was not negatively affected by plant ageing. Seeds of the oldest plants contained significantly higher salicylic acid, jasmonic acid, and vitamin E levels compared with those from younger plants. Despite vigour (in terms of plant growth) being severely reduced due to harsh environmental conditions in the natural population, the oldest individuals produced seeds with no decline in viability. Seed biomass was instead positively correlated with seed viability. In conclusion, increased plant size may explain the loss of seed viability in the experimental field, but older smaller individuals in natural populations can escape senescence in terms of seed viability loss.
Introduction
It is well documented that annual plants enter a controlled senescence programme. In most cases, this is associated with flowering in monocarpic plants (annuals, biennials, and some perennials with a single reproductive episode). However, it is a matter of current debate as to whether or not iteroparous perennial plants, such as trees and shrubs, senesce (Munné-Bosch, 2008; Wingler et al., 2009; Peñuelas and Munné-Bosch, 2010; de Witte and Stöcklin, 2010; Thomas, 2013). While some recent studies have shown symptoms of senescence at the whole-plant level in iteroparous perennials (Ally et al., 2010; Herrera and Jovani, 2010), other studies failed to report senescence symptoms with plant ageing (García et al., 2011; Morales et al., 2013). These results present new challenges for general theories of biological ageing, as they question whether or not ageing of living organisms is a universal pattern in nature. The arguments against these theories are based, at least in part, on the fact that perennials maintain the capacity to develop new leaves and grow throughout their life, due to the presence of meristems. Unless damaged, meristems can potentially give rise to new structures throughout the entire life of a plant. Therefore, some individuals can live for millennia, even without using clonal growth (e.g. the oldest known bristlecone pine has been dated to >4500 years). However, have these organisms suffered age-associated physiological deterioration, or are they young, potentially immortal individuals?
Plants grow vigorously in the early stages of development, until a maximum height is attained at a certain age. Growth rates then tend to decline. With increasing age and size, relative leaf growth and photosynthetic rates in woody perennials tend to slow down. Increased size, rather than meristem ageing, has been proposed as a factor that determines age-related reductions in growth and photosynthetic rates in leaves (Mencuccini et al., 2005; Vanderklein et al., 2007; Oñate and Munné-Bosch, 2008). Similarly, flower production increases with size and total leaf area at early stages of plant development. When advanced developmental ages are reached, flower production becomes stable. For instance, the production of flowers increases with total leaf area in the herbaceous perennial Corydalis intermedia. It then reaches a plateau around an age of 11 years, after which the number of flowers produced remains constant (Ehlers and Olesen, 2004). This study suggests that, given a limited amount of resources, an individual plant allocates a fraction of its resources to reproduction and the remaining resources to survival and growth. Another study found that flower production in the herbaceous perennial Potentilla recta correlates much better with site elevation than with ageing (Perkins et al., 2006). Furthermore, it has been shown that flower production remains constant in 5- to 10-year-old C. albidus plants, even though flower bud vigour decreases with age. This suggests symptoms of senescence at the whole-plant level (Oñate and Munné-Bosch, 2010). Unfortunately, studies of age-related changes in flower production in woody perennials (shrubs or trees) are limited, particularly in older individuals. This is partly because of the intrinsic limitation of such studies: as time elapses and plants age, so the mortality risk increases. To the authors’ knowledge, the effects of plant ageing on seed production in woody perennials has not yet been investigated.
The aim of the present study was to determine whether or not C. albidus plants show symptoms of senescence at the whole-plant level when individuals of sufficiently old ages are sampled. The focus was on senescence symptoms in seed production. Two populations were studied: the first under controlled conditions in experimental plots in which F2, F1, and F0 plants from the same population were assessed, while the second one was studied under natural conditions in the Montserrat Mountains (NE Spain).
Materials and methods
Plant material and sampling
Cistus albidus was selected as the experimental model. This is a common Mediterranean shrub that is widely distributed in the western Mediterranean from sea level to 1400 m (Blasco and Mateu, 1995). This shrub is resistant to drought stress and has a high capacity to grow in degraded environments. The life span of this species is thought to be ~15 years (Roy and Sonié, 1992), so it was possible to evaluate the effects of plant ageing by using plants at advanced developmental stages.
Two independent studies were performed. The first study was conducted using three groups of C. albidus plants of different ages (3-, 8-, and 13-year-old plants) in the Experimental Fields of the Faculty of Biology at the University of Barcelona (Barcelona, Catalonia, NE Spain) in 2011. All specimens were considered mature, since this plant becomes reproductive for the first time in the second year of life. The three plant groups corresponded to three consecutive generations of plants obtained under controlled conditions. The F0 generation (13-year-old plants) corresponded to individuals obtained from seeds that germinated during 1998 and were grown in 0.5 litre pots containing a mixture of soil:peat:perlite (1:1:1, v/v/v), maintained in a greenhouse with controlled temperature and watering for 1 year and then transferred to plots in the Experimental Fields, in which the plants grew for 12 years before the study began. The F1 and F2 generations (8- and 3-year-old plants) corresponded to individuals also sampled during 2011 but obtained from seeds of the preceding generation that germinated during 2008 and 2003, and were grown in 0.5 litre pots containing a mixture of soil:peat:perlite (1:1:1, v/v/v), maintained in a greenhouse with controlled temperature and watering for 1 year and then transferred to plots in the Experimental Fields, in which the plants grew for 7 years and 2 years, respectively, before the study began. Although not identical, due to annual variation, all three plant groups were exposed to Mediterranean climatic conditions during their life histories and were grown in three plots of calcic Luvisol (FAO) of exactly the same characteristics. Plants were separated by 2 m. They were not watered manually other than when first transferred to the soil, at which point all plant groups were watered equally and treated with N:P:K (1:1:1) fertilizer at a rate of 100kg ha–1. Fertilizer was applied again only when mineral deficiencies were detected (maximum application: once a year), but always equally to the three plant groups. All groups were therefore exposed to identical climatic and soil conditions, with the exception of the time spent in these conditions before sampling. Therefore, the effects of ageing were evaluated using three generations grown under the same climatic and soil conditions.
