Preclinical studies suggest that ketamine-induced psychopathology is mediated, in part, by increased glutamate release, hypothesized to occur via inhibition of GABAergic interneurons. Using proton magnetic resonance spectroscopy (1H-MRS), we tested this hypothesis in healthy humans. Ketamine increased anterior cingulate cortex glutamate levels, which correlated with the degree of positive psychotic symptoms. Ketamine did not affect subcortical gamma-aminobutyric acid (GABA) levels.
Ketamine, an uncompetitive N-methyl-D-aspartate glutamate receptor antagonist, induces perceptual and behavioral responses resembling the positive, negative and cognitive symptoms of schizophrenia.1 In rodents, increases in cortical glutamate have been shown following acute systemic ketamine administration, as measured by microdialysis,2 and also following chronic ketamine administration, as measured by 1H-MRS.3 In humans, increased glutamine levels in anterior cingulate have been detected using 1H-MRS following ketamine administration.4 Ketamine has been suggested to increase cortical glutamate release through inhibition of GABAergic interneurons,5 possibly in thalamus.6 In this study, we used 1H-MRS to test the hypothesis that ketamine administration in humans reduces subcortical GABA levels and increases cortical glutamate and glutamine levels, and that these changes are related to ketamine-induced psychotic symptoms.
Methods are fully described in Supplementary Materials. Briefly, 1H-MRS data were acquired, using a 3 Tesla magnetic resonance imaging system, from 13 healthy male volunteers. GABA-edited MR spectra using the MEGA-PRESS method were acquired from a 30 × 30 × 30 mm3 volume positioned medially over the thalami (bilaterally) and surrounding subcortical structures (see Supplementary Figure S1), followed by a PRESS acquisition (TE = 30), to estimate gluta-mate levels, acquired from a 20 × 20 × 20 mm3 midline volume positioned over the anterior cingulate 13 mm above the genu of corpus callosum (see Supplementary Figure S2). A dynamically modeled intravenous infusion of ketamine was commenced (target plasma level: 150 ng ml–1, Supplementary Figure S3), and the GABA and glutamate acquisitions were repeated at 25 and 35 min after the start of the infusion, respectively. After completing the scan, ketamine-induced effects were measured using the positive and negative syndrome scale (PANSS).
1H-MRS data were analysed using LCModel (PRESS) and in-house software developed in Matlab (MEGA-PRESS). Adequate spectrum quality was confirmed by visual inspection (Supplementary Figures S3 and S4). PRESS metabolites with a reported Cramer-Rao Lower Bound (CRLB) greater than 20% were excluded from further analysis. Following estimation of metabolite levels, data were corrected for voxel CSF and water content. Normality of data was checked using the Shapiro–Wilk test. Changes in anterior cingulate glutamate and in subcortical GABA were analyzed using paired t-tests. Correlations between metabolite levels and PANSS scores were analyzed using Pearson's product moment correlation coefficient.
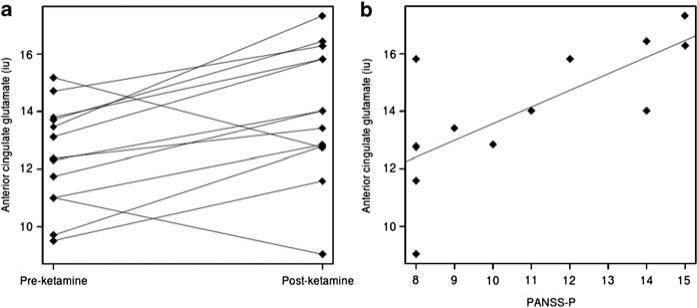
Following ketamine administration, mean (s.d.) PANSS positive, negative and general subscales increased to 10.7 (2.89), 10.07 (3.43) and 20.15 (3.53), respectively. Anterior cingulate glutamine quantification was of poor quality (CRLB > 20%) in 63% of scans, and so data were insufficient to examine whether ketamine had any effect on this metabolite. Ketamine led to a significant increase in glutamate in anterior cingulate (t = 3.11, degree of freedom = 12, r = 0.67, P = 0.009; Figure 1), but did not affect anterior cingulate glutamate+glutamine, or subcortical GABA levels (see Supplementary Table S1). Anterior cingulate glutamate following ketamine administration was significantly related to PANSS positive (r = 0.72, P = 0.005; Figure 1), but not PANSS negative or general subscales. PANSS subscales were not related to baseline anterior cingulate glutamate levels nor to GABA levels at either time point.
Figure 1.
Glutamate levels in anterior cingulate before and after ketamine (a; P = 0.009), and relationship of anterior cingulate glutamate levels with positive and negative syndrome scale (PANSS) positive scores following ketamine administration (b; P = 0.005).
This study provides support for the hypothesis that ketamine leads to increases in glutamatergic neuro-transmission in anterior cingulate cortex. One limitation is the lack of placebo-control. However, our results are in keeping with the finding of increased glutamate in frontal cortex in rats following ketamine administration,3 and converge with an earlier finding of increased glutamine in anterior cingulate in the first 10 min following ketamine administration in humans.4 It has been suggested that increased glutamine may be the first marker of increased glutamate release, with increases in glutamate becoming detectable as glutamine is converted back to glutamate,3 which may explain the difference between our finding and that of Rowland et al.4 The lack of an effect of ketamine on subcortical GABA is in keeping with an earlier study that failed to show any effect of ketamine on glutamate or GABA levels in occipital cortex,7 but it is possible that ketamine effects on GABA neuron activity occur at an earlier time point or in a different brain region.
Increased glutamate transmission, as indexed by glutamine levels in anterior cingulate, has been reported in individuals at risk of psychosis and in patients with first-episode schizophrenia,8,9 whereas in depression, reduced glutamate levels are generally reported.10 The current study suggests that ketamine-induced psychological effects may share a common neurochemical basis with idiopathic psychoses and that increases in glutamatergic neuro-transmission may underlie ketamine's antidepressant effect.
Supplementary Material
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam B, Adams B, Verma A, Daly D. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee DW, et al. NMR Biomed. 2011 doi: 10.1002/nbm.1681. doi:10.1002/nbm.1681, PMID 21560175 (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 5.Homayoun H, Moghaddam B. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber NB, Jiang X, Dikranian K, Nemmers B. Brain Res. 2003;993:90–100. doi: 10.1016/j.brainres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Biol Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 10.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.