In a second experiment, a natural population of C. albidus was studied. Ninety-one individuals growing naturally in the Natural Park of the Montserrat Mountains (50 km northwest of Barcelona, Catalonia, NE Spain) were sampled. More specifically, the plants were found between 1000 m and 1149 m above sea level (UTM: 401.2012,4.606.724) on a north-facing site. The trunk perimeter of the sampled individuals ranged from 2cm to 22cm. The Montserrat Mountains are exposed to Mediterranean climatic conditions, but summers are drier and winters colder than at the Experimental Fields in Barcelona. The individuals sampled were on the sunny side of the mountain and the soil was a mixture of conglomerate, sandstone, and red shale. Mature seeds from both populations were obtained from plants during September 2011 in the Experimental Fields and in 2012 in the Montserrat Mountains, when fruits were fully mature.
Age estimation
The trunk perimeter of all individuals growing in the Experimental Fields (for several years) and in the Natural Park (during 2012) was measured ~4cm from the trunk base with a measuring tape. Since the age of individuals growing in the Experimental Fields was already known (based on sowing time; see previous section) and the age of 15 individuals growing naturally in the Natural Park was estimated by counting the trunk rings, a regression of the trunk perimeter with the age of individuals was calculated.
Flower and fruit production
The number of flowers produced per individual was counted every day during the flowering period (February–June) in plants growing in the Experimental Fields. Fruit biomass and number of seeds per fruit were estimated from 50 fruits per individual. The percentage of aborted fruits was also estimated by marking 50 flowers (at anthesis) per individual and evaluating the number of mature fruits formed. Fruit and seed biomass were also estimated by weighing the samples both in the Experimental Fields and under natural field conditions. The number of seeds per fruit was also counted.
Seed germination and viability tests
Seed germination and viability tests were performed using seeds collected from both the Experimental Fields and natural field conditions. For the study conducted at the Experimental Fields, 250 seeds per age group were used (five subsamples of 50 seeds). Under natural field conditions, 50 seeds per individual were used.
Germination tests were carried out by sterilizing the seeds in an aqueous solution of bleach and Tween-20 (50:0.15, v/v) for 10min. The seeds were then imbibed for 24h in Milli Q water before being subjected to a heat shock (100 °C, 5min). The seeds were germinated at 17 °C. All steps of the germination test were performed in the dark.
Viability tests were performed as follows. Seeds were imbibed for 24h in the dark and a heat shock was applied as described above. The seeds were then soaked in a tetrazolium solution at 1% and incubated at 37 ºC in darkness for 2 d. Subsequently, seeds were bleached for 10min at 80% and the viable and non-viable seeds were counted. Seeds were considered to be alive when the embryo was intact and fully stained with rich formazan red. Seeds were considered to be non-viable when staining of the embryo was patchy, weak (pink), or absent. A fraction of these seeds were empty (aborted seeds).
Vitamin E and hormonal profiling
The extraction and analyses of tocopherols and tocotrienols were performed as described by Cela et al. (2011). The extraction and analyses of endogenous concentrations of phytohormones, including gibberellins (GAs), abscisic acid (ABA), auxin [indole-3-acetic acid (IAA)], jasmonic acid (JA), salicylic acid (SA), and cytokinins, were performed as described by Müller and Munné-Bosch (2011).
Elemental and fatty acid analyses
Total C and N concentrations as well as the fatty acid profile were measured in seeds of plants growing in the Experimental Fields. Elemental analyses were measured using the Dumas method and an NA2100 protein nitrogen analyser (Thermo, Milan, Italy). The extraction and analyses of fatty acids were performed as described by Vrinten et al. (2005).
Statistical analyses
Differences between age groups were evaluated using analysis of variance (ANOVA), with DMS’s post-hoc test for plants grown in the Experimental Fields. Results were considered significant at a probability level of P ≤ 0.05. The significance of correlations between parameters measured in seeds obtained from plants growing in the Natural Park was tested using Spearman’s rank correlation analyses, and correlations were considered significant when P was ≤0.05.
Results
Flower, fruit, and seed production in the Experimental Fields
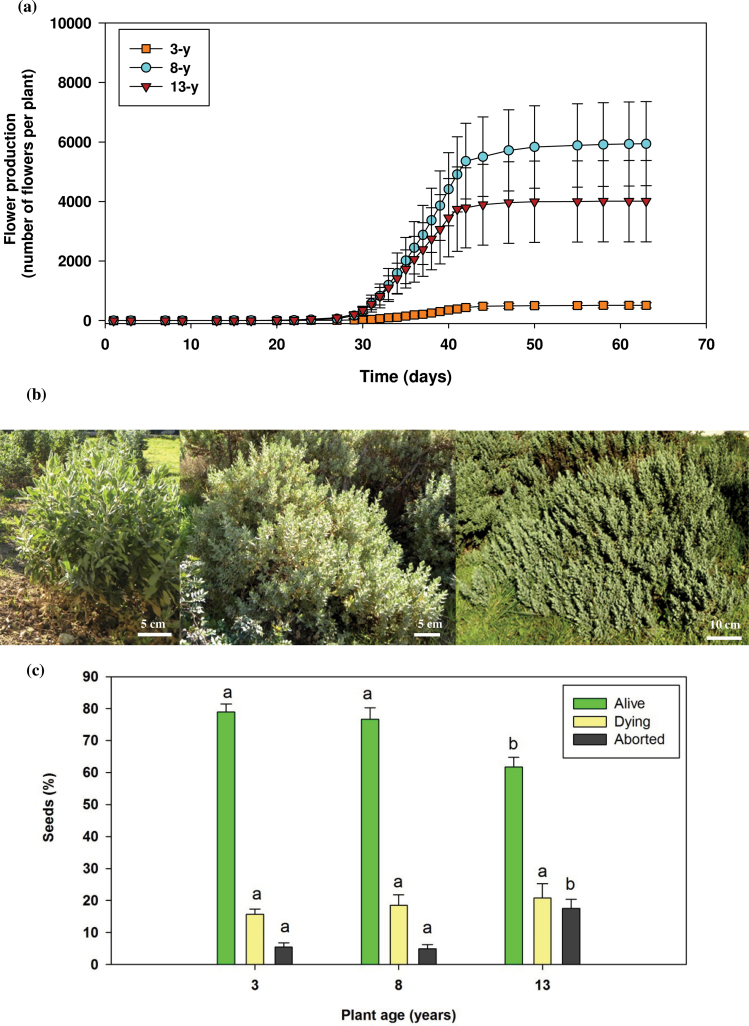
Flower production was affected by plant age in the Experimental Fields. The highest rate of flower production was observed in 8-year-old plants, with an average of ~6000 flowers, followed by the oldest plant group with 4000 flowers, and the youngest plant group with 500 flowers per plant. The first flower was observed in the 8-year-old plants. The 13-year-old plants began to flower 6 d later and the smaller 3-year-old plants 16 d later (Fig. 1a). This timing was consistent with plant size, with the 13- and 8-year-old plants being of similar size, but much bigger than the 3-year-old plants (Fig. 1b). Therefore, reproduction was reduced and delayed in the youngest plant group. However, the high flower production in the two oldest plant groups seemed to affect the biomass and the number of seeds per fruit negatively (Table 1). Thirteen- and 8-year-old plants produced 16% and 40% lower fruit biomass and 31% and 53% fewer seeds per fruit, respectively, than the youngest plant group. Seed biomass decreased significantly with increasing plant age (Table 1). There were no differences between the 3- and 8-year-old plants in the number of alive, dying, and aborted seeds. However, 13-year-old plants produced 20% fewer viable seeds and 3-fold more aborted seeds than the younger plants (Fig. 1c). Nevertheless, comparison of the seed germination capacity revealed no significant variation between the three plant groups (Supplementary Fig. S1 available at JXB online).
Fig. 1.
(a) Flower production (number of flowers) per individual in 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields. Time corresponds to days elapsed since the first flower was observed. Arrows indicate the average date of start of flowering in the three plant groups (24.3±1.5, 7.6±3.5, and 13.0±4.2 d for 3-, 8-, and 13-year-old plants, respectively). Data are the mean ±SE of n=16 individuals for 3-year-old plants and n=4 individuals for 8- and 13-year-old plants. Statistical analyses indicated significant differences between 3- and 8- or 13-year-old plants, but not between 8- and 13-year-old plants in either flower production or time of flowering (P ≤ 0.05). (b) Photographs of plants growing in the experimental fields. Left to right: 3-, 8-, and 13-year-old plants. (c) Percentage of live, dying, and aborted seeds during fruit production in 3-, 8-, and 13-year-old C. albidus plants. Data are the mean ±SE of n=4 individuals with an analysis of 50 seeds per individual. Different letters indicate significant differences between age groups (ANOVA, P ≤ 0.05). (This figure is available in colour at JXB online.)
Table 1.
Fruit biomass, percentage of aborted fruits (from anthesis), number of seeds per fruit, and seed biomass in 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields
| Plant age (years) | Fruit biomass (mg FW fruit–1) | Fruit abortion (%) | No. of seeds per fruit | Seed biomass (mg FW) |
|---|---|---|---|---|
| 3 | 94.3±2.7 a | 1.1±0.6 a | 66.6±2.4 a | 50.8±0.9 a |
| 8 | 56.1±3.3 b | None | 31.3±3.5 b | 43.3±0.6 b |
| 13 | 79.0±3.0 c | 1.1±0.7 a | 45.7±2.3 c | 39.3±0.7 c |
Data are the mean ±SE of n=4 with an analysis of 50 fruits per individual. Different letters indicate significant differences between age groups (P ≤ 0.05).
Biochemical seed composition in the Experimental Fields
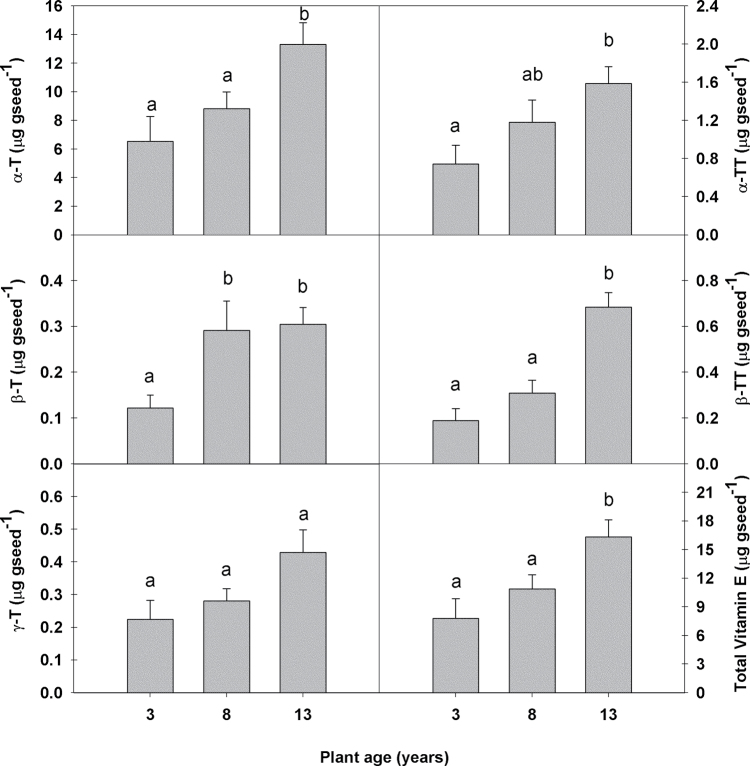
A screen of the fatty acid composition showed that the seeds of the oldest plant group had significantly higher total polyunsaturated fatty acid (PUFA) levels than the seeds from 3- and 8-year-old plants. This was mainly due to their higher levels of linoleic acid (C18:2) (Supplementary Table S1 at JXB online). The seeds of the oldest plant group also showed a tendency to synthesize higher concentrations of very long chain saturated fatty acids. However, only the concentrations of tetracosanoic acid (C24:0) were significantly higher (~17%) than in the seeds of the younger plant groups. Interestingly, the seeds of the oldest plant group had significantly higher total vitamin E levels at 17.6 μg (g DW)–1 than the seeds from 3- and 8-year-old plants, at 8.3 and 11.6 μg (g DW)–1, respectively (Fig. 2). The main vitamin E compound in C. albidus seeds was α-tocopherol. δ-Tocopherol, δ-tocotrienol, and γ-tocotrienol were not detected.
Fig. 2.
Tocopherol, tocotrienol, and total vitamin E content in seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields. Data are the mean ±SE of n=4 individuals with an analysis of 50mg of seeds per individual. Different letters indicate significant differences between age groups (ANOVA, P ≤ 0.05).
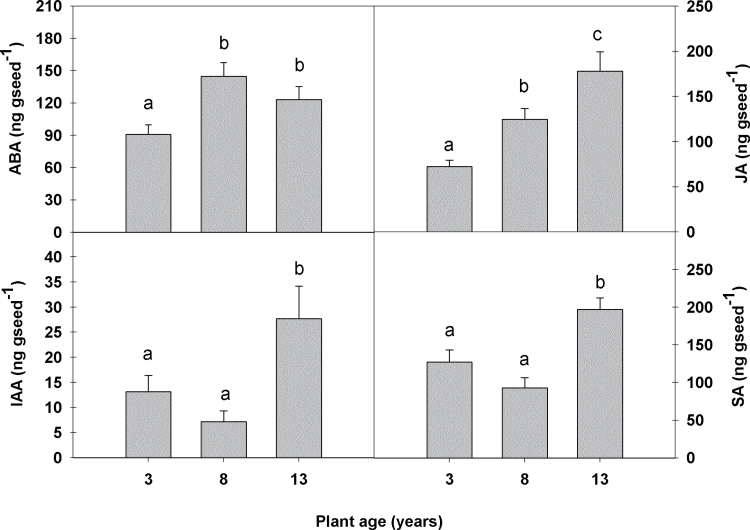
No significant variation in GA4 or the precursor GA24 was found between seeds from any of the plant groups. However, the concentration of the precursor GA9 was significantly lower in the seeds of 3-year-old plants (Supplementary Fig. S2 at JXB online). Endogenous concentrations of ABA were significantly higher in seeds from 8- and 13-year-old plants [154.6ng (g DW)–1 and 133.28ng (g DW)–1, respectively] than in seeds from 3-year-old plants [96.92ng (g DW)–1] (Fig. 3). However, JA levels also increased significantly with increasing plant age: seeds from 8- and 13-year-old plants contained 42% and 60% higher levels, respectively, than seeds from 3-year-old plants. In contrast, the IAA and SA content was only significantly higher in the seeds of the oldest plants (for IAA, 2- and 4-fold; and for SA, 1.58- and 2.15-fold higher than in seeds from 3- and 8-year-old plants, respectively). Within the cytokinins, no differences were found between the three plant age groups, with the exception of levels of zeatin (Z) (Supplementary Fig. S3). Z is the major cytokinin form found in C. albidus seeds, and was ~5-fold higher in seeds from 8-year-old plants than in seeds from 3-year-old plants. The Z levels found in seeds from 13-year-old plants did not differ significantly from those in the other two plant groups.
Fig. 3.
Abscisic acid (ABA), indole-3-acetic acid (IAA), jasmonic acid (JA), and salicylic acid (SA) content in seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields. Data are the mean ±SE of n=4 individuals with an analysis of 50mg of seeds per individual. Different letters indicate significant differences between age groups (ANOVA, P ≤ 0.05).
No significant variation in C and N concentrations or the C/N ratio was found in seeds of any plant group (Supplementary Fig. S4 at JXB online).
Fruit and seed production in a natural population
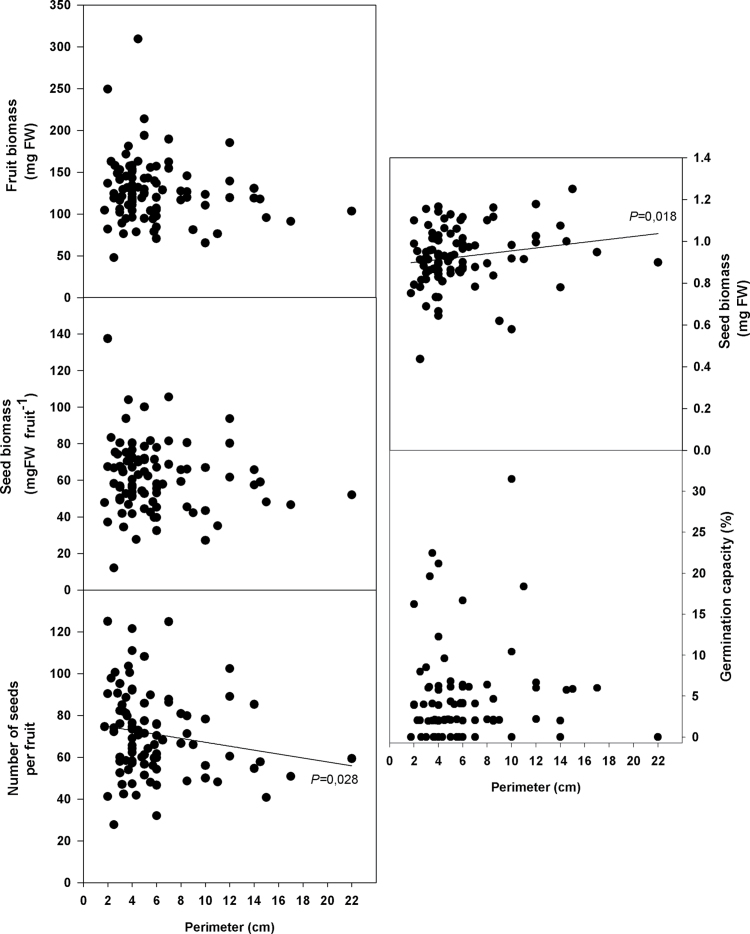
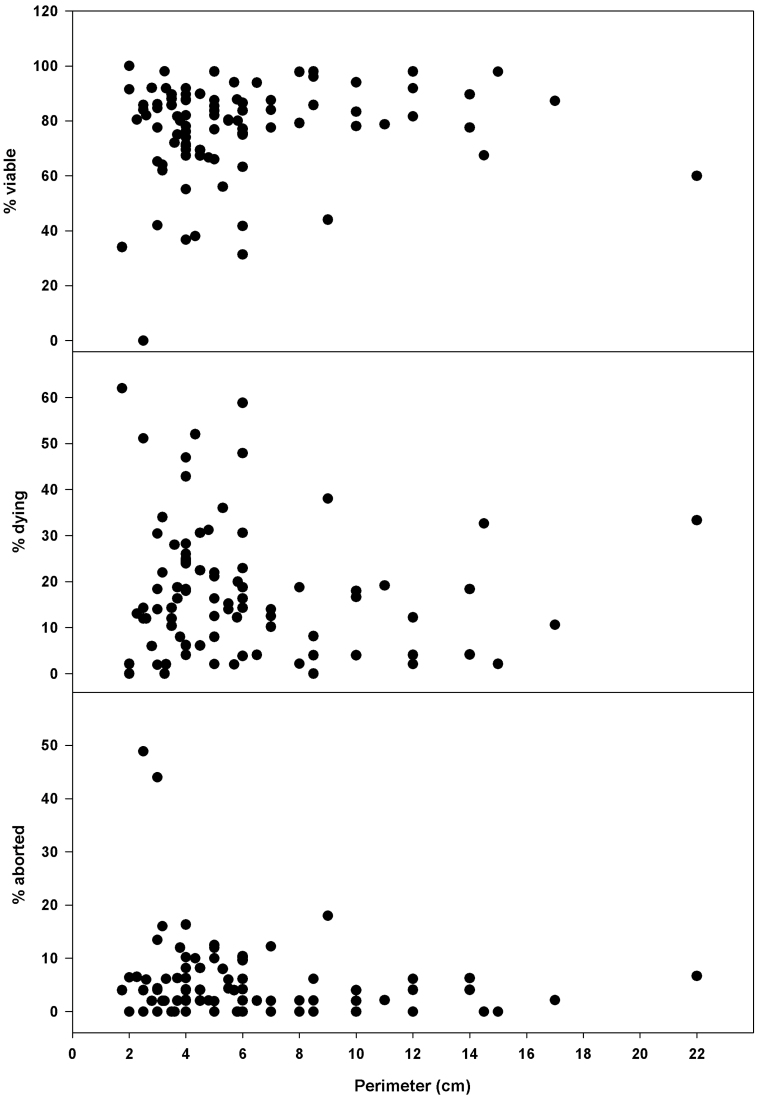
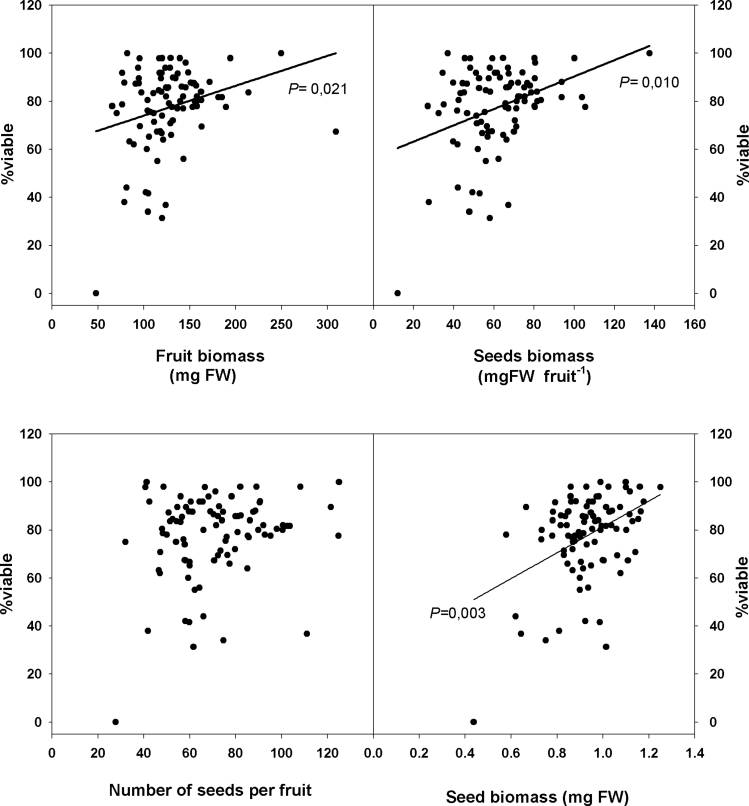
Fruit biomass and seed biomass per fruit were not correlated with the trunk perimeter of the individuals sampled in the Natural Park. In contrast, individuals with larger perimeters produced fewer seeds per fruit (from 79 down to 60 seeds) but larger seeds (from 0.9mg to 1mg fresh matter per seed; Fig. 4). All individuals (except one with a perimeter of 2.5cm) had viable seeds, most of them (75 from 85 individuals) with a seed viability ranging from 60% to 100% (Fig. 5). The percentage of dying seeds ranged between 0% and 60%, but in general the number of dying seeds was low. There were very few aborted seeds except for two individuals. Most importantly, seed viability was not correlated with trunk perimeter. In other words, since trunk perimeter positively correlated with plant ageing (Supplementary Fig. S5 at JXB online), seed viability was not negatively affected by plant age. Instead, seed viability was correlated with fruit biomass, seed biomass, and seed biomass per fruit (Fig. 6). The larger the fruits and seeds, the more viable the seeds produced by individuals in the natural population. Seed germination capacity, which was in general lower than in individuals grown under controlled conditions, was also not affected by plant age (Fig. 4).
Fig. 4.
Fruit biomass, number of seeds per fruit, seed biomass per fruit, biomass of seeds, and seed germination capacity of C. albidus plants with different perimeters growing in a natural population in the Montserrat Mountains (NE Spain). P-values were calculated by Spearman’s rank correlation, and significant values (P ≤ 0.05) are shown.
Fig. 5.
Percentage of live, dying, and aborted seeds of C. albidus plants with different perimeters growing in a natural population in the Montserrat Mountains (NE Spain). Spearman’s rank correlations were not significant for any studied parameter (P > 0.05).
Fig. 6.
Correlation between seed viability and fruit biomass, seed biomass per fruit, number of seeds per fruit, and seed biomass in a natural population in the Montserrat Mountains (NE Spain). P-values were calculated by Spearman’s rank correlation, and significant values (P ≤ 0.05) are shown.
Biochemical seed composition in a natural population
The vitamin E profiling of seeds of C. albidus growing in the Montserrat Mountains confirmed that α-tocopherol is the major vitamin E compound in seeds. The amounts of tocopherols and tocotrienols were not correlated with the perimeter (Supplementary Table S2 at JXB online). δ-Tocopherol, γ-tocotrienol, and δ-tocotrienol were not detected. Likewise there was much variability in plant hormones between individuals with different perimeters and even between individuals with the same perimeter, and thus there was no correlation with perimeter or therefore with age. Levels of GA4, GA9, and GA24, ABA, auxin, SA, JA, and cytokinins were not correlated with the perimeter (Supplementary Table S2).
Discussion
The life span of plants ranges from a few weeks for annuals to thousands of years for some trees (Bliss, 1971; Brundu et al., 2008). Understanding the mechanisms underlying the wide range of longevity in plants or any other organism is fundamental to our understanding of life history, population dynamics, and evolutionary fitness (Vaupel et al., 2004). For most annuals and biennials, reproduction is one of the key factors leading to whole-plant senescence. However, little is known about whole-plant senescence in perennials. To the authors’ knowledge, this is the first study to analyse the effect of mother plant age on seed production and quality in woody perennials.
The study performed in the Experimental Fields showed clear signs of senescence in the 13-year-old plants, in which flower production was 40% lower than in the 8-year-old plant group. The oldest plant group produced more seeds per fruit, but seed production per individual was 13% lower overall. This latter result is based on the assumption that flower vigour was similar in both plant groups, but this is not the case. In a previous study, it was shown that flower bud vigour declines with ageing (Oñate and Munné-Bosch, 2008); therefore, seed production per individual may be even more than 13% lower in 13-year-old compared with 8-year-old plants. Furthermore, seed abortion was higher in the oldest plant group in the Experimental Fields, therefore indicating senescence at the whole-plant level under controlled conditions. This is not surprising, since it has been well documented that when perennials reach an optimal plant size, plant ageing leads to a reduction in growth and photosynthetic rates in leaves (Bond, 2000; Koch et al., 2004; Mencuccini et al., 2005; Oñate and Munné-Bosch, 2008); however, evidence of reproductive senescence (i.e. reproductive decline with ageing) in non-clonal woody perennials is limited. In the perennial Corydalis intermedia, an increase in flower production and total leaf area was observed at early stages of plant development. Around the age of 11 years, flowering reached a plateau and the plants then produced a constant number of flowers (Ehlers and Olesen, 2004). Similar results were found for C. albidus plants, in which flower production was almost identical in 5- and 10-year-old plants of similar sizes (Oñate and Munné-Bosch, 2010). It has been suggested that maternal regulation of offspring quality (Stephenson, 1981) or genetic load (Stanton, 1984; Wiens et al., 1987) are possible mechanisms for determining seed abortion when resources are limited. Intrafruit resource competition could also lead to seed reduction and arise from the fact that the maternal plant predominantly invests resources in zygotes that have higher probabilities of maturing (Shaanker et al., 1988; De Jong and Klinkhamer, 2005). Therefore, it is not surprising that physiological deterioration occurs in seeds with ageing of the mother plant. However, seeds from 13-year-old plants showed similar germination capacity to seeds from the younger individuals. This suggests that a compensatory mechanism may enable similar reproductive fitness in plants growing in the Experimental Fields, such that the offspring are not affected. The higher levels of vitamin E in seeds from 13-year-old plants could explain the similar germination capacity of this group, as α-tocopherol is an important compound for germination (Sattler et al., 2004). Seed viability and vigour are important aspects determining the success of seed germination, which is a key trait for the survival of a species (Nonogaki et al., 2010), but, at the same time, seed germination is a very sophisticated process that requires the concerted action and interaction of diverse plant hormones (Kucera et al., 2005). Although there were no differences in either GA or cytokinin content, the higher levels of ABA, JA, IAA, and SA indicate that an altered hormonal balance might be behind the improved germination capacity, despite the increased proportion of aborted seeds. JA and SA are associated with increased resistance to insects and fungi (Davies, 2010), respectively; therefore, increases in the levels of these phytohormones in seeds of the oldest plants could serve a compensatory mechanism and increase the survival of the seeds. Although a complete understanding of the complex hormonal cross-talk and signalling events leading to this phenotype remains elusive, the present results suggest that vitamin E and hormones may underlie the similar germination capacity despite greater embryo abortion in the oldest plant group.
In the second part of this study, the focus was on confirming these results under natural field conditions. However, plants from a natural population sampled in the Montserrat Mountains (NE Spain) did not show the same symptoms of senescence at the organism level. Seed vigour was not reduced with ageing and, although the number of seeds per fruit decreased with larger perimeters, the seed biomass increased, providing a compensatory mechanism to achieve similar reproductive fitness. Roy and Sonié (1992) found that the annual seed production of 5-year-old Cistus albidus and Cistus monspeliensis plants was comparable with that of older plants. In addition, the viability and germination capacity were similar in plants of all sizes and the number of aborted seeds was always low and not age dependent. So, what was the difference between the population grown in controlled conditions and one growing naturally? The most likely explanation is the size effect, as can be illustrated using the oldest living individual found in the Montserrat Mountains (Fig. 7), which was 19 years old. This individual comprised a large amount of necromass and a small amount of biomass. Compared with the individuals in the Experimental Fields, which had a large biomass (Fig. 1b), even in the youngest group, the biomass of C. albidus growing in the Montserrat Mountains was very low. Note that this pattern was not only found in the oldest individual, but also in other individuals with different ages and trunk perimeters (Supplementary Fig. S6 at JXB online). All individuals, including the oldest living C. albidus from the Montserrat Mountains, had less biomass than the 3-year-old plants growing in the Experimental Fields. This is probably because plants growing in natural conditions are exposed to more severe environmental conditions, such as extreme droughts and contrasting temperatures (winter/summer). Due to the potential for frost damage, C. albidus plants usually have a short stem with three or five branches inserted (Barry, 1960). This pattern was found in some of the sampled plants, especially in older individuals. In natural populations, mortality is greatly influenced by the environment (Picó and Retana, 2008), and C. albidus plants usually die due to natural causes (Roy and Sonié, 1992), for example due to competition for water during drought years or biotic causes. Age, size, and growth can also interact with the environment to influence mortality and life span when the environment is stressful (Roach, 2012). Stress also had an effect on the plants in this study as observed with the correlation of the perimeter of the trunk and the age of the plant: individuals growing in natural conditions with similar ages to those in the Experimental Fields had smaller trunks (Supplementary Fig. S5). Furthermore, the oldest living individual found in the Natural Park was 19 years old, while the oldest one found dead was 25 years old. This was the first time that a C. albidus older than 15 years old (Roy and Sonié, 1992) has been found. It should be noted that the Montserrat Mountains suffered two important fires, one in 1986 and the other in 1994. The fire of 1986, which occurred 27 years ago, razed much of the forest to the ground. This explains why the oldest individuals are no more than 25 years old.
Fig. 7.
(a) Phenotype of the oldest living individual found in a natural population in the Montserrat Mountains (NE Spain) (perimeter of 22cm). Approximately half of the photosynthetic biomass was lost. (b) Estimation of the perimeter at the base of the trunk. (This figure is available in colour at JXB online.)
The C. albidus plants growing in the Montserrat Mountains, which are exposed to hard environmental conditions, showed severe biomass loss (including entire branches) and appeared to use their resources to make fewer fruits and seeds but of higher quality. In contrast, in the Experimental Fields, plants produced more fruits and seeds but of lower quality. Although the oldest individuals in the Experimental Fields were 13 years old and were not as old as they could be, the effect of size was apparent. The viability of the seeds was not affected by the perimeter of the trunk in the natural population (Supplementary Table S2 at JXB online), but instead was correlated with fruit biomass, seed biomass, and seed biomass per fruit (Fig. 6). Thus the higher the fruit and the seed biomass, the more viable the seed. Furthermore, seed germination capacity was lower than under controlled conditions, suggesting a higher degree of dormancy.
In conclusion, the oldest individuals with a large amount of biomass presented symptoms of senescence at the organism level, as indicated by lower seed production and loss of seed viability, but old individuals that have reduced their size, due to reduced growth and photosynthetic biomass loss, produce seeds of higher quality in natural populations. Such plants are less productive in terms of photosynthetic and seed biomass, but the seeds that they produce are of better quality.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Germination capacity of seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields.
Figure S2. Gibberellin (GA) content, including that of GA4, GA9, and GA24, in seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields.
Figure S3. Cytokinin content, including that of zeatin (Z), zeatin riboside (ZR), isopentenyladenosine (IPA), dihydrozeatin (DHZ), dihydrozeatin riboside (DHZR), and 2-isopentenyladenine (2-IP), in seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields.
Figure S5. Correlation between plant age and trunk perimeter in C. albidus plants growing in the Experimental Fields and Montserrat Mountains.
Figure S6. Comparison of the plant biomass between C. albidus from the Experimental Fields and Montserrat Mountains.
Table S1. Fatty acid composition of seeds of 3-, 8-, and 13-year-old C. albidus plants growing in the Experimental Fields.
Table S2. Correlation coefficient (r 2) and P-values of Spearman rank correlation analysis between the trunk perimeter and all measured parameters in seeds of C. albidus plants growing in a natural population in the Montserrat Mountains (NE Spain).
Acknowledgements
Support for this research was received through grants BFU2012-32057, BFU2009-07294, BFU2009-06045, and CSD2008-00040 from the Spanish Government, and the ICREA Academia prize to SM-B, which is funded by the Catalan Government. We thank Jordi Calaf (Natural Park of the Montserrat Mountains) for help provided in the sampling of individuals for counting the trunk rings. We also thank Iker Hernández (University of Barcelona) for his help in sampling and counting the trunk rings; and Emilia Gutiérrez (University of Barcelona) for her advice on cutting the trunks and sample treatment. We are indebted to Toffa Evans for English correction of the manuscript.
Glossary
Abbreviations:
- ABA
abscisic acid
- GA
gibberellin
- IAA
indole-3-acetic acid
- JA
jasmonic acid
- SA
salicylic acid
- Z
zeatin.
References
- Ally D, Ritland K, Otto SP. 2010. Aging in a long-lived clonal tree. PLoS Biology 8, e1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela J, Chang C, Munné-Bosch S. 2011. Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana . Plant and Cell Physiology 52, 1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JP. 1960. Contribution à l’étude de la végétation de la région de Nîmes. III. Les stades préforestiers. Année Biologique 36, 311–540 [Google Scholar]
- Blasco S, Mateu I. 1995. Flowering and fruiting phenology and breeding system of Cistus albidus L. Acta Botanica Gallica 142, 245–251 [Google Scholar]
- Bliss LC. 1971. Artic and alpine plant life cycles. Annual Review of Ecology and Systematics 2, 405–438 [Google Scholar]
- Bond BJ. 2000. Age-related changes in photosynthesis of woody plants. Trends in Plant Science 5, 349–353 [DOI] [PubMed] [Google Scholar]
- Brundu G, Lupi R, Zapelli I, Fossati T, Patrignani G, Camarda I, Sala F, Castiglione S. 2008. The origin of clonal diversity and structure of Populus alba in Sardinia: evidence from nuclear and plastid microsatellite markers. Annals of Botany 102, 997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. 2010. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, ed. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer, 1–15 [Google Scholar]
- De Jong TJ, Klinkhamer PGL. 2005. Evolutionary ecology of plant reproductive strategies. New York: Cambridge University Press [Google Scholar]
- de Witte LC, Stöcklin J. 2010. Longevity of clonal plants: why it matters and how to measure it. Annals of Botany 106, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers BK, Olesen JM. 2004. Flower production in relation to individual plant age and leaf production among different patches of Corydalis intermedia . Plant Ecology 174, 71–78 [Google Scholar]
- García MB, Dahlgren JP, Ehrlén J. 2011. No evidence of senescence in a 300-year-old mountain herb. Journal of Ecology 99, 1424–1430 [Google Scholar]
- Herrera CM, Jovani R. 2010. Lognormal distribution of individual lifetime fecundity: insights from a 23-year study. Ecology 91, 422–430 [DOI] [PubMed] [Google Scholar]
- Koch GW, Sillet SC, Jennings GM, Davis SD. 2004. The limits to tree height. Nature 428, 851–854 [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. 2005. Plant hormone interactions during seed dormancy release and germination. Seed Science Research 15, 281–307 [Google Scholar]
- Mencuccini M, Martínez-Vilalta J, Vanderklein D, Hamid HA, Korakaki E, Lee S, Michiels B. 2005. Size-mediated ageing reduces vigor in trees. Ecology Letters 8, 1183–1190 [DOI] [PubMed] [Google Scholar]
- Morales M, Oñate M, García MB, Munné-Bosch S. 2013. Photo-oxidative stress markers reveal absence of physiological deterioration with ageing in Borderea pyrenaica, an extraordinarily long-lived herb. Journal of Ecology 101, 555–565 [Google Scholar]
- Müller M, Munné-Bosch S. 2011. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectometry. Plant Methods 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S. 2008. Do perennials really senesce? Trends in Plant Science 13, 216–220 [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Bassel GW, Bewley JD. 2010. Germination—still a mystery. Plant Science 179, 574–581 [Google Scholar]
- Oñate M, Munné-Bosch S. 2008. Meristem aging is not responsible for age-related changes in growth and abscisic acid levels in the Mediterranean shrub, Cistus clusii . Plant Biology 10 (Suppl. 1), 148–155 [DOI] [PubMed] [Google Scholar]
- Oñate M, Munné-Bosch S. 2010. Loss of flower bud vigor in the Mediterranean shrub, Cistus albidus L. at advanced developmental stages. Plant Biology 12, 475–483 [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Munné-Bosch S. 2010. Potentially immortal? New Phytologist 187, 564–567 [DOI] [PubMed] [Google Scholar]
- Perkins DL, Parks CG, Dwire KA, Endress BA, Johnson KL. 2006. Age structure and age-related performance of sulfur cinquefoil (Potentilla recta). Weed Science 54, 87–93 [Google Scholar]
- Picó FX, Retana J. 2008. Age-specific, density-dependent and environmental-based mortality of a short-lived perennial herb. Journals of Ecology 97, 1000–1009 [DOI] [PubMed] [Google Scholar]
- Roach DA. 2012. Age, growth and size interact with stress to determine life span and mortality. Experimental Gerontology 47, 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Sonié L. 1992. Germination and population dynamics of Cistus sp. in relation to fire. Journal of Applied Ecology 29, 647–655 [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. 2004. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. The Plant Cell 16, 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaanker U, Ganeshaiah KN, Bawa KS. 1988. Parent–offspring confilct, sibling rivalry, and brood size patterns in plants. Annual Review of Ecology and Systematics 19, 177–205 [Google Scholar]
- Stanton ML. 1984. Developmental and genetic sources of seed weight variation in Raphanus raphanistrum L. (Brassicaceae). American Journal of Botany 7, 1090–1098 [Google Scholar]
- Stephenson AG. 1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12, 253–279 [Google Scholar]
- Thomas H. 2013. Senescence, ageing and death of the whole plant. New Phytologist 197, 696–711 [DOI] [PubMed] [Google Scholar]
- Vanderklein D, Martínez-Vilalta J, Lee S, Mencuccini M. 2007. Plant size, not age, regulates growth and gas exchange in grafted Scots pine trees. Tree Physiology 27, 71–79 [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Baudisch A, Dölling M, Roach DA, Gampe J. 2004. The case for negative senescence. Theoretical Population Biology 65, 339–351 [DOI] [PubMed] [Google Scholar]
- Vrinten P, Hu Z, Munchinsky M-A, Rowland G, Qiu X. 2005. Two FAD3 desaturase genes control the level of linolenic acid in flax seed. Plant Physiology 139, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens D, Calvin CL, Wilson CA, Davern CI, Frank D, Seacey SR. 1987. Reproduction success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia 71, 501–509 [DOI] [PubMed] [Google Scholar]
- Wingler A, Masclaux-Daubresse C, Fischer AM. 2009. Sugars, senescence, and ageing in plants and heterotrophic organisms. Journal of Experimental Botany 60, 1063–1066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